Left Ventricular “Longitudinal Rotation” and Conduction Abnormalities—A New Outlook on Dyssynchrony
Abstract
1. Introduction
2. Methods
2.1. Patient Selection
2.2. Echocardiography
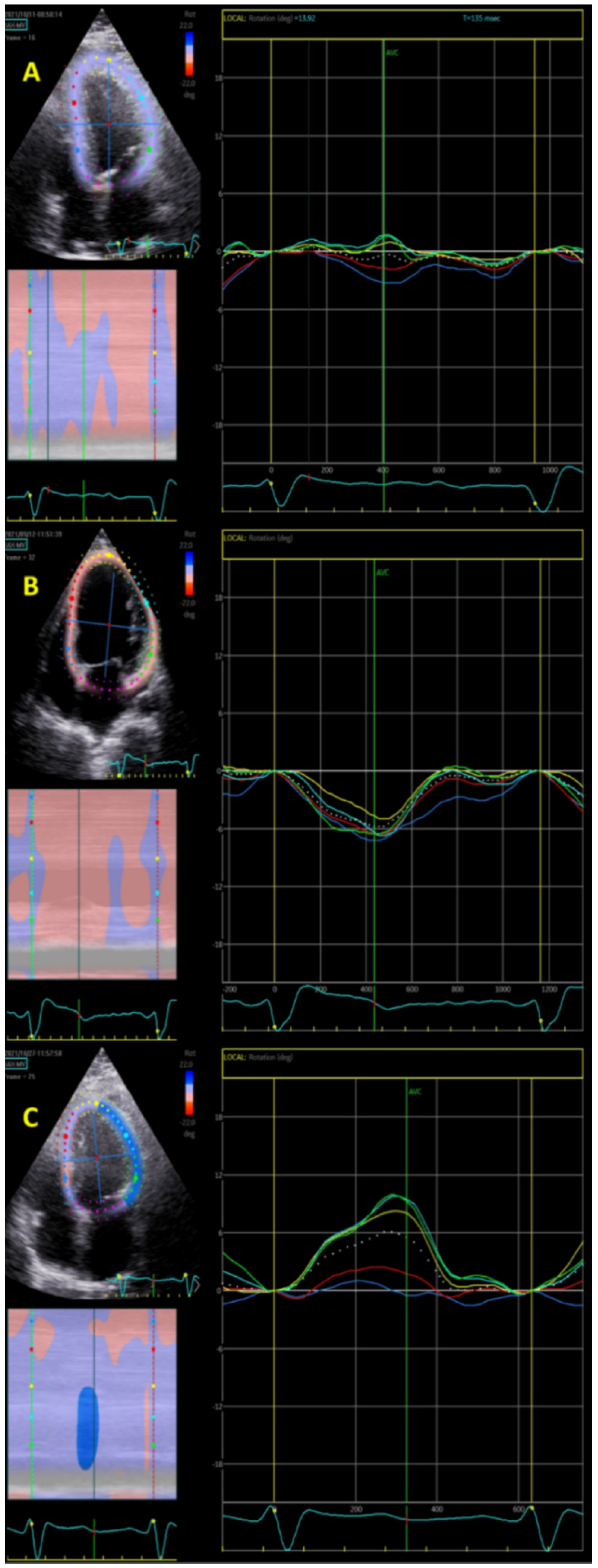
2.3. Longitudinal Rotation Quantification
2.4. Statistical Analysis
3. Results
3.1. Clinical and Echocardiographic Data
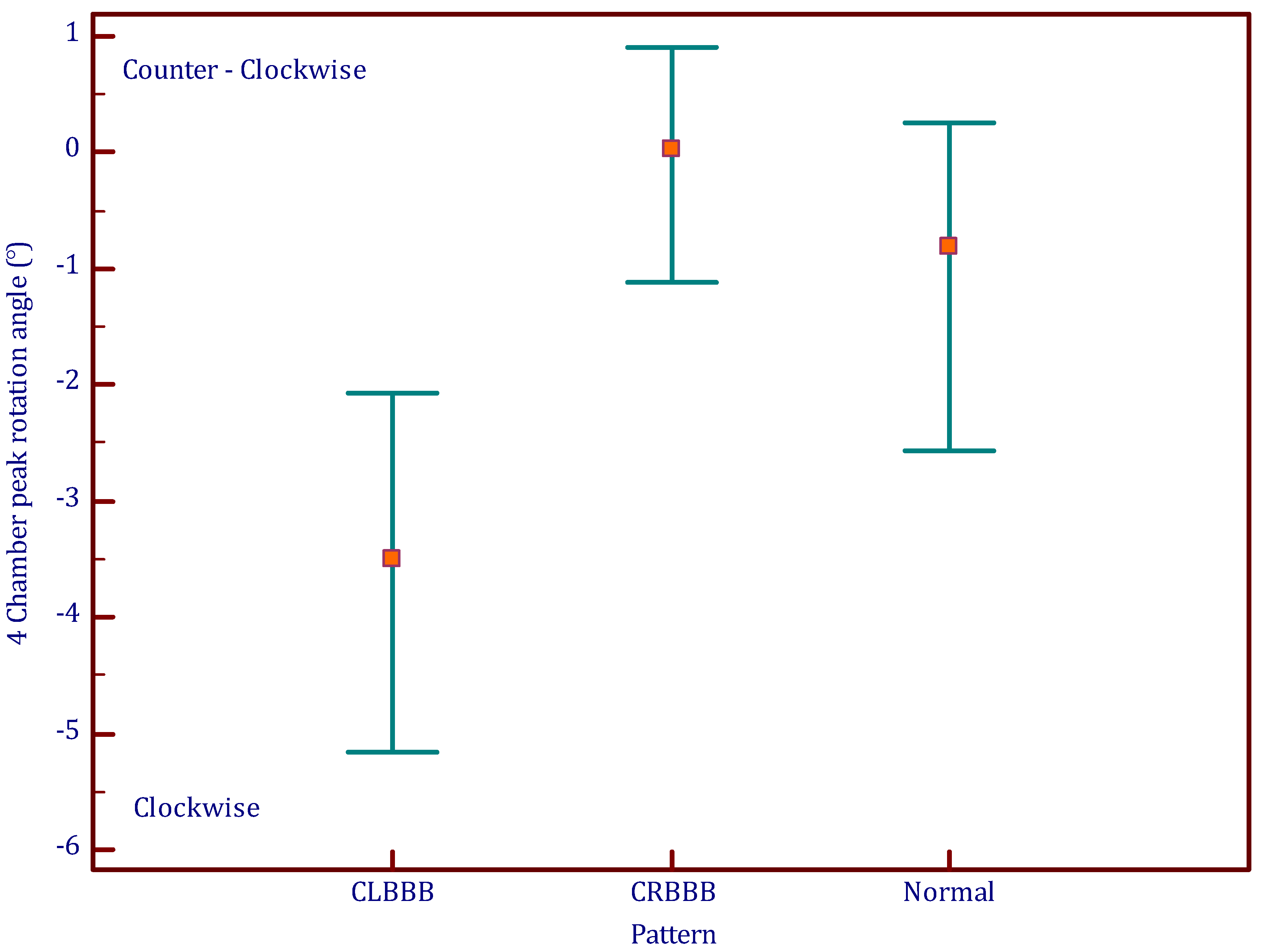
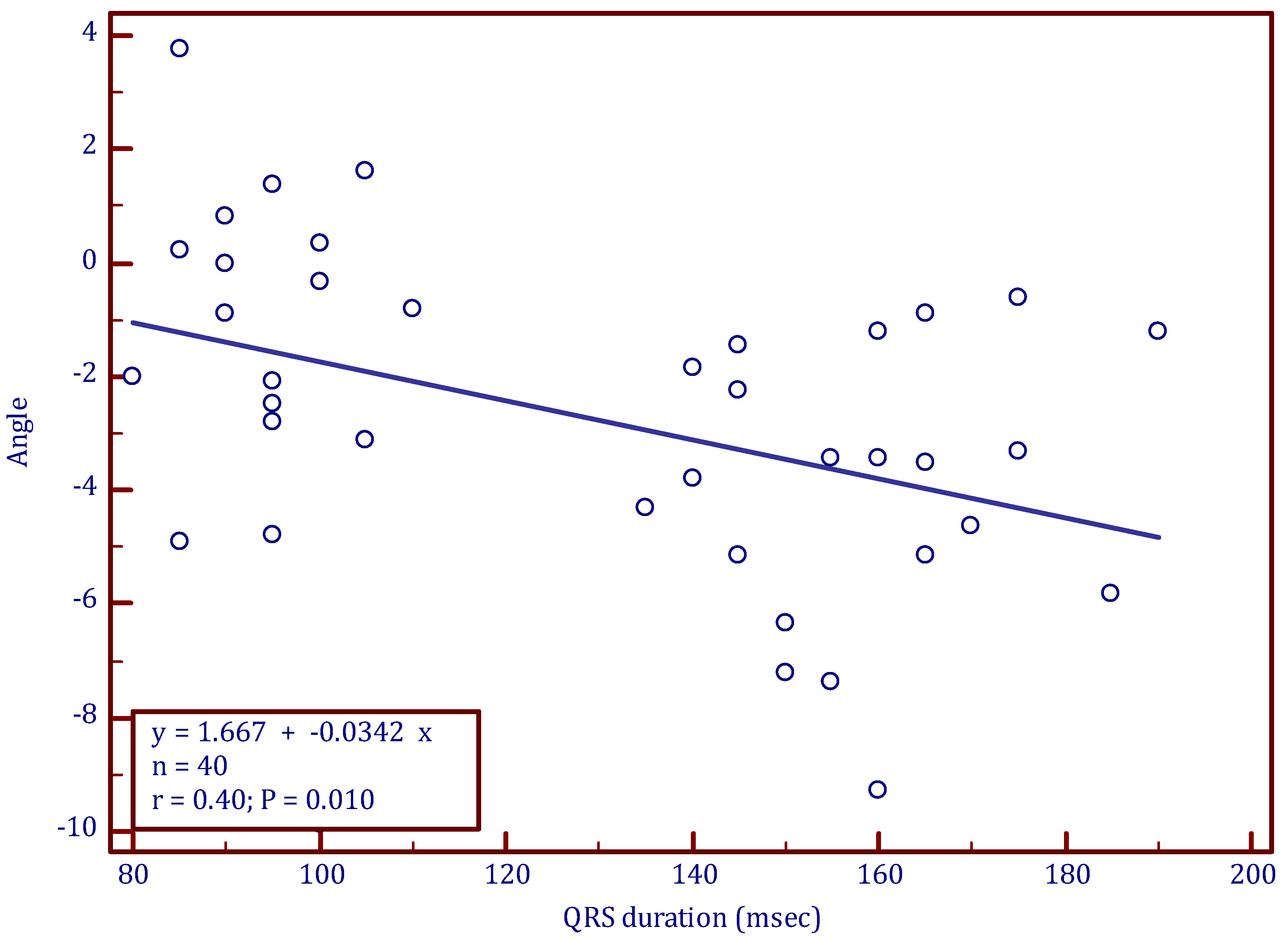
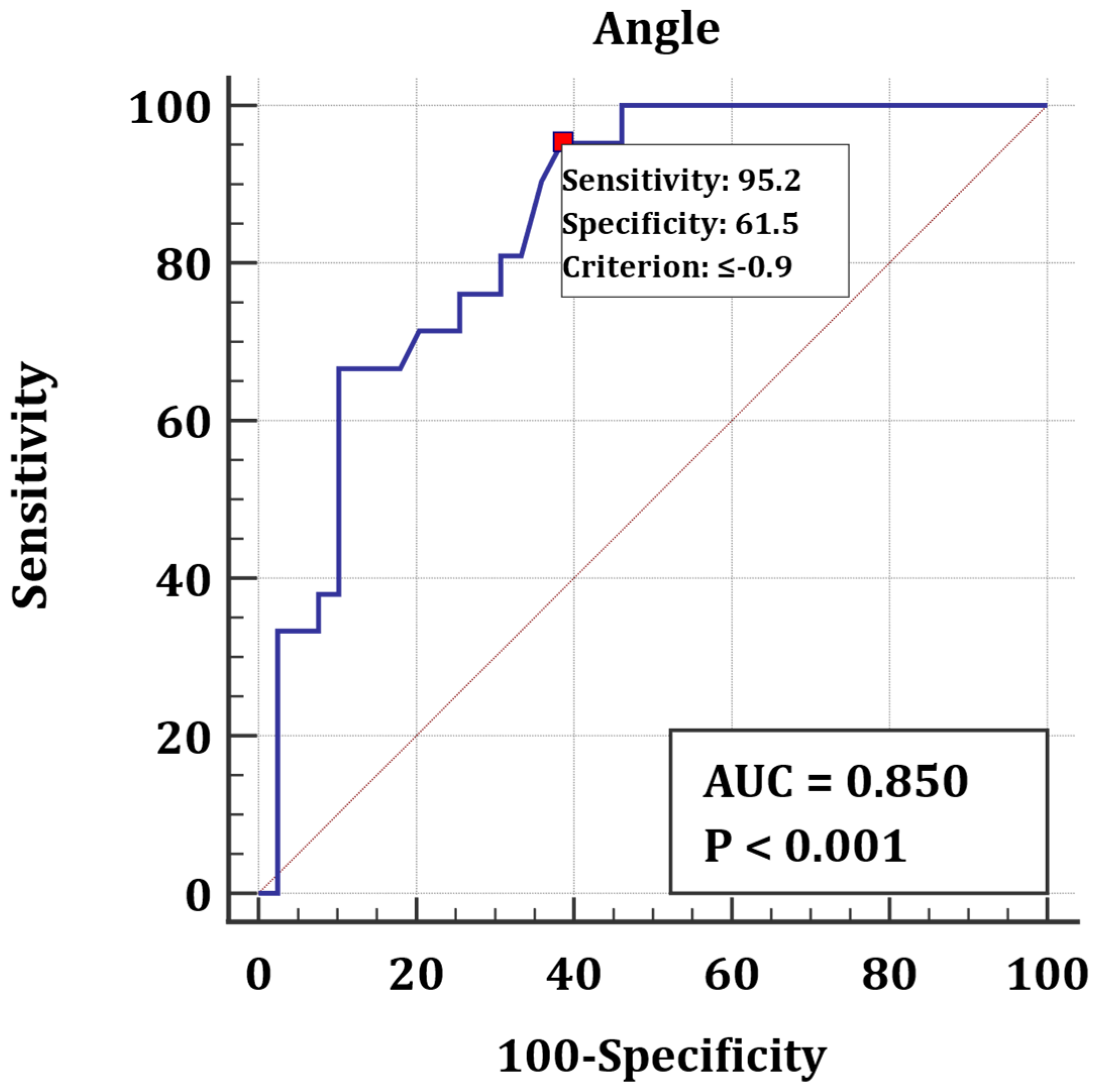
3.2. Longitudinal Rotation
4. Discussion
4.1. Main Findings
4.2. Longitudinal Rotation
4.3. Clinical Outlook
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kashani, A.; Barold, S.S. Significance of QRS Complex Duration in Patients with Heart Failure. J. Am. Coll. Cardiol. 2005, 46, 2183–2192. [Google Scholar] [CrossRef]
- Bax, J.J.; Ansalone, G.; Breithardt, O.A.; Derumeaux, G.; Leclercq, C.; Schalij, M.J.; Sogaard, P.; John Sutton, M.S.; Petros Nihoyannopoulos MD, FRCP, FACC. Echocardiographic evaluation of cardiac resynchronization therapy: Ready for routine clinical use? A critical appraisal. J. Am. Coll. Cardiol. 2004, 44, 1–9. [Google Scholar] [CrossRef]
- Pitzalis, M.V.; Iacoviello, M.; Romito, R.; Massari, F.; Rizzon, B.; Luzzi, G.; Guida, P.; Andriani, A.; Mastropasqua, F.; Rizzon, P. Cardiac resynchronization therapy tailored by echocardiographic evaluation of ventricular asynchrony. J. Am. Coll. Cardiol. 2002, 40, 1615–1622. [Google Scholar] [CrossRef]
- Yu, C.-M.; Fung, J.W.-H.; Zhang, Q.; Chan, C.-K.; Chan, Y.-S.; Lin, H.; Kum, L.C.; Kong, S.-L.; Zhang, Y.; Sanderson, J.E.; et al. Tissue Doppler Imaging Is Superior to Strain Rate Imaging and Postsystolic Shortening on the Prediction of Reverse Remodeling in Both Ischemic and Nonischemic Heart Failure After Cardiac Resynchronization Therapy. Circulation 2004, 110, 66–73. [Google Scholar] [CrossRef]
