Paraoxonase 1 and Chronic Kidney Disease: A Meta-Analysis
Abstract
1. Introduction
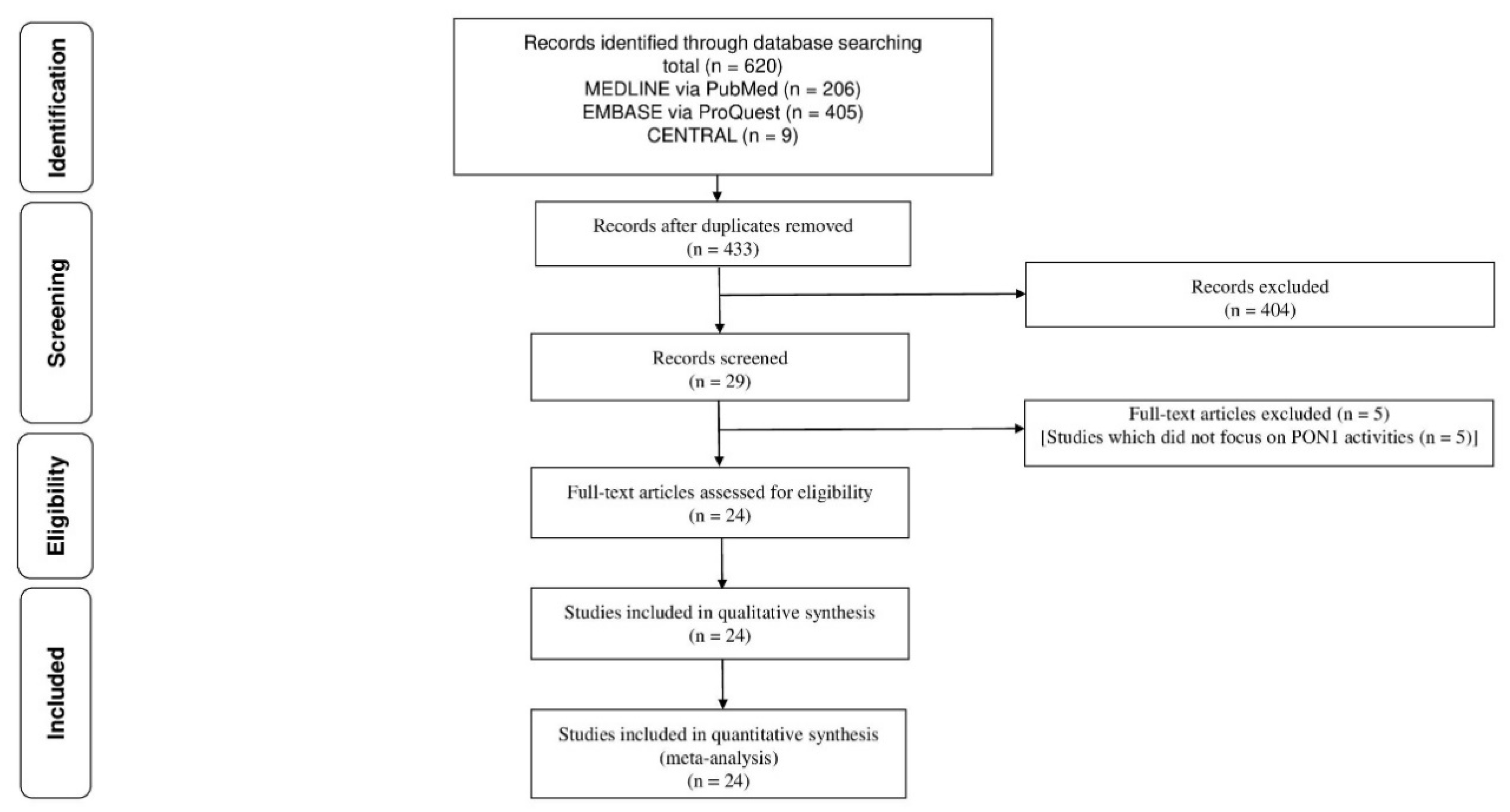
2. Materials and Methods
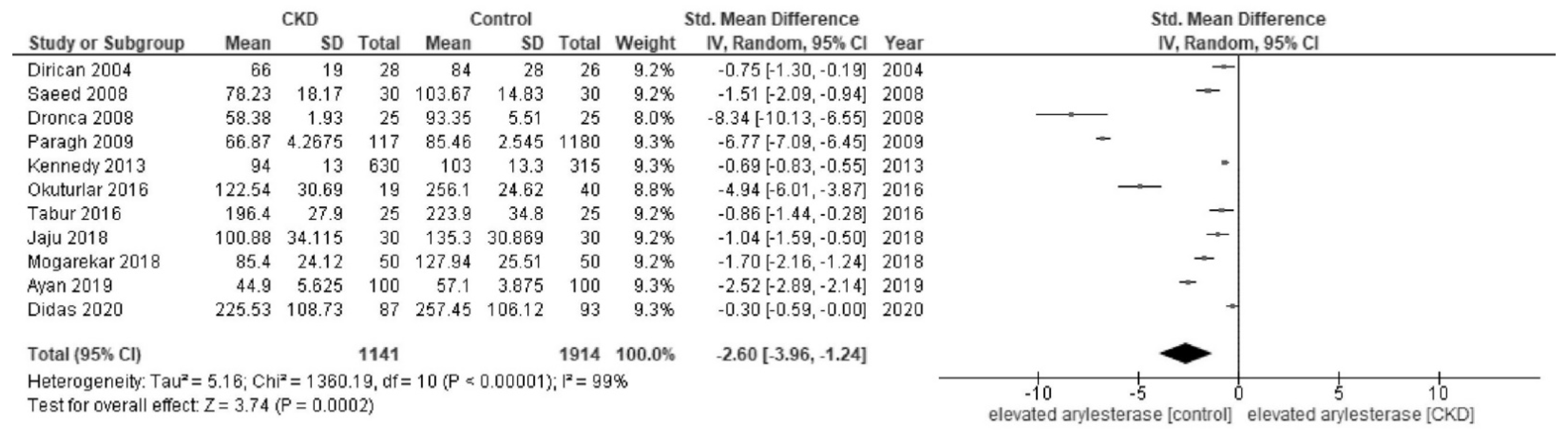
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Search Strategy
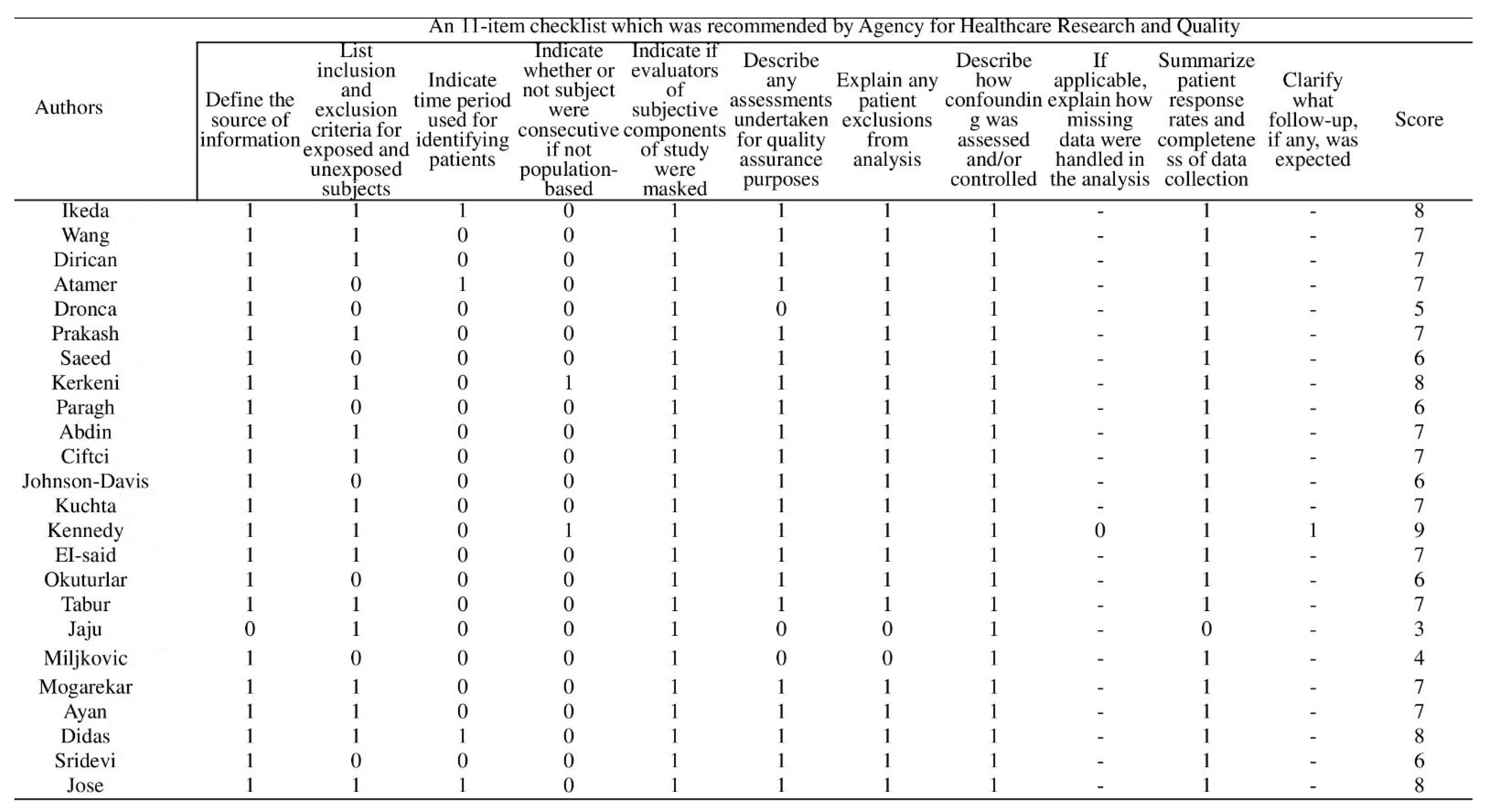
Appendix B. Details of Risk of Bias Assessment
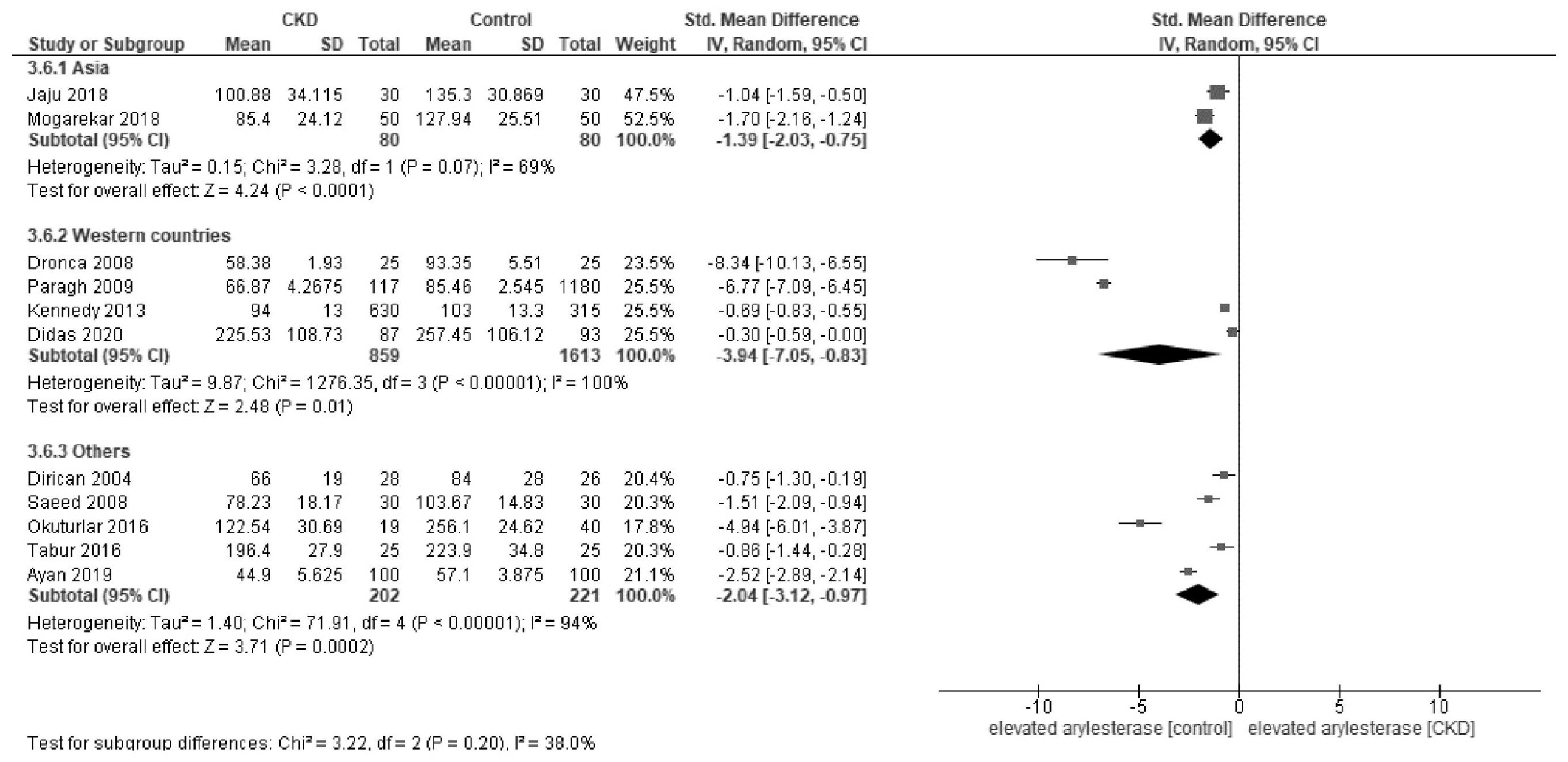
Appendix C. Forest Plot of Paraoxonase and Arylesterase Activity Stratified by Diabetes Status and Regions
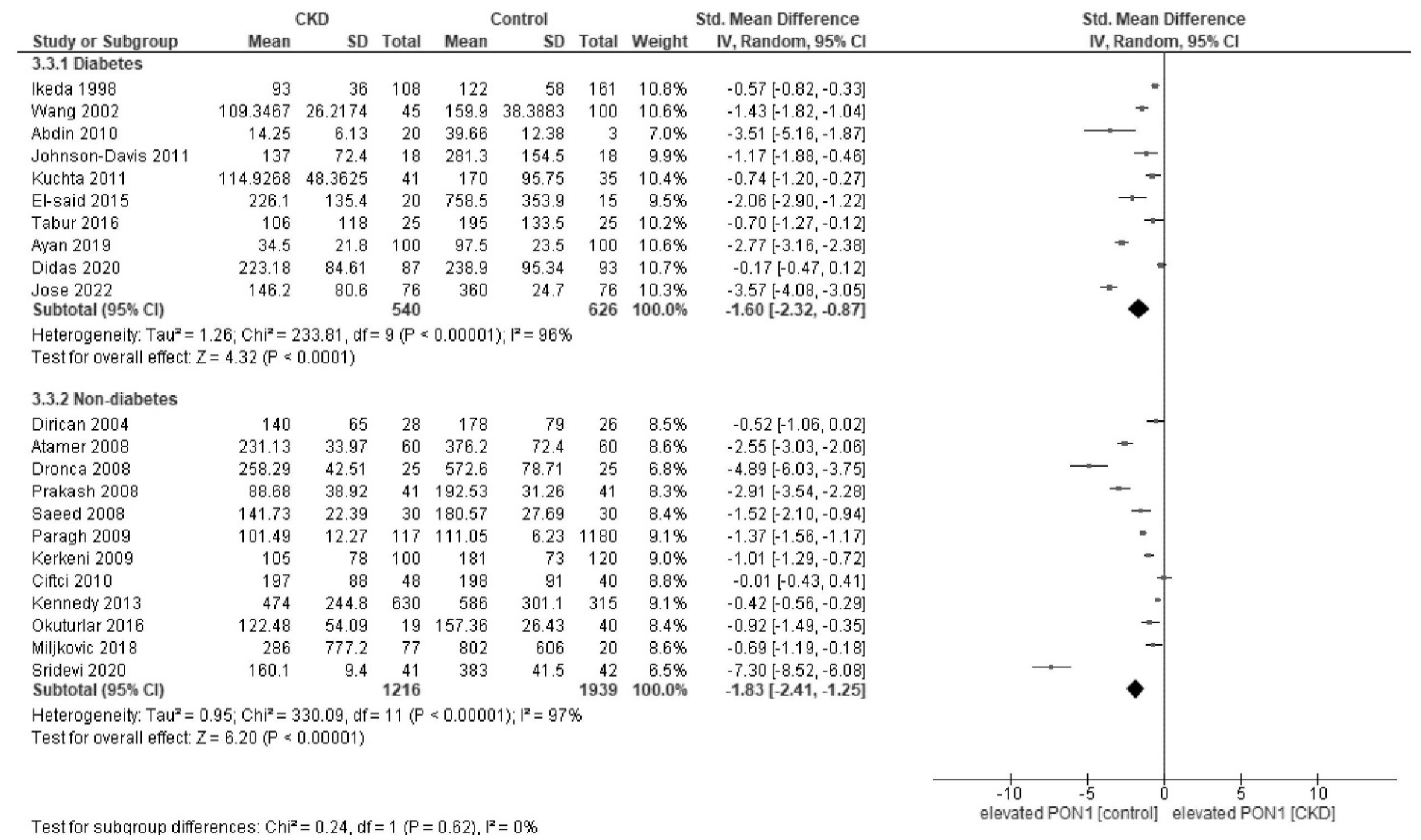


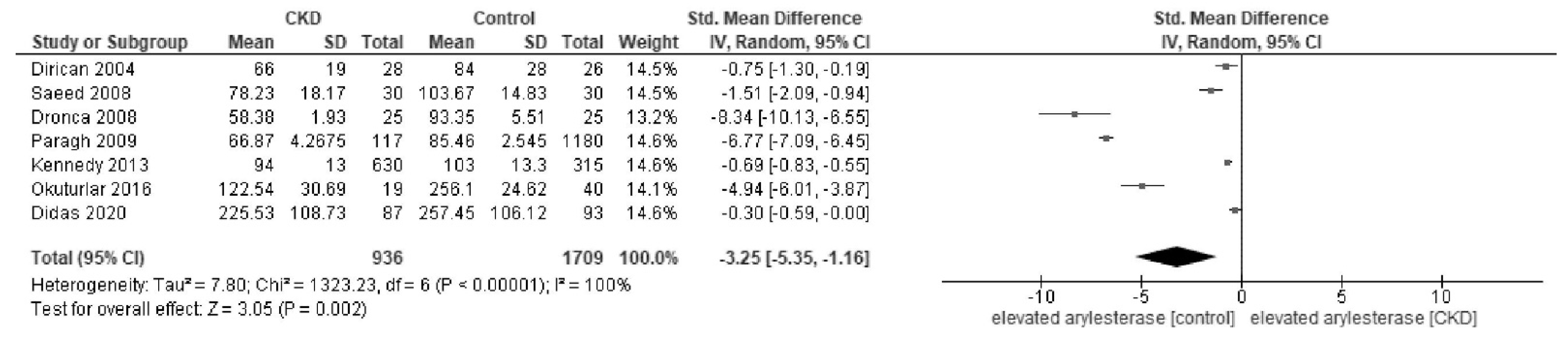
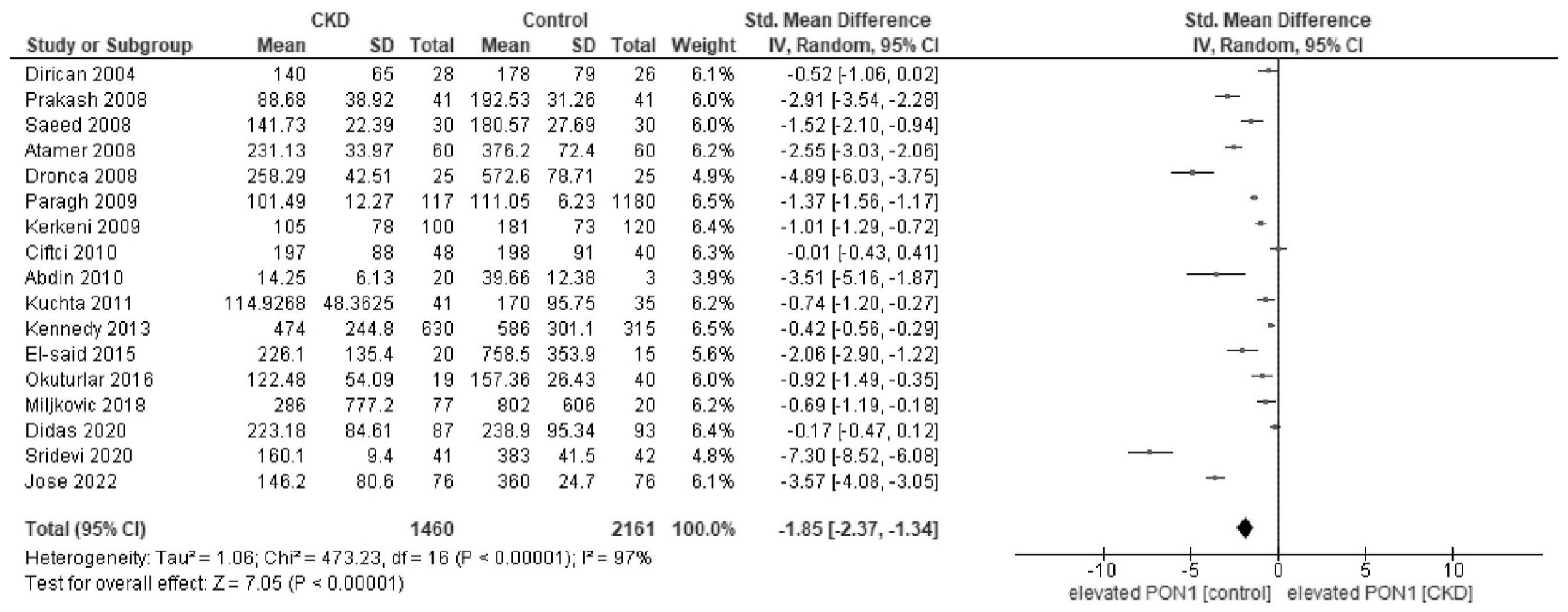
Appendix D. Forest Plot of Paraoxonase and Arylesterase Activity in Sensitivity Analysis
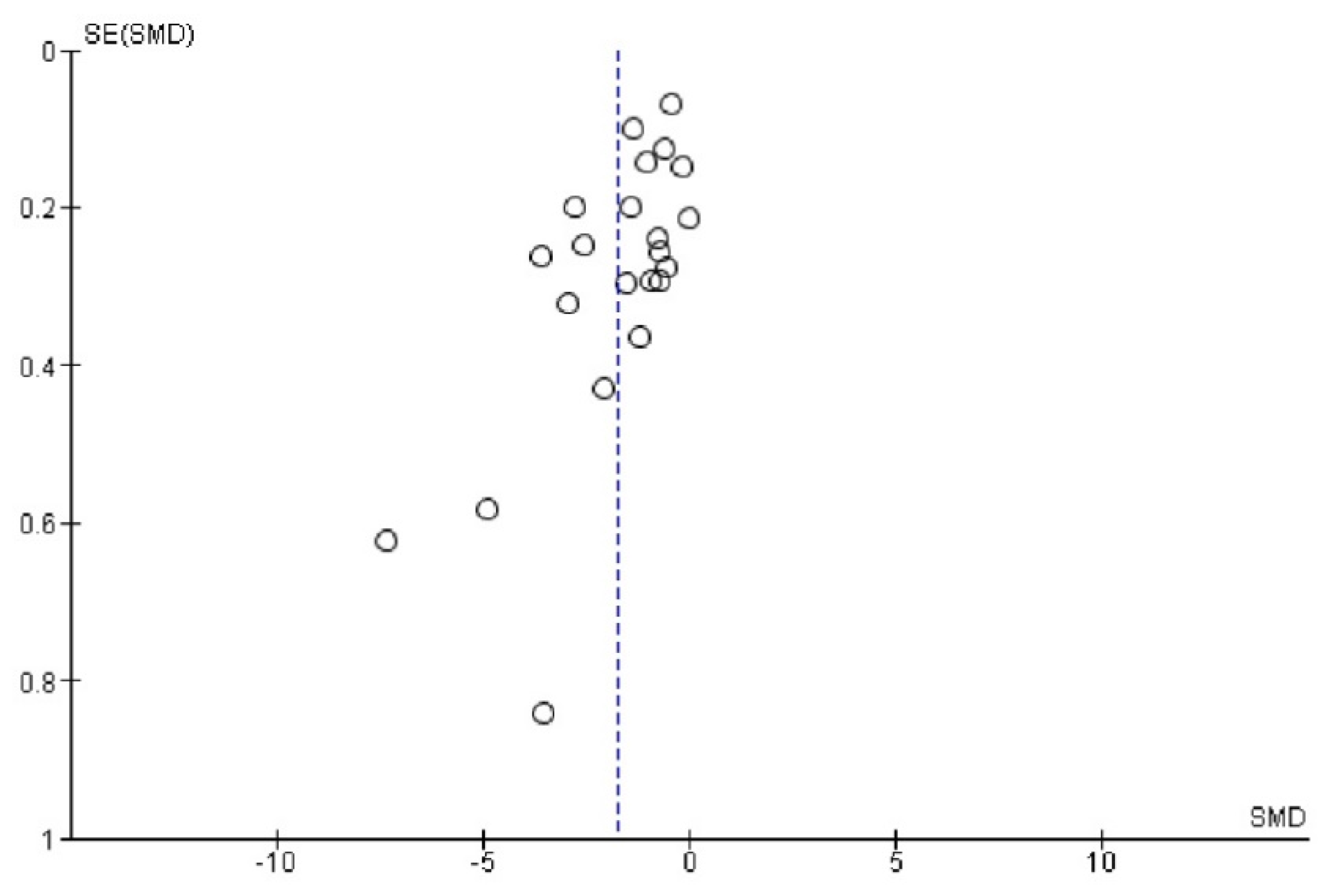
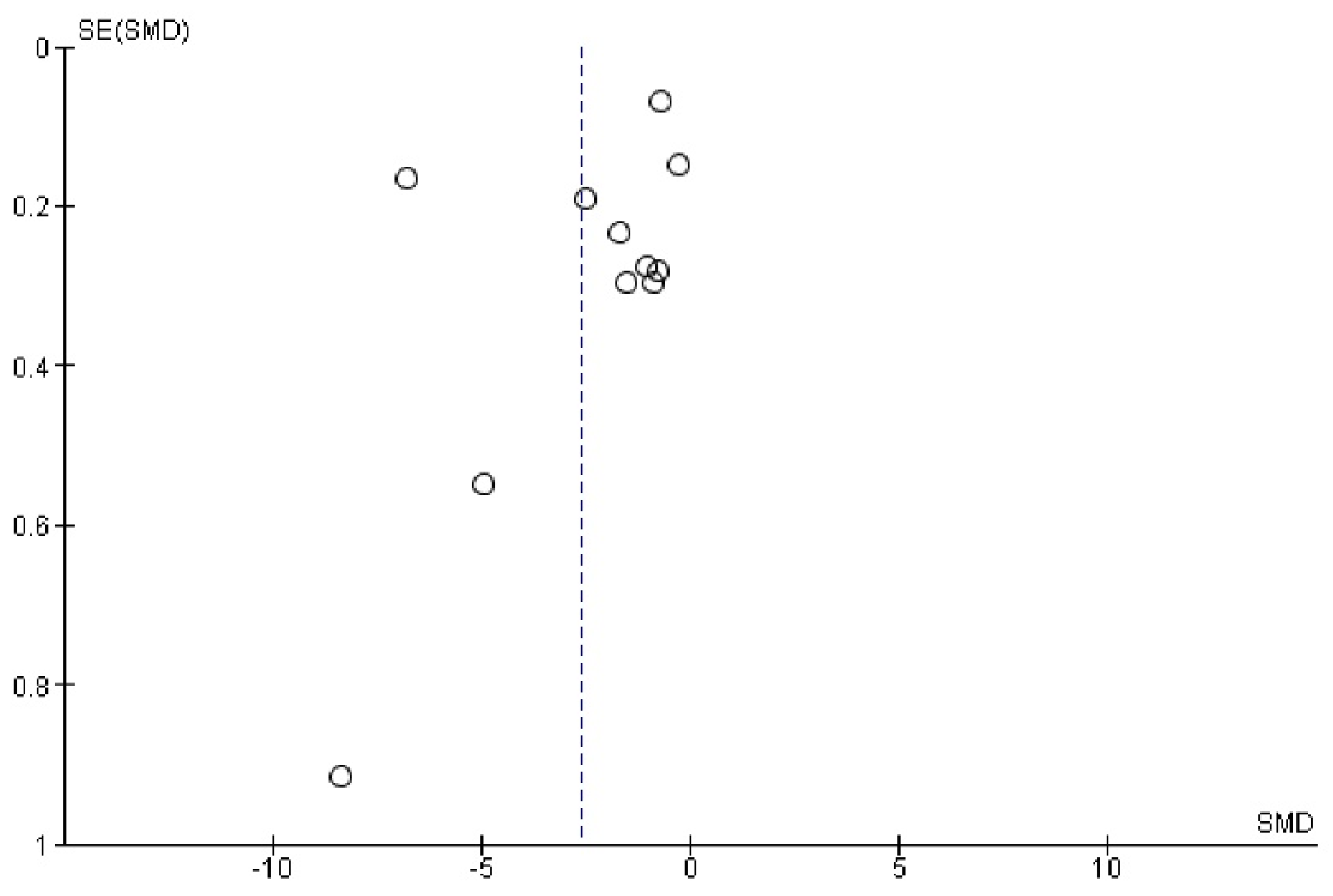
Appendix E. Funnel Plot of Paraoxonase and Arylesterase Activity
References
- Matsushita, K.; Coresh, J.; Sang, Y.; Chalmers, J.; Fox, C.; Guallar, E.; Jafar, T.; Jassal, S.K.; Landman, G.W.D.; Muntner, P.; et al. Estimated Glomerular Filtration Rate and Albuminuria for Prediction of Cardiovascular Outcomes: A Collaborative Meta-Analysis of Individual Participant Data. Lancet Diabetes Endocrinol. 