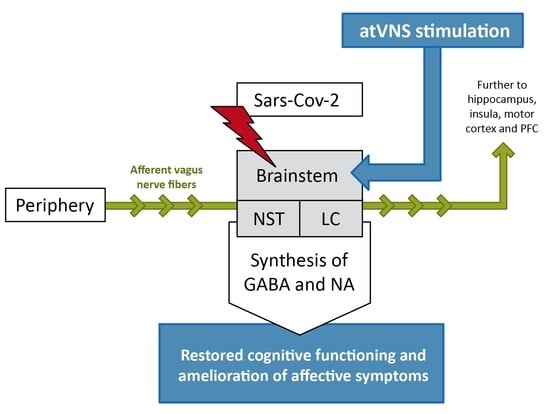
A Prospect to Ameliorate Affective Symptoms and to Enhance Cognition in Long COVID Using Auricular Transcutaneous Vagus Nerve Stimulation
Abstract
1. Introduction
2. The Role of Brainstem and Vagus Nerve Signaling in Long COVID, Affective Disorders and Cognition
3. Cognitive Deficits in Long COVID
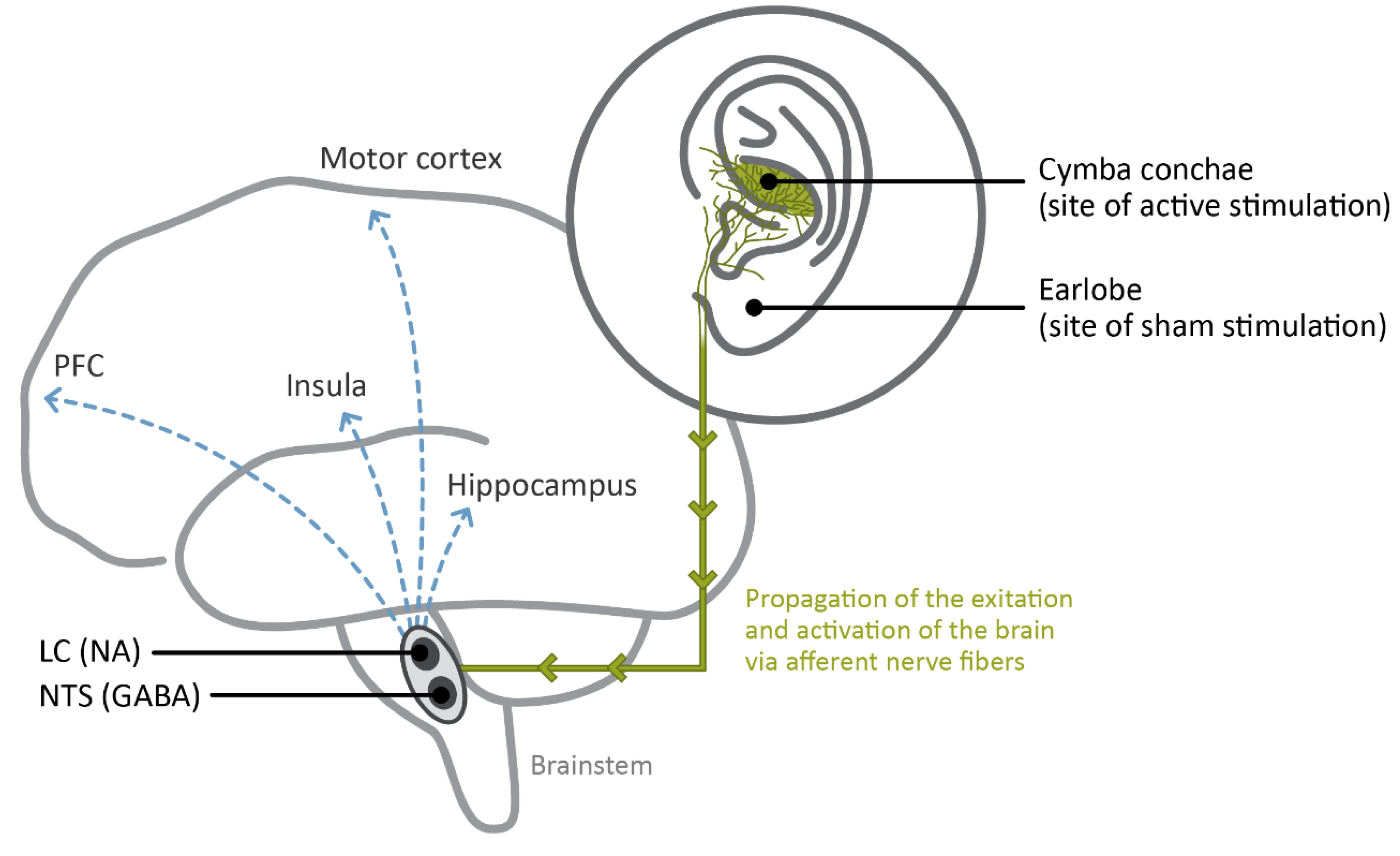
4. Clinical Studies Employing atVNS
5. Neurobiological, Affective and Cognitive Effects of atVNS
6. AtVNS as Adjuvant Treatment to Pharmacotherapy?
7. AtVNS Safety and Feasibility in Real-World Settings
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jarrott, B.; Head, R.; Pringle, K.G.; Lumbers, E.R.; Martin, J.H. “LONG COVID”—A Hypothesis for Understanding the Biological Basis and Pharmacological Treatment Strategy. Pharmacol. Res. Perspect. 2022, 10, e00911. [Google Scholar] [CrossRef] [PubMed]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising Long COVID: A Living Systematic Review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef] [PubMed]
- Orfei, M.D.; Porcari, D.E.; D’Arcangelo, S.; Maggi, F.; Russignaga, D.; Ricciardi, E. A New Look on Long-COVID Effects: The Functional Brain Fog Syndrome. J. Clin. Med. 2022, 11, 5529. [Google Scholar] [CrossRef] [PubMed]
- Bowe, B.; Xie, Y.; Al-Aly, Z. Acute and Postacute Sequelae Associated with SARS-CoV-2 Reinfection. Nat. Med. 2022, 28, 2398–2405. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. Long-COVID Treatments: Why the World Is Still Waiting. Nature 2022, 608, 258–260. [Google Scholar] [CrossRef]
- Pilloni, G.; Bikson, M.; Badran, B.W.; George, M.S.; Kautz, S.A.; Okano, A.H.; Baptista, A.F.; Charvet, L.E. Update on the Use of Transcranial Electrical Brain Stimulation to Manage Acute and Chronic COVID-19 Symptoms. Front. Hum. Neurosci. 2020, 14, 595567. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Rowe, J.W.; Kahn, R.L. Successful Aging. Gerontologist 1997, 37, 433–440. [Google Scholar] [CrossRef]
- McTeague, L.M.; Goodkind, M.S.; Etkin, A. Transdiagnostic Impairment of Cognitive Control in Mental Illness. J. Psychiatr. Res. 2016, 83, 37–46. [Google Scholar] [CrossRef]
- Guedj, E.; Campion, J.Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H.; Guis, S.; Barthelemy, F.; Habert, P.; Ceccaldi, M.; et al. 18F-FDG Brain PET Hypometabolism in Patients with Long COVID. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2823–2833. [Google Scholar] [CrossRef]
- Guedj, E.; Million, M.; Dudouet, P.; Tissot-Dupont, H.; Bregeon, F.; Cammilleri, S.; Raoult, D. 18F-FDG Brain PET Hypometabolism in Post-SARS-CoV-2 Infection: Substrate for Persistent/Delayed Disorders? Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 592–595. [Google Scholar] [CrossRef]
- Hugon, J.; Queneau, M.; Sanchez Ortiz, M.; Msika, E.F.; Farid, K.; Paquet, C. Cognitive Decline and Brainstem Hypometabolism in Long COVID: A Case Series. Brain Behav. 2022, 12, e2513. [Google Scholar] [CrossRef]