- Yu, C.-M.; Chau, E.; Sanderson, J.E.; Fan, K.; Tang, M.-O.; Fung, W.-H.; Lin, H.; Kong, S.-L.; Lam, Y.-M.; Hill, M.R.; et al. Tissue Doppler Echocardiographic Evidence of Reverse Remodeling and Improved Synchronicity by Simultaneously Delaying Regional Contraction After Biventricular Pacing Therapy in Heart Failure. Circulation 2002, 105, 438–445. [Google Scholar] [CrossRef]
- Søgaard, P.; Egeblad, H.; Kim, W.Y.; Jensen, H.K.; Pedersen, A.K.; Kristensen, B.Ø.; Mortensen, P.T. Tissue Doppler imaging predicts improved systolic performance and reversed left ventricular remodeling during long-term cardiac resynchronization therapy. J. Am. Coll. Cardiol. 2002, 40, 723–730. [Google Scholar] [CrossRef]
- Van Bommel, R.J.; Ypenburg, C.; Borleffs, C.J.; Delgado, V.; Marsan, N.A.; Bertini, M.; Holman, E.R.; Schalij, M.J.; Bax, J.J. Value of tissue Doppler echocardiography in predicting response to cardiac resynchronization therapy in patients with heart failure. Am. J. Cardiol. 2010, 105, 1153–1158. [Google Scholar] [CrossRef]
- Gorcsan, J., III; Tanabe, M.; Bleeker, G.B.; Suffoletto, M.S.; Thomas, N.C.; Saba, S.; Tops, L.F.; Schalij, M.J.; Bax, J.J. Combined longitudinal and radial dyssynchrony predicts ventricular response after resynchronization therapy. J. Am. Coll. Cardiol. 2007, 50, 1476–1483. [Google Scholar] [CrossRef]