2015, 3, 514–525. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.K.; Chang, T.I.; Cushman, W.C.; Furth, S.L.; Hou, F.F.; Ix, J.H.; Knoll, G.A.; Muntner, P.; Pecoits-Filho, R.; Sarnak, M.J. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021, 99, S1–S87. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Ene-Iordache, B.; Perico, N.; Bikbov, B.; Carminati, S.; Remuzzi, A.; Perna, A.; Islam, N.; Bravo, R.F.; Aleckovic-Halilovic, M.; Zou, H.; et al. Chronic Kidney Disease and Cardiovascular Risk in Six Regions of the World (ISN-KDDC): A Cross-Sectional Study. Lancet Glob. Health 2016, 4, e307–e319. [Google Scholar] [CrossRef]
- Isakova, T.; Nickolas, T.L.; Denburg, M.; Yarlagadda, S.; Weiner, D.E.; Gutiérrez, O.M.; Bansal, V.; Rosas, S.E.; Nigwekar, S.; Yee, J.; et al. KDOQI US Commentary on the 2017 KDIGO Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Am. J. Kidney Dis. 2017, 70, 737–751. [Google Scholar] [CrossRef]
- Matsushita, K.; Ballew, S.H.; Wang, A.Y.-M.; Kalyesubula, R.; Schaeffner, E.; Agarwal, R. Epidemiology and Risk of Cardiovascular Disease in Populations with Chronic Kidney Disease. Nat. Rev. Nephrol. 