- Morand, A.; Campion, J.-Y.; Lepine, A.; Bosdure, E.; Luciani, L.; Cammilleri, S.; Chabrol, B.; Guedj, E. Similar Patterns of [18F]-FDG Brain PET Hypometabolism in Paediatric and Adult Patients with Long COVID: A Paediatric Case Series. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J. Persistent Brainstem Dysfunction in Long-COVID: A Hypothesis. ACS Chem. Neurosci. 2021, 12, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-Acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef] [PubMed]
- Sodagar, A.; Javed, R.; Tahir, H.; Razak, S.I.A.; Shakir, M.; Naeem, M.; Yusof, A.H.A.; Sagadevan, S.; Hazafa, A.; Uddin, J.; et al. Pathological Features and Neuroinflammatory Mechanisms of SARS-CoV-2 in the Brain and Potential Therapeutic Approaches. Biomolecules 2022, 12, 971. [Google Scholar] [CrossRef]
- Acanfora, D.; Nolano, M.; Acanfora, C.; Colella, C.; Provitera, V.; Caporaso, G.; Rodolico, G.R.; Bortone, A.S.; Galasso, G.; Casucci, G. Impaired Vagal Activity in Long-COVID-19 Patients. Viruses 2022, 14, 1035. [Google Scholar] [CrossRef]
- Colzato, L.; Beste, C. A Literature Review on the Neurophysiological Underpinnings and Cognitive Effects of Transcutaneous Vagus Nerve Stimulation: Challenges and Future Directions. J. Neurophysiol. 2020, 123, 1739–1755. [Google Scholar] [CrossRef]
- Karemaker, J.M. The Multibranched Nerve: Vagal Function beyond Heart Rate Variability. Biol. Psychol. 2022, 172, 108378. [Google Scholar] [CrossRef]
- Yakunina, N.; Kim, S.S.; Nam, E.-C. Optimization of Transcutaneous Vagus Nerve Stimulation Using Functional MRI. Neuromodulation 2017, 20, 290–300. [Google Scholar] [CrossRef]
- Yakunina, N.; Kim, S.S.; Nam, E.-C. BOLD FMRI Effects of Transcutaneous Vagus Nerve Stimulation in Patients with Chronic Tinnitus. PLoS ONE 2018, 13, e0207281. [Google Scholar] [CrossRef]
- Dietrich, S.; Smith, J.; Scherzinger, C.; Hofmann-Preiss, K.; Freitag, T.; Eisenkolb, A.; Ringler, R. A Novel Transcutaneous Vagus Nerve Stimulation Leads to Brainstem and Cerebral Activations Measured by Functional MRI. BioMed. Tech. 2008, 53, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.; Kiess, O.; Hösl, K.; Terekhin, P.; Kornhuber, J.; Forster, C. CNS BOLD FMRI Effects of Sham-Controlled Transcutaneous Electrical Nerve Stimulation in the Left Outer Auditory Canal—A Pilot Study. Brain Stimul. 2013, 6, 798–804. [Google Scholar] [CrossRef]
- Frangos, E.; Ellrich, J.; Komisaruk, B.R. Non-Invasive Access to the Vagus Nerve Central Projections via Electrical Stimulation of the External Ear: FMRI Evidence in Humans. Brain Stimul. 2015, 8, 624–636. [Google Scholar] [CrossRef]
- Alicart, H.; Heldmann, M.; Göttlich, M.; Obst, M.A.; Tittgemeyer, M.; Münte, T.F. Modulation of Visual Processing of Food by Transcutaneous Vagus Nerve Stimulation (TVNS). Brain Imaging Behav. 2020, 15, 1886–1897. [Google Scholar] [CrossRef] [PubMed]
- Borgmann, D.; Rigoux, L.; Kuzmanovic, B.; Edwin Thanarajah, S.; Münte, T.F.; Fenselau, H.; Tittgemeyer, M. Technical Note: Modulation of FMRI Brainstem Responses by Transcutaneous Vagus Nerve Stimulation. Neuroimage 2021, 244, 118566. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, J.; Xu, C.; Zhang, X.; Qiao, L.; Wang, X.; Zhang, X.; Yan, X.; Ni, D.; Yu, T.; et al. The Functional Connectivity Study on the Brainstem-Cortical/Subcortical Structures in Responders Following Cervical Vagus Nerve Stimulation. Int. J. Dev. Neurosci. 2020, 80, 679–686. [Google Scholar] [CrossRef]