- Yu, C.M.; Gorcsan, J., III; Bleeker, G.B.; Zhang, Q.; Schalij, M.J.; Suffoletto, M.S.; Fung, J.W.; Schwartzman, D.; Chan, Y.S.; Tanabe, M.; et al. Usefulness of tissue Doppler velocity and strain dyssynchrony for predicting left ventricular reverse remodeling response after cardiac resynchronization therapy. Am. J. Cardiol. 2007, 100, 1263–1270. [Google Scholar] [CrossRef]
- Carasso, S.; Rakowski, H.; Witte, K.K.; Smith, P.; Carasso, D.; Garceau, P.; Sasson, Z.; Parker, J.D. Left ventricular strain patterns in dilated cardiomyopathy predict response to cardiac resynchronization therapy: Timing is not everything. J. Am. Soc. Echocardiogr. 2009, 22, 242–250. [Google Scholar] [CrossRef]
- Popovic, Z.; A Grimm, R.; Ahmad, A.; Agler, D.; Favia, M.; Dan, G.; Lim, P.; Casas, F.; Greenberg, N.L.; Thomas, J.D. Longitudinal rotation: An unrecognised motion pattern in patients with dilated cardiomyopathy. Heart 2008, 94, e11. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Cameli, M.; Mandoli, G.E.; Sciaccaluga, C.; Mondillo, S. More than 10 years of speckle tracking echocardiography: Still a novel technique or a definite tool for clinical practice? Echocardiography 2019, 36, 958–970. [Google Scholar] [CrossRef]
- Rao, H.B.; Krishnaswami, R.; Kalavakolanu, S.; Calambur, N. Ventricular dyssynchrony patterns in left bundle branch block, with and without heart failure. Indian Pacing Electrophysiol. J. 2010, 10, 115–121. [Google Scholar]
- Risum, N.; Jons, C.; Olsen, N.T.; Fritz-Hansen, T.; Bruun, N.E.; Hojgaard, M.V.; Valeur, N.; Kronborg, M.B.; Kisslo, J.; Sogaard, P. Simple regional strain pattern analysis to predict response to cardiac resynchronization therapy: Rationale, initial results, and advantages. Am. Heart J. 2012, 163, 697–704. [Google Scholar] [CrossRef]