2022, 18, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Ferro, C.J.; Mark, P.B.; Kanbay, M.; Sarafidis, P.; Heine, G.H.; Rossignol, P.; Massy, Z.A.; Mallamaci, F.; Valdivielso, J.M.; Malyszko, J.; et al. Lipid Management in Patients with Chronic Kidney Disease. Nat. Rev. Nephrol. 2018, 14, 727–749. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar]
- Noels, H.; Lehrke, M.; Vanholder, R.; Jankowski, J. Lipoproteins and Fatty Acids in Chronic Kidney Disease: Molecular and Metabolic Alterations. Nat. Rev. Nephrol. 2021, 17, 528–542. [Google Scholar] [CrossRef]
- Mackness, M.; Mackness, B. Targeting Paraoxonase-1 in Atherosclerosis. Expert Opin. Ther. Targets 2013, 17, 829–837. [Google Scholar] [CrossRef]
- Vivekananthan, D.P.; Penn, M.S.; Sapp, S.K.; Hsu, A.; Topol, E.J. Use of Antioxidant Vitamins for the Prevention of Cardiovascular Disease: Meta-Analysis of Randomised Trials. Lancet 2003, 361, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Márquez, A.B.; Nazir, S.; van der Vorst, E.P.C. High-Density Lipoprotein Modifications: A Pathological Consequence or Cause of Disease Progression? Biomedicines 2020, 8, 549. [Google Scholar] [CrossRef] [PubMed]