- Wittbrodt, M.T.; Gurel, N.Z.; Nye, J.A.; Shandhi, M.M.H.; Gazi, A.H.; Shah, A.J.; Pearce, B.D.; Murrah, N.; Ko, Y.-A.; Shallenberger, L.H.; et al. Noninvasive Cervical Vagal Nerve Stimulation Alters Brain Activity During Traumatic Stress in Individuals with Posttraumatic Stress Disorder. Psychosom. Med. 2021, 83, 969–977. [Google Scholar] [CrossRef]
- Gazi, A.H.; Wittbrodt, M.T.; Harrison, A.B.; Sundararaj, S.; Gurel, N.Z.; Nye, J.A.; Shah, A.J.; Vaccarino, V.; Bremner, J.D.; Inan, O.T. Robust Estimation of Respiratory Variability Uncovers Correlates of Limbic Brain Activity and Transcutaneous Cervical Vagus Nerve Stimulation in the Context of Traumatic Stress. IEEE Trans. BioMed. Eng. 2022, 69, 849–859. [Google Scholar] [CrossRef]
- Lerman, I.; Klaming, R.; Spadoni, A.; Baker, D.G.; Simmons, A.N. Non-Invasive Cervical Vagus Nerve Stimulation Effects on Reaction Time and Valence Image Anticipation Response. Brain Stimul. 2022, 15, 946–956. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Z.; Qu, Y.; Zhao, Y.; Yang, Y.; Du, J.; Yang, C. Cognitive Function and Brain Activation before and after Transcutaneous Cervical Vagus Nerve Stimulation in Healthy Adults: A Concurrent TcVNS-FMRI Study. Front. Psychol. 2022, 13, 1003411. [Google Scholar] [CrossRef] [PubMed]
- Clancy, J.A.; Mary, D.A.; Witte, K.K.; Greenwood, J.P.; Deuchars, S.A.; Deuchars, J. Non-Invasive Vagus Nerve Stimulation in Healthy Humans Reduces Sympathetic Nerve Activity. Brain Stimul. 2014, 7, 871–877. [Google Scholar] [CrossRef] [PubMed]
- De Couck, M.; Cserjesi, R.; Caers, R.; Zijlstra, W.P.; Widjaja, D.; Wolf, N.; Luminet, O.; Ellrich, J.; Gidron, Y. Effects of Short and Prolonged Transcutaneous Vagus Nerve Stimulation on Heart Rate Variability in Healthy Subjects. Auton. Neurosci. 2017, 203, 88–96. [Google Scholar] [CrossRef]
- Machetanz, K.; Berelidze, L.; Guggenberger, R.; Gharabaghi, A. Transcutaneous Auricular Vagus Nerve Stimulation and Heart Rate Variability: Analysis of Parameters and Targets. Auton. Neurosci. 2021, 236, 102894. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, P.; Fu, H.; Chen, W.; Cui, S.; Lu, L.; Tang, C. Transcutaneous Auricular Vagus Nerve Stimulation in Treating Major Depressive Disorder: A Systematic Review and Meta-Analysis. Medicine 2018, 97, e13845. [Google Scholar] [CrossRef] [PubMed]
- Ridgewell, C.; Heaton, K.J.; Hildebrandt, A.; Couse, J.; Leeder, T.; Neumeier, W.H. The Effects of Transcutaneous Auricular Vagal Nerve Stimulation on Cognition in Healthy Individuals: A Meta-Analysis. Neuropsychology 2021, 35, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and Cognitive Impairment in Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- McIntire, L.K.; McKinley, R.A.; Goodyear, C.; McIntire, J.P.; Brown, R.D. Cervical Transcutaneous Vagal Nerve Stimulation (CtVNS) Improves Human Cognitive Performance under Sleep Deprivation Stress. Commun. Biol. 2021, 4, 634. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Huang, Y.-C.; Huang, L.-T.; Chen, R.-M.; Chen, C. Cervical Noninvasive Vagus Nerve Stimulation for Migraine and Cluster Headache: A Systematic Review and Meta-Analysis. Neuromodulation 2020, 23, 721–731. [Google Scholar] [CrossRef]