- Risum, N.; Tayal, B.; Hansen, T.F.; Bruun, N.E.; Jensen, M.T.; Lauridsen, T.K.; Saba, S.; Kisslo, J.; Gorcsan, J.; Sogaard, P. Identification of Typical Left Bundle Branch Block Contraction by Strain Echocardiography Is Additive to Electrocardiography in Prediction of Long-Term Outcome After Cardiac Resynchronization Therapy. J. Am. Coll. Cardiol. 2015, 66, 631–641. [Google Scholar] [CrossRef]
- Emerek, K.; Friedman, D.J.; Sørensen, P.L.; Hansen, S.M.; Larsen, J.M.; Risum, N.; Thøgersen, A.M.; Graff, C.; Atwater, B.D.; Kisslo, J.; et al. The Association of a classical left bundle Branch Block Contraction Pattern by vendor-independent strain echocardiography and outcome after cardiac resynchronization therapy. Cardiovasc. Ultrasound 2019, 17, 10. [Google Scholar] [CrossRef]
- Calle, S.; Kamoen, V.; De Buyzere, M.; De Pooter, J.; Timmermans, F. A Strain-Based Staging Classification of Left Bundle Branch Block-Induced Cardiac Remodeling. JACC Cardiovasc. Imaging 2021, 14, 1691–1702. [Google Scholar] [CrossRef]
- Eriksson, J.; Zajac, J.; Alehagen, U.; Bolger, A.F.; Ebbers, T.; Carlhäll, C.-J. Left ventricular hemodynamic forces as a marker of mechanical dyssynchrony in heart failure patients with left bundle branch block. Sci. Rep. 2017, 7, 2971. [Google Scholar] [CrossRef]
- Khan, F.Z.; Virdee, M.S.; Palmer, C.R.; Pugh, P.J.; O’Halloran, D.; Elsik, M.; Read, P.A.; Begley, D.; Fynn, S.P.; Dutka, D.P. Targeted Left Ventricular Lead Placement to Guide Cardiac Resynchronization Therapy: The TARGET Study: A Randomized, Controlled Trial. J. Am. Coll. Cardiol. 2012, 59, 1509–1518. [Google Scholar] [CrossRef]