- Soran, H.; Younis, N.N.; Charlton-Menys, V.; Durrington, P. Variation in Paraoxonase-1 Activity and Atherosclerosis. Curr. Opin. Lipidol. 2009, 20, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, T.; Nicholls, S.J.; Topol, E.J.; Zhang, R.; Yang, X.; Schmitt, D.; Fu, X.; Shao, M.; Brennan, D.M.; Ellis, S.G.; et al. Relationship of Paraoxonase 1 (PON1) Gene Polymorphisms and Functional Activity with Systemic Oxidative Stress and Cardiovascular Risk. JAMA 2008, 299, 1265–1276. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Sahebkar, A. Effect of Statin Therapy on Paraoxonase-1 Status: A Systematic Review and Meta-Analysis of 25 Clinical Trials. Prog. Lipid Res. 2015, 60, 50–73. [Google Scholar] [CrossRef]
- Watanabe, J.; Kotani, K.; Gugliucci, A. Paraoxonase 1 and Chronic Obstructive Pulmonary Disease: A Meta-Analysis. Antioxidants 2021, 10, 1891. [Google Scholar] [CrossRef]
- Kotani, K.; Watanabe, J.; Miura, K.; Gugliucci, A. Paraoxonase 1 and Non-Alcoholic Fatty Liver Disease: A Meta-Analysis. Molecules 2021, 26, 2323. [Google Scholar] [CrossRef]
- Bassu, S.; Mangoni, A.A.; Argiolas, D.; Carru, C.; Pirina, P.; Fois, A.G.; Zinellu, A. A Systematic Review and Meta-Analysis of Paraoxonase-1 Activity in Asthma. Clin. Exp. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A.; Mehlhaff, K.; Kinugasa, E.; Ogata, H.; Hermo, R.; Schulze, J.; Kimura, S. Paraoxonase-1 Concentrations in End-Stage Renal Disease Patients Increase after Hemodialysis: Correlation with Low Molecular AGE Adduct Clearance. Clin. Chim. Acta 2007, 377, 213–220. [Google Scholar] [CrossRef]
- Gugliucci, A.; Kinugasa, E.; Kotani, K.; Caccavello, R.; Kimura, S. Serum Paraoxonase 1 (PON1) Lactonase Activity Is Lower in End-Stage Renal Disease Patients than in Healthy Control Subjects and Increases after Hemodialysis. Clin. Chem. Lab. Med. 2011, 49, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A.; Kotani, K.; Kimura, S. Paraoxonase 1 in Chronic Kidney Failure. J. Lipids 2012, 2012, 726048. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Viswanathan, M.; Ansari, M.T.; Berkman, N.D.; Chang, S.; Hartling, L.; McPheeters, M.; Santaguida, P.L.; Shamliyan, T.; Singh, K.; Tsertsvadze, A.; et al. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. Agency for Healthcare Research and Quality Methods Guide for Comparative Effectiveness Reviews. 2012. Available online: https://effectivehealthcare.ahrq.gov/ (accessed on 1 December 2022).