- Buonsenso, D.; Piazza, M.; Boner, A.L.; Bellanti, J.A. Long COVID: A Proposed Hypothesis-Driven Model of Viral Persistence for the Pathophysiology of the Syndrome. Allergy Asthma Proc. 2022, 43, 187–193. [Google Scholar] [CrossRef]
- Khamsi, R. Rogue Antibodies Could Be Driving Severe COVID-19. Nature 2021, 590, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Willyard, C. Could Tiny Blood Clots Cause Long COVID’s Puzzling Symptoms? Nature 2022, 608, 662–664. [Google Scholar] [CrossRef]
- Lukiw, W.J.; Pogue, A.; Hill, J.M. SARS-CoV-2 Infectivity and Neurological Targets in the Brain. Cell Mol. Neurobiol. 2022, 42, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Matschke, J.; Lütgehetmann, M.; Hagel, C.; Sperhake, J.P.; Schröder, A.S.; Edler, C.; Mushumba, H.; Fitzek, A.; Allweiss, L.; Dandri, M.; et al. Neuropathology of Patients with COVID-19 in Germany: A Post-Mortem Case Series. Lancet Neurol. 2020, 19, 919–929. [Google Scholar] [CrossRef]
- Solomon, I.H.; Normandin, E.; Bhattacharyya, S.; Mukerji, S.S.; Keller, K.; Ali, A.S.; Adams, G.; Hornick, J.L.; Padera, R.F.; Sabeti, P. Neuropathological Features of Covid-19. N. Engl. J. Med. 2020, 383, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Itoi, K.; Sugimoto, N. The Brainstem Noradrenergic Systems in Stress, Anxiety and Depression. J. Neuroendocr. 2010, 22, 355–361. [Google Scholar] [CrossRef]
- Porges, S.W. Vagal Tone: A Physiologic Marker of Stress Vulnerability. Pediatrics 1992, 90, 498–504. [Google Scholar] [CrossRef]
- Iseger, T.A.; van Bueren, N.E.R.; Kenemans, J.L.; Gevirtz, R.; Arns, M. A Frontal-Vagal Network Theory for Major Depressive Disorder: Implications for Optimizing Neuromodulation Techniques. Brain Stimul. 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Li, C.; Chen, W.; Lin, F.; Li, W.; Wang, P.; Liao, G.; Zhang, L. Functional Two-Way Crosstalk Between Brain and Lung: The Brain–Lung Axis. Cell. Mol. Neurobiol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Clancy, J.A.; Deuchars, S.A.; Deuchars, J. The Wonders of the Wanderer. Exp. Physiol. 2013, 98, 38–45. [Google Scholar] [CrossRef]
- Mather, M.; Clewett, D.; Sakaki, M.; Harley, C.W. Norepinephrine Ignites Local Hotspots of Neuronal Excitation: How Arousal Amplifies Selectivity in Perception and Memory. Behav. Brain Sci. 2016, 39, e200. [Google Scholar] [CrossRef] [PubMed]
- Sara, S.J. The Locus Coeruleus and Noradrenergic Modulation of Cognition. Nat. Rev. Neurosci. 2009, 10, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Wilcke, T.; Fuchs, E.; Funke, K.; Vlachos, A.; Müller-Dahlhaus, F.; Puts, N.A.J.; Harris, R.E.; Edden, R.A.E. GABA—From Inhibition to Cognition: Emerging Concepts. Neuroscientist 2018, 24, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, J.; Hinson, E.L.; Divanbeighi Zand, A.P.; Rizov, A.; Emir, U.E.; Stagg, C.J. The Dynamics of Cortical GABA in Human Motor Learning. J. Physiol. 2019, 597, 271–282. [Google Scholar] [CrossRef]
- Heaney, C.F.; Kinney, J.W. Role of GABAB Receptors in Learning and Memory and Neurological Disorders. Neurosci. Biobehav. Rev. 2016, 63, 1–28. [Google Scholar] [CrossRef]