- Sommer, A.; Kronborg, M.B.; Nørgaard, B.L.; Poulsen, S.H.; Bouchelouche, K.; Böttcher, M.; Jensen, H.K.; Jensen, J.M.; Kristensen, J.; Gerdes, C.; et al. Multimodality imaging-guided left ventricular lead placement in cardiac resynchronization therapy: A randomized controlled trial. Eur. J. Heart Fail. 2016, 18, 1365–1374. [Google Scholar] [CrossRef]
- Lunati, M.; Magenta, G.; Cattafi, G.; Moreo, A.; Falaschi, G.; Contardi, D.; Locati, E. Clinical Relevance of Systematic CRT Device Optimization. J. Atr. Fibrillation 2014, 7, 1077. [Google Scholar]
- Zhang, K.; Sheu, R.; Zimmerman, N.M.; Alfirevic, A.; Sale, S.; Gillinov, A.M.; Duncan, A.E. A Comparison of Global Longitudinal, Circumferential, and Radial Strain to Predict Outcomes After Cardiac Surgery. J. Cardiothorac. Vasc. Anesthesia 2019, 33, 1315–1322. [Google Scholar] [CrossRef]
- Delgado, V.; Ypenburg, C.; van Bommel, R.J.; Tops, L.F.; Mollema, S.A.; Marsan, N.A.; Bleeker, G.B.; Schalij, M.J.; Bax, J.J. Assessment of Left Ventricular Dyssynchrony by Speckle Tracking Strain Imaging: Comparison Between Longitudinal, Circumferential, and Radial Strain in Cardiac Resynchronization Therapy. J. Am. Coll. Cardiol. 2008, 51, 1944–1952. [Google Scholar] [CrossRef]
- Helm, R.H.; Leclercq, C.; Faris, O.P.; Ozturk, C.; McVeigh, E.; Lardo, A.; Kass, D.A. Cardiac dyssynchrony analysis using circumferential versus longitudinal strain: Implications for assessing cardiac resynchronization. Circulation 2005, 111, 2760–2767. [Google Scholar] [CrossRef]
- Wang, C.-L.; Wu, C.-T.; Yeh, Y.-H.; Wu, L.-S.; Chan, Y.-H.; Kuo, C.-T.; Chu, P.-H.; Hsu, L.-A.; Ho, W.-J. Left bundle-branch block contraction patterns identified from radial-strain analysis predicts outcomes following cardiac resynchronization therapy. Int. J. Cardiovasc. Imaging 2017, 33, 869–877. [Google Scholar] [CrossRef]