- Higgins, J.P.T.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3. 2022. Available online: https://training.cochrane.org/handbook/current (accessed on 17 May 2022).
- Ikeda, Y.; Suehiro, T.; Inoue, M.; Nakauchi, Y.; Morita, T.; Arii, K.; Ito, H.; Kumon, Y.; Hashimoto, K. Serum Paraoxonase Activity and Its Relationship to Diabetic Complications in Patients with Non-Insulin-Dependent Diabetes Mellitus. Metabolism 1998, 47, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Deng, H.; Liu, W. The effects of paraoxonase-1 and oxidized low density lipoprotein on nephropathy in type-2 diabetes mellitus. Zhonghua Nei Ke Za Zhi 2002, 41, 179–182. [Google Scholar]
- Dirican, M.; Akca, R.; Sarandol, E.; Dilek, K. Serum Paraoxonase Activity in Uremic Predialysis and Hemodialysis Patients. J. Nephrol. 2004, 17, 813–818. [Google Scholar] [PubMed]
- Atamer, A.; Kocyigit, Y.; Ecder, S.A.; Selek, S.; Ilhan, N.; Ecder, T.; Atamer, Y. Effect of Oxidative Stress on Antioxidant Enzyme Activities, Homocysteine and Lipoproteins in Chronic Kidney Disease. J. Nephrol. 2008, 21, 924–930. [Google Scholar] [PubMed]
- Dronca, M.; Paşca, S.P.; Nemeş, B.; Vlase, L.; Vladutiu, D. Serum Paraoxonase 1 Activities and Homocysteinemia in Hemodialysis Patients. Clin. Chem. Lab. Med. 2008, 46, 880–881. [Google Scholar] [CrossRef]
- Prakash, M.; Shetty, J.K.; Rao, L.; Sharma, S.; Rodrigues, A.; Prabhu, R. Serum Paraoxonase Activity and Protein Thiols in Chronic Renal Failure Patients. Indian J. Nephrol. 2008, 18, 13–16. [Google Scholar] [CrossRef]
- Saeed, S.A.; Elsharkawy, M.; Elsaeed, K.; Fooda, O. Paraoxonase-1 (PON1) Activity as a Risk Factor for Atherosclerosis in Chronic Renal Failure Patients. Hemodial. Int. 2008, 12, 471–479. [Google Scholar] [CrossRef]
- Kerkeni, M.; Letaief, A.; Achour, A.; Miled, A.; Trivin, F.; Maaroufi, K. Hyperhomocysteinemia, Paraoxonase Concentration and Cardiovascular Complications in Tunisian Patients with Nondiabetic Renal Disease. Clin. Biochem. 2009, 42, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Paragh, G.; Seres, I.; Harangi, M.; Pocsai, Z.; Asztalos, L.; Locsey, L.; Szeles, G.; Kardos, L.; Varga, E.; Karpati, I.; et al. Discordance in Human Paraoxonase-1 Gene between Phenotypes and Genotypes in Chronic Kidney Disease. Nephron Clin. Pract. 2009, 113, c46–c53. [Google Scholar] [CrossRef] [PubMed]
- Abdin, A.A.; Hassanien, M.A.; Ibrahim, E.A.; El-Noeman, S.E.-D.A.A. Modulating Effect of Atorvastatin on Paraoxonase 1 Activity in Type 2 Diabetic Egyptian Patients with or without Nephropathy. J. Diabetes Complicat. 2010, 24, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, H.; Savas, M.; Yeni, E.; Verit, A.; Celik, H.; Oncel, H. Serum Paraoxonase Activity in Patients with Low Glomerular Filtration Rates. Ren. Fail. 2010, 32, 562–565. [Google Scholar] [CrossRef]
- Johnson-Davis, K.L.; Fernelius, C.; Eliason, N.B.; Wilson, A.; Beddhu, S.; Roberts, W.L. Blood Enzymes and Oxidative Stress in Chronic Kidney Disease: A Cross Sectional Study. Ann. Clin. Lab. Sci. 2011, 41, 331–339. [Google Scholar] [PubMed]
- Kuchta, A.; Pacanis, A.; Kortas-Stempak, B.; Cwiklińska, A.; Ziętkiewicz, M.; Renke, M.; Rutkowski, B. Estimation of Oxidative Stress Markers in Chronic Kidney Disease. Kidney Blood Press. Res. 2011, 34, 12–19. [Google Scholar] [CrossRef]
- Kennedy, D.J.; Tang, W.H.W.; Fan, Y.; Wu, Y.; Mann, S.; Pepoy, M.; Hazen, S.L. Diminished Antioxidant Activity of High-Density Lipoprotein-Associated Proteins in Chronic Kidney Disease. J. Am. Heart Assoc. 2013, 2, e000104. [Google Scholar] [CrossRef] [PubMed]
- El-said, N.H.; Nasr-Allah, M.M.; Sadik, N.A.; Sharaf, S.A. Paraoxonase-1 Activity in Type 2 Diabetes Mellitus with and without Nephropathy. Egypt. J. Intern. Med. 2015, 27, 63–68. [Google Scholar] [CrossRef]
- Okuturlar, Y.; Akalin, N.; Kaptanogullari, O.H.; Guner, N.T.; Yilmaz, D.; Gedikbasi, A.; Soyluk, O.; Mert, M.; Serin, S.O.; Kocoglu, H.; et al. Comparison of Serum Paraoxonase and Arylesterase Activities between Iron Deficiency Anemia Patients and Chronic Kidney Disease Patients with Anemia. Ren. Fail. 2016, 38, 781–786. [Google Scholar] [CrossRef]