- Haag, L.; Quetscher, C.; Dharmadhikari, S.; Dydak, U.; Schmidt-Wilcke, T.; Beste, C. Interrelation of Resting State Functional Connectivity, Striatal GABA Levels, and Cognitive Control Processes. Hum. Brain Mapp. 2015, 36, 4383–4393. [Google Scholar] [CrossRef]
- Quetscher, C.; Yildiz, A.; Dharmadhikari, S.; Glaubitz, B.; Schmidt-Wilcke, T.; Dydak, U.; Beste, C. Striatal GABA-MRS Predicts Response Inhibition Performance and Its Cortical Electrophysiological Correlates. Brain Struct. Funct. 2015, 220, 3555–3564. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Cohen, J.D. An Integrative Theory of Locus Coeruleus-Norepinephrine Function: Adaptive Gain and Optimal Performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef]
- Chmielewski, W.X.; Mückschel, M.; Ziemssen, T.; Beste, C. The Norepinephrine System Affects Specific Neurophysiological Subprocesses in the Modulation of Inhibitory Control by Working Memory Demands. Hum. Brain Mapp. 2017, 38, 68–81. [Google Scholar] [CrossRef]
- Mückschel, M.; Chmielewski, W.; Ziemssen, T.; Beste, C. The Norepinephrine System Shows Information-Content Specific Properties during Cognitive Control—Evidence from EEG and Pupillary Responses. Neuroimage 2017, 149, 44–52. [Google Scholar] [CrossRef]
- Mückschel, M.; Gohil, K.; Ziemssen, T.; Beste, C. The Norepinephrine System and Its Relevance for Multi-Component Behavior. Neuroimage 2017, 146, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Houben, S.; Bonnechère, B. The Impact of COVID-19 Infection on Cognitive Function and the Implication for Rehabilitation: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 7748. [Google Scholar] [CrossRef]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.-M. Mid and Long-Term Neurological and Neuropsychiatric Manifestations of Post-COVID-19 Syndrome: A Meta-Analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Rodríuez-Jiménez, J.; Palacios-Ceña, M.; Velasco-Arribas, M.; Guijarro, C.; de-la-Llave-Rincón, A.I.; Fuensalida-Novo, S.; Elvira-Martínez, C.M.; et al. Long-Term Post-COVID Symptoms and Associated Risk Factors in Previously Hospitalized Patients: A Multicenter Study. J. Infect. 2021, 83, 237–279. [Google Scholar] [CrossRef] [PubMed]
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E.; et al. Post-Discharge Persistent Symptoms and Health-Related Quality of Life after Hospitalization for COVID-19. J. Infect. 2020, 81, e4–e6. [Google Scholar] [CrossRef]
- Mattioli, F.; Stampatori, C.; Righetti, F.; Sala, E.; Tomasi, C.; De Palma, G. Neurological and Cognitive Sequelae of Covid-19: A Four Month Follow-Up. J. Neurol. 2021, 268, 4422–4428. [Google Scholar] [CrossRef]
- Pilotto, A.; Cristillo, V.; Cotti Piccinelli, S.; Zoppi, N.; Bonzi, G.; Sattin, D.; Schiavolin, S.; Raggi, A.; Canale, A.; Gipponi, S.; et al. Long-Term Neurological Manifestations of COVID-19: Prevalence and Predictive Factors. Neurol. Sci. 2021, 42, 4903–4907. [Google Scholar] [CrossRef]
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 Symptom Burden: What Is Long-COVID and How Should We Manage It? Lung 2021, 199, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, H.M.; Shaaban, H.M.; Tawfik, A.M. Post-COVID-19 Syndrome in Egyptian Healthcare Staff: Highlighting the Carers Sufferings. Electron. J. Gen. Med. 2021, 18, em291. [Google Scholar] [CrossRef]