- Delgado-Montero, A.; Tayal, B.; Goda, A.; Ryo, K.; Marek, J.J.; Sugahara, M.; Qi, Z.; Althouse, A.D.; Saba, S.; Schwartzman, D.; et al. Additive Prognostic Value of Echocardiographic Global Longitudinal and Global Circumferential Strain to Electrocardiographic Criteria in Patients with Heart Failure Undergoing Cardiac Resynchronization Therapy. Circ. Cardiovasc. Imaging 2016, 9, e004241. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Torp, H.; Opdahl, A.; Haugaa, K.H.; Urheim, S. Myocardial strain imaging: How useful is it in clinical decision making? Eur. Heart J. 2016, 37, 1196–1207. [Google Scholar] [CrossRef]
- Olsen, F.J.; Biering-Sørensen, T. Myocardial Strain and Dyssynchrony: Incremental Value? Heart Fail. Clin. 2019, 15, 167–178. [Google Scholar] [CrossRef]
- Sareen, N.; Ananthasubramaniam, K. Strain Imaging: From Physiology to Practical Applications in Daily Practice. Cardiol. Rev. 2016, 24, 56–69. [Google Scholar] [CrossRef]
| Normal (n = 19) | LBBB (n = 21) | p Value (LBBB vs. Normal) | RBBB (n = 20) | p Value (RBBB vs. Normal) | p Value (LBBB vs. RBBB) | |
|---|---|---|---|---|---|---|
| Age | 61 ± 14 | 67 ± 12 | 0.0497 | 71 ± 13 | 0.008 | 0.16 |
| Female gender n, % | 6 (31.6) | 7 (33.3) | 0.9 | 5 (25) | 0.65 | 0.56 |
| QRS duration (ms) | 95 ± 8 | 159 ± 15 | 0.00001 | 141 ± 11 | 0.00001 | 0.00001 |
| Heart rate (min−1) | 70 ± 12 | 78 ± 21 | 0.17 | 75 ± 13 | 0.24 | 0.56 |
| Ejection fraction (%) | 62 ± 6 | 45 ± 17 | 0.0001 | 55 ± 14 | 0.038 | 0.043 |
| LVEDD (mm) | 53 ± 4 | 61 ± 12 | 0.004 | 54 ± 10 | 0.2 | 0.04 |
| LVESD (mm) | 34 ± 5 | 47 ± 14 | 0.0002 | 38 ± 12 | 0.06 | 0.02 |