- Tabur, S.; Korkmaz, H.; Eren, M.A.; Oğuz, E.; Sabuncu, T.; Kul, S.; Aksoy, N. Can Visfatin Be Considered as a Diagnostic Marker for Diabetic Nephropathy? Turk. J. Endocrinol. Metab. 2016, 20, 10–15. [Google Scholar] [CrossRef]
- Jaju, J.B.; Dhabe, M.G. Study of Serum Carbamylated Total Protein and Paraoxonase 1 Levels in Chronic Kidney Disease. Indian J. Clin. Biochem. 2018, 33, S44. [Google Scholar]
- Miljkovic, M.; Stefanovic, A.; Simic-Ogrizovic, S.; Vekic, J.; Bogavac-Stanojevic, N.; Cerne, D.; Kocbek, P.; Marc, J.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V.; et al. Association of Dyslipidemia, Oxidative Stress, and Inflammation With Redox Status in VLDL, LDL, and HDL Lipoproteins in Patients With Renal Disease. Angiology 2018, 69, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Mogarekar, M.R.; Dhabe, M.G.; Palmate, M.M. Paraoxonase 1 Activity and Its Polymorphism in Type 2 Diabetic Nephropathy. Turk. J. Endocrinol. Metab. 2018, 22, 151–157. [Google Scholar] [CrossRef]
- Ayan, D.; Şeneş, M.; Çaycı, A.B.; Söylemez, S.; Eren, N.; Altuntaş, Y.; Öztürk, F.Y. Evaluation of Paraoxonase, Arylesterase, and Homocysteine Thiolactonase Activities in Patients with Diabetes and Incipient Diabetes Nephropathy. J. Med. Biochem. 2019, 38, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Didas, N.; Thitisopee, W.; Porntadavity, S.; Jeenduang, N. Arylesterase Activity but Not PCSK9 Levels Is Associated with Chronic Kidney Disease in Type 2 Diabetes. Int. Urol. Nephrol. 2020, 52, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Sridevi, C.; Sowjanya, U.; Selvi, V.S.K.; Rajakumari, D.M.M.; Babu, K. Serum Paraoxonase with HDL-C as a Predictor of Atherosclerosis in Patients of Chronic Kidney Disease. Biomedicine 2021, 40, 442–446. [Google Scholar] [CrossRef]
- Jose, S.; Kc, R.; Chandran, S. Oxidative Stress in Patients with Diabetic Nephropathy. Natl. J. Physiol. Pharm. Pharmacol. 2022, 12, 1709. [Google Scholar] [CrossRef]
- Ebert, T.; Neytchev, O.; Witasp, A.; Kublickiene, K.; Stenvinkel, P.; Shiels, P.G. Inflammation and Oxidative Stress in Chronic Kidney Disease and Dialysis Patients. Antioxid. Redox Signal. 2021, 35, 1426–1448. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M.; Billecke, S.; Erogul, J.; Sorenson, R.; Bisgaier, C.L.; Newton, R.S.; La Du, B. Human Serum Paraoxonase (PON 1) Is Inactivated by Oxidized Low Density Lipoprotein and Preserved by Antioxidants. Free Radic. Biol. Med. 1999, 26, 892–904. [Google Scholar] [CrossRef]
- Camps, J.; Marsillach, J.; Joven, J. The Paraoxonases: Role in Human Diseases and Methodological Difficulties in Measurement. Crit. Rev. Clin. Lab. Sci. 2009, 46, 83–106. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Chen, L.-S.; Zhang, L.-C.; Zhou, T.-B. Meta-Analysis of the Relationship between ACE I/D Gene Polymorphism and End-Stage Renal Disease in Patients with Diabetic Nephropathy. Nephrology 2012, 17, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Tziastoudi, M.; Cholevas, C.; Theoharides, T.C.; Stefanidis, I. Meta-Analysis and Bioinformatics Detection of Susceptibility Genes in Diabetic Nephropathy. Int. J. Mol. Sci. 2021, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Gbandjaba, N.Y.; Ghalim, N.; Hassar, M.; Berrougui, H.; Labrazi, H.; Taki, H.; Saile, R.; Khalil, A. Paraoxonase Activity in Healthy, Diabetic, and Hemodialysis Patients. Clin. Biochem. 2012, 45, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Dirican, M.; Sarandol, E.; Serdar, Z.; Ocak, N.; Dilek, K. Oxidative Status and Prevalent Cardiovascular Disease in Patients with Chronic Renal Failure Treated by Hemodialysis. Clin. Nephrol. 2007, 68, 144–150. [Google Scholar] [CrossRef]
- Suematsu, Y.; Goto, M.; Park, C.; Nunes, A.C.F.; Jing, W.; Streja, E.; Rhee, C.M.; Cruz, S.; Kashyap, M.L.; Vaziri, N.D.; et al. Association of Serum Paraoxonase/Arylesterase Activity with All-Cause Mortality in Maintenance Hemodialysis Patients. J. Clin. Endocrinol. Metab. 2019, 104, 4848–4856. [Google Scholar] [CrossRef]