- Woo, M.S.; Malsy, J.; Pöttgen, J.; Seddiq Zai, S.; Ufer, F.; Hadjilaou, A.; Schmiedel, S.; Addo, M.M.; Gerloff, C.; Heesen, C.; et al. Frequent Neurocognitive Deficits after Recovery from Mild COVID-19. Brain Commun. 2020, 2, fcaa205. [Google Scholar] [CrossRef]
- Mazza, M.G.; Palladini, M.; De Lorenzo, R.; Magnaghi, C.; Poletti, S.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; Benedetti, F. Persistent Psychopathology and Neurocognitive Impairment in COVID-19 Survivors: Effect of Inflammatory Biomarkers at Three-Month Follow-Up. Brain Behav. Immun. 2021, 94, 138–147. [Google Scholar] [CrossRef]
- Writing Committee for the COMEBAC Study Group; Morin, L.; Savale, L.; Pham, T.; Colle, R.; Figueiredo, S.; Harrois, A.; Gasnier, M.; Lecoq, A.-L.; Meyrignac, O.; et al. Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA 2021, 325, 1525–1534. [Google Scholar] [CrossRef]
- Darley, D.R.; Dore, G.J.; Byrne, A.L.; Plit, M.L.; Brew, B.J.; Kelleher, A.; Matthews, G.V. Limited Recovery from Post-Acute Sequelae of SARS-CoV-2 at 8 Months in a Prospective Cohort. ERJ Open Res. 2021, 7, 00384. [Google Scholar] [CrossRef] [PubMed]
- Elkan, M.; Dvir, A.; Zaidenstein, R.; Keller, M.; Kagansky, D.; Hochman, C.; Koren, R. Patient-Reported Outcome Measures After Hospitalization During the COVID-19 Pandemic: A Survey Among COVID-19 and Non-COVID-19 Patients. Int. J. Gen. Med. 2021, 14, 4829–4836. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, R.; Dini, M.; Groppo, E.; Rosci, C.; Reitano, M.R.; Bai, F.; Poletti, B.; Brugnera, A.; Silani, V.; D’Arminio Monforte, A.; et al. Long-Lasting Cognitive Abnormalities after COVID-19. Brain Sci. 2021, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Havervall, S.; Rosell, A.; Phillipson, M.; Mangsbo, S.M.; Nilsson, P.; Hober, S.; Thålin, C. Symptoms and Functional Impairment Assessed 8 Months After Mild COVID-19 Among Health Care Workers. JAMA 2021, 325, 2015–2016. [Google Scholar] [CrossRef]
- Jacobson, K.B.; Rao, M.; Bonilla, H.; Subramanian, A.; Hack, I.; Madrigal, M.; Singh, U.; Jagannathan, P.; Grant, P. Patients with Uncomplicated Coronavirus Disease 2019 (COVID-19) Have Long-Term Persistent Symptoms and Functional Impairment Similar to Patients with Severe COVID-19: A Cautionary Tale During a Global Pandemic. Clin. Infect. Dis. 2021, 73, e826–e829. [Google Scholar] [CrossRef]
- Dressing, A.; Bormann, T.; Blazhenets, G.; Schroeter, N.; Walter, L.I.; Thurow, J.; August, D.; Hilger, H.; Stete, K.; Gerstacker, K.; et al. Neuropsychologic Profiles and Cerebral Glucose Metabolism in Neurocognitive Long COVID Syndrome. J. Nucl. Med. 2022, 63, 1058–1063. [Google Scholar] [CrossRef]
- Hampshire, A.; Trender, W.; Chamberlain, S.R.; Jolly, A.E.; Grant, J.E.; Patrick, F.; Mazibuko, N.; Williams, S.C.; Barnby, J.M.; Hellyer, P.; et al. Cognitive Deficits in People Who Have Recovered from COVID-19. EClinicalMedicine 2021, 39, 101044. [Google Scholar] [CrossRef]
- Henneghan, A.M.; Lewis, K.A.; Gill, E.; Kesler, S.R. Cognitive Impairment in Non-Critical, Mild-to-Moderate COVID-19 Survivors. Front. Psychol. 2022, 13, 770459. [Google Scholar] [CrossRef]
- Johnsen, S.; Sattler, S.M.; Miskowiak, K.W.; Kunalan, K.; Victor, A.; Pedersen, L.; Andreassen, H.F.; Jørgensen, B.J.; Heebøll, H.; Andersen, M.B.; et al. Descriptive Analysis of Long COVID Sequelae Identified in a Multidisciplinary Clinic Serving Hospitalised and Non-Hospitalised Patients. ERJ Open Res. 2021, 7, 00205–02021. [Google Scholar] [CrossRef] [PubMed]
- Miskowiak, K.W.; Johnsen, S.; Sattler, S.M.; Nielsen, S.; Kunalan, K.; Rungby, J.; Lapperre, T.; Porsberg, C.M. Cognitive Impairments Four Months after COVID-19 Hospital Discharge: Pattern, Severity and Association with Illness Variables. Eur. Neuropsychopharmacol. 2021, 46, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Tobinick, E.; Spengler, R.N.; Ignatowski, T.A.; Wassel, M.; Laborde, S. Rapid Improvement in Severe Long COVID Following Perispinal Etanercept. Curr. Med. Res. Opin. 2022, 38, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elfattah, H.M.; Abdelazeim, F.H.; Elshennawy, S. Physical and Cognitive Consequences of Fatigue: A Review. J. Adv. Res. 2015, 6, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Hartung, T.J.; Neumann, C.; Bahmer, T.; Chaplinskaya-Sobol, I.; Endres, M.; Geritz, J.; Haeusler, K.G.; Heuschmann, P.U.; Hildesheim, H.; Hinz, A.; et al. Fatigue and Cognitive Impairment after COVID-19: A Prospective Multicentre Study. eClinicalMedicine 2022, 53, 101651. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Mudge, J.D.; Kasole, M.; Chen, R.C.; Blanz, S.L.; Trevathan, J.K.; Lovett, E.G.; Williams, J.C.; Ludwig, K.A. Auricular Vagus Neuromodulation—A Systematic Review on Quality of Evidence and Clinical Effects. Front. Neurosci. 2021, 15, 664740. [Google Scholar] [CrossRef]
- Hein, E.; Nowak, M.; Kiess, O.; Biermann, T.; Bayerlein, K.; Kornhuber, J.; Kraus, T. Auricular Transcutaneous Electrical Nerve Stimulation in Depressed Patients: A Randomized Controlled Pilot Study. J. Neural. Transm. 2013, 120, 821–827. [Google Scholar] [CrossRef]
- Rong, P.; Liu, J.; Wang, L.; Liu, R.; Fang, J.; Zhao, J.; Zhao, Y.; Wang, H.; Vangel, M.; Sun, S.; et al. Effect of Transcutaneous Auricular Vagus Nerve Stimulation on Major Depressive Disorder: A Nonrandomized Controlled Pilot Study. J. Affect. Disord. 2016, 195, 172–179. [Google Scholar] [CrossRef]
- Fang, J.; Rong, P.; Hong, Y.; Fan, Y.; Liu, J.; Wang, H.; Zhang, G.; Chen, X.; Shi, S.; Wang, L.; et al. Transcutaneous Vagus Nerve Stimulation Modulates Default Mode Network in Major Depressive Disorder. Biol. Psychiatry 2016, 79, 266–273. [Google Scholar] [CrossRef]
- Liu, J.; Fang, J.; Wang, Z.; Rong, P.; Hong, Y.; Fan, Y.; Wang, X.; Park, J.; Jin, Y.; Liu, C.; et al. Transcutaneous Vagus Nerve Stimulation Modulates Amygdala Functional Connectivity in Patients with Depression. J. Affect Disord. 2016, 205, 319–326. [Google Scholar] [CrossRef]
- Fang, J.; Egorova, N.; Rong, P.; Liu, J.; Hong, Y.; Fan, Y.; Wang, X.; Wang, H.; Yu, Y.; Ma, Y.; et al. Early Cortical Biomarkers of Longitudinal Transcutaneous Vagus Nerve Stimulation Treatment Success in Depression. Neuroimage Clin. 2016, 14, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Stefan, H.; Kreiselmeyer, G.; Kerling, F.; Kurzbuch, K.; Rauch, C.; Heers, M.; Kasper, B.S.; Hammen, T.; Rzonsa, M.; Pauli, E.; et al. Transcutaneous Vagus Nerve Stimulation (t-VNS) in Pharmacoresistant Epilepsies: A Proof of Concept Trial. Epilepsia 2012, 53, e115–e118. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Jing, X.; Wang, X.; Rong, P.; Li, L.; Shi, H.; Shang, H.; Wang, Y.; Zhang, J.; Zhu, B. Transcutaneous Auricular Vagus Nerve Stimulation as a Complementary Therapy for Pediatric Epilepsy: A Pilot Trial. Epilepsy Behav. 2013, 28, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Baier, H.; Baumgartner, C.; Bohlmann, K.; Fauser, S.; Graf, W.; Hillenbrand, B.; Hirsch, M.; Last, C.; Lerche, H.; et al. Transcutaneous Vagus Nerve Stimulation (TVNS) for Treatment of Drug-Resistant Epilepsy: A Randomized, Double-Blind Clinical Trial (CMPsE02). Brain Stimul. 2016, 9, 356–363. [Google Scholar] [CrossRef]
- Boon, P.; De Cock, E.; Mertens, A.; Trinka, E. Neurostimulation for Drug-Resistant Epilepsy: A Systematic Review of Clinical Evidence for Efficacy, Safety, Contraindications and Predictors for Response. Curr. Opin. Neurol. 2018, 31, 198–210. [Google Scholar] [CrossRef]
- Lehtimäki, J.; Hyvärinen, P.; Ylikoski, M.; Bergholm, M.; Mäkelä, J.P.; Aarnisalo, A.; Pirvola, U.; Mäkitie, A.; Ylikoski, J. Transcutaneous Vagus Nerve Stimulation in Tinnitus: A Pilot Study. Acta Otolaryngol. 2013, 133, 378–382. [Google Scholar] [CrossRef]
- De Ridder, D.; Vanneste, S.; Engineer, N.D.; Kilgard, M.P. Safety and Efficacy of Vagus Nerve Stimulation Paired with Tones for the Treatment of Tinnitus: A Case Series. Neuromodulation 2014, 17, 170–179. [Google Scholar] [CrossRef]
- Shim, H.J.; Kwak, M.Y.; An, Y.-H.; Kim, D.H.; Kim, Y.J.; Kim, H.J. Feasibility and Safety of Transcutaneous Vagus Nerve Stimulation Paired with Notched Music Therapy for the Treatment of Chronic Tinnitus. J. Audiol. Otol. 2015, 19, 159–167. [Google Scholar] [CrossRef]
- Kreuzer, P.M.; Landgrebe, M.; Resch, M.; Husser, O.; Schecklmann, M.; Geisreiter, F.; Poeppl, T.B.; Prasser, S.J.; Hajak, G.; Rupprecht, R.; et al. Feasibility, Safety and Efficacy of Transcutaneous Vagus Nerve Stimulation in Chronic Tinnitus: An Open Pilot Study. Brain Stimul. 2014, 7, 740–747. [Google Scholar] [CrossRef]
- Jacobs, H.I.L.; Riphagen, J.M.; Razat, C.M.; Wiese, S.; Sack, A.T. Transcutaneous Vagus Nerve Stimulation Boosts Associative Memory in Older Individuals. Neurobiol. Aging 2015, 36, 1860–1867. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Neuhuber, W.L. Functional and Chemical Anatomy of the Afferent Vagal System. Auton. Neurosci. 2000, 85, 1–17. [Google Scholar] [CrossRef]
- Rutecki, P. Anatomical, Physiological, and Theoretical Basis for the Antiepileptic Effect of Vagus Nerve Stimulation. Epilepsia 1990, 31 (Suppl. S2), S1–S6. [Google Scholar] [CrossRef]
- Farmer, A.D.; Strzelczyk, A.; Finisguerra, A.; Gourine, A.V.; Gharabaghi, A.; Hasan, A.; Burger, A.M.; Jaramillo, A.M.; Mertens, A.; Majid, A.; et al. International Consensus Based Review and Recommendations for Minimum Reporting Standards in Research on Transcutaneous Vagus Nerve Stimulation (Version 2020). Front. Hum. Neurosci. 2021, 14, 568