| Severe valvular disease n, % | 1 (5.3) | 5 (23.8) | 0.1 | 6 (30) | 0.1 | 0.9 |
| Regional WMA n, % | 2 (10.5) | 8 (38.1) | 0.044 | 7 (35) | 0.07 | 0.8 |
| CHF n, % | 1 (5.3) | 14 (66.7) | 0.00006 | 6 (30) | 0.044 | 0.02 |
| Clockwise rotation n, % | 11 (58) | 21 (100) | <0.005 | 7 (35) | 0.15 | <0.0005 |
| Longitudinal rotation angle (°) | −1.4 ± 3 | −3.9 ± 2.4 | 0.005 | 0.1 ± 2.2 | 0.093 | 0.00001 |
| Time to peak rotation (ms) | 395 ± 98 | 339 ± 135 | 0.1447 | 367 ± 89 | 0.356 | 0.437 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marai, I.; Haddad, R.; Andria, N.; Kinany, W.; Hazanov, Y.; Kleinberg, B.M.; Birati, E.; Carasso, S. Left Ventricular “Longitudinal Rotation” and Conduction Abnormalities—A New Outlook on Dyssynchrony. J. Clin. Med. 2023, 12, 745. https://doi.org/10.3390/jcm12030745
Marai I, Haddad R, Andria N, Kinany W, Hazanov Y, Kleinberg BM, Birati E, Carasso S. Left Ventricular “Longitudinal Rotation” and Conduction Abnormalities—A New Outlook on Dyssynchrony. Journal of Clinical Medicine. 2023; 12(3):745. https://doi.org/10.3390/jcm12030745
Chicago/Turabian StyleMarai, Ibrahim, Rabea Haddad, Nizar Andria, Wadi Kinany, Yevgeni Hazanov, Bruce M. Kleinberg, Edo Birati, and Shemy Carasso. 2023. "Left Ventricular “Longitudinal Rotation” and Conduction Abnormalities—A New Outlook on Dyssynchrony" Journal of Clinical Medicine 12, no. 3: 745. https://doi.org/10.3390/jcm12030745
APA StyleMarai, I., Haddad, R., Andria, N., Kinany, W., Hazanov, Y., Kleinberg, B. M., Birati, E., & Carasso, S. (2023). Left Ventricular “Longitudinal Rotation” and Conduction Abnormalities—A New Outlook on Dyssynchrony. Journal of Clinical Medicine, 12(3), 745. https://doi.org/10.3390/jcm12030745