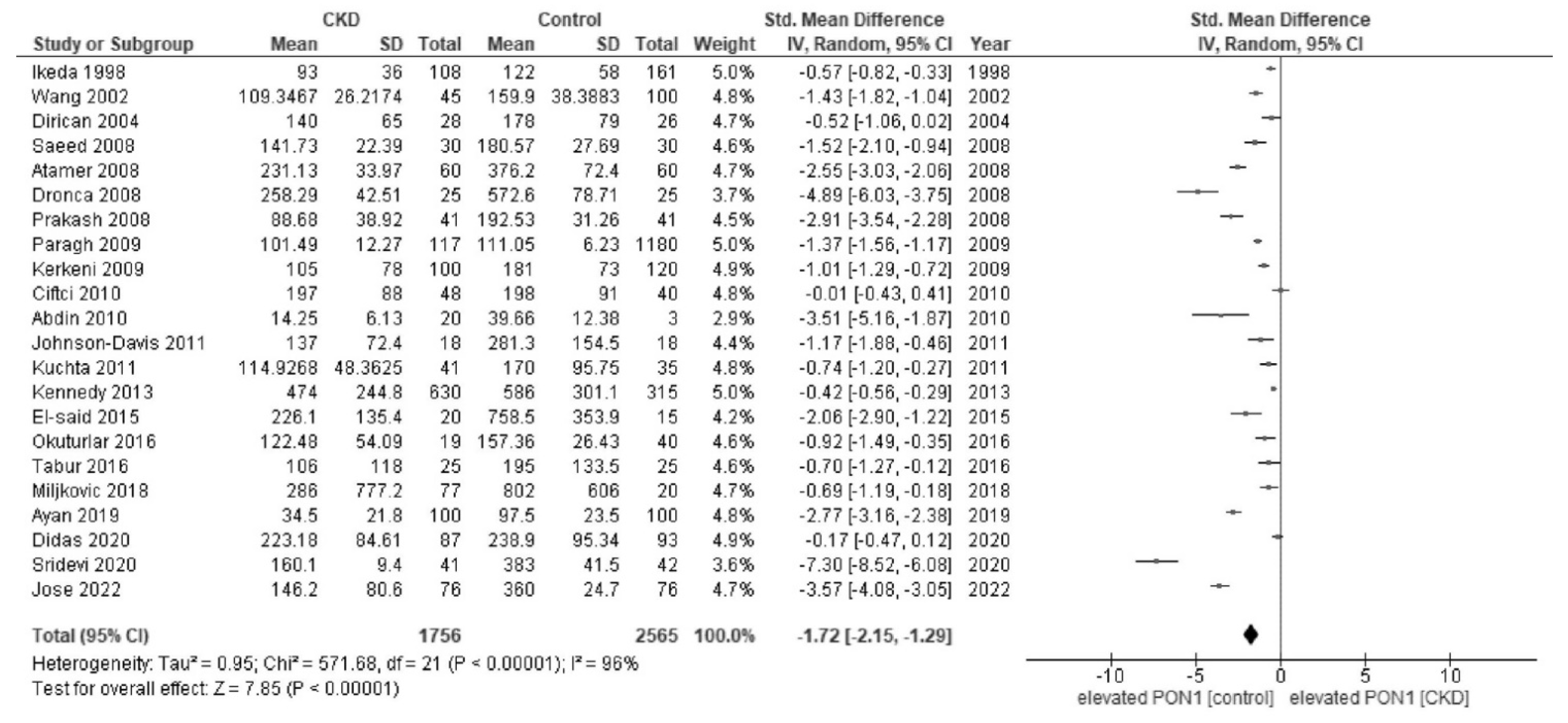


| First Author | Year [Ref. No.] | Country | Subject No. | Age | Unit | Activity in CKD | Activity in Healthy Controls | CKD/CKF Stages |
|---|---|---|---|---|---|---|---|---|
| Paraoxonase | ||||||||
| Ikeda | 1998 [26] | Japan | 169 | 57 | U/L | 93 ± 36.0 | 122.0 ± 58.0 | CKD |
| Wang | 2002 [27] | China | 145 | 59 | U/mL | 109.3 ± 26.2 | 159.9 ± 38.3 | CKD |
| Dirican | 2004 [28] | Turkey | 72 | 47 | U/L | 140.0 ± 65.0 | 178.0 ± 79.0 | CKF |
| Atamer | 2008 [29] | Turkey | 60 | 53 | U/L | 231.1 ± 34.0 | 376.2 ± 72.4 | CKF |
| Dronca | 2008 [30] | Romania | 50 | 51 | U/L | 258.3 ± 42.5 | 572.6 ± 78.7 | CKF |
| Prakash | 2008 [31] | India | 89 | 53 | U/L | 88.7 ± 38.9 | 192.5 ± 31.3 | CKF |
| Saeed | 2008 [32] | Egypt | 60 | 42 | U/L | 141.7 ± 22.4 | 180.6 ± 27.7 | CKF |
| Kerkeni | 2009 [33] | Tunisia | 220 | 51 | U/mL | 105.0 ± 78.0 | 181.0 ± 73.0 | CKD |
| Paragh | 2009 [34] | Hungary | 1297 | 48 | U/L | 101.5 ± 12.3 | 111.1 ± 6.2 | CKF |
| Abdin | 2010 [35] | Egypt | 50 | 54 | U/mL | 14.3 ± 6.1 | 39.7 ± 12.4 | CKF |
| Ciftci | 2010 [36] | Turkey | 88 | 31 | U/L | 197.0 ± 88.0 | 198.0 ± 91.0 | CKD |
| Johnson-Davis | 2011 [37] | USA | 36 | 18–84 | U/L | 137.0 ± 72.4 | 281.3 ± 154.5 | CKD |
| Kuchta | 2011 [38] | Poland | 76 | 58 | U/L | 114.9 ± 48.4 | 170.0 ± 95.8 | CKD/CKF |
| Kennedy | 2013 [39] | USA | 945 | 69 | U/L | 474.0 ± 244.8 | 586.0 ± 301.1 | CKD |
| EI-said | 2015 [40] | Egypt | 35 | 50 | U/L | 226.1 ± 135.4 | 758.5 ± 353.9 | CKD |
| Okuturlar | 2016 [41] | Turkey | 59 | 55 | U/L | 122.5 ± 54.1 | 157.4 ± 26.4 | CKD |
| Tabur | 2016 [42] | Turkey | 50 | 51 | U/mL | 106.0 ± 118.0 | 115.0 ± 101.0 | CKD |
| Miljkovic | 2018 [44] | Serbia | 41 | 57 | U/L | 286.0 ± 777.2 | 802.0 ± 606.0 | CKD |
| Ayan | 2019 [46] | Turkey | 200 | 55 | U/L | 34.5 ± 21.8 | 97.5 ± 23.5 | CKD |
| Didas | 2020 [47] | Thailand | 180 | 68 | U/L | 223.2 ± 84.6 | 238.9 ± 95.3 | CKD |
| Sridevi | 2021 [48] | India | 81 | 20–60 | U/L | 160.1 ± 9.4 | 383.0 ± 41.5 | CKF |
| Jose | 2022 [49] | India | 152 | 51 | U/L | 146.2 ± 80.6 | 360.0 ± 24.7 | CKF |
| Arylesterase | ||||||||
| Dirican | 2004 [28] | Turkey | 72 | 47 | kU/L | 66.0 ± 19.0 | 84.0 ± 28.0 | CKF |
| Dronca | 2008 [30] | Romania | 50 | 51 | U/L | 58.4 ± 1.9 | 93.4 ± 5.5 | CKF |
| Saeed | 2008 [32] | Egypt | 60 | 42 | kU/L | 78.2 ± 18.2 | 103.7 ± 14.8 | CKF |
| Paragh | 2009 [34] | Hungary | 1297 | 47.6 | U/mL | 66.9 ± 4.3 | 85.5 ± 2.5 | CKD |
| Kennedy | 2013 [39] | USA | 945 | 69 | U/mL | 94.0 ± 13.0 | 103.0 ± 13.3 | CKD |
| Okuturlar | 2016 [41] | Turkey | 59 | 55 | U/L | 122.5 ± 30.7 | 256.1 ± 24.6 | CKD |
| Tabur | 2016 [42] | Turkey | 50 | 51 | U/mL | 196.4 ± 27.9 | 223.9 ± 34.8 | CKD |
| Jaju | 2018 [43] | India | 60 | NR | NR | 100.9 ± 34.2 | 135.3 ± 30.9 | CKD |
| Mogarekar | 2018 [45] | India | 100 | 59 | kU/L | 85.4 ± 24.1 | 127.9 ± 25.5 | CKD |
| Ayan | 2019 [46] | Turkey | 200 | 55 | kU/L | 44.9 ± 5.6 | 57.1 ± 3.9 | CKD |
| Didas | 2020 [47] | Thailand | 87 | 68 | kU/L | 225.5 ± 108.7 | 257.5 ± 106.1 | CKD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, J.; Kotani, K.; Gugliucci, A. Paraoxonase 1 and Chronic Kidney Disease: A Meta-Analysis. J. Clin. Med. 2023, 12, 1199. https://doi.org/10.3390/jcm12031199
Watanabe J, Kotani K, Gugliucci A. Paraoxonase 1 and Chronic Kidney Disease: A Meta-Analysis. Journal of Clinical Medicine. 2023; 12(3):1199. https://doi.org/10.3390/jcm12031199
Chicago/Turabian StyleWatanabe, Jun, Kazuhiko Kotani, and Alejandro Gugliucci. 2023. "Paraoxonase 1 and Chronic Kidney Disease: A Meta-Analysis" Journal of Clinical Medicine 12, no. 3: 1199. https://doi.org/10.3390/jcm12031199
APA StyleWatanabe, J., Kotani, K., & Gugliucci, A. (2023). Paraoxonase 1 and Chronic Kidney Disease: A Meta-Analysis. Journal of Clinical Medicine, 12(3), 1199. https://doi.org/10.3390/jcm12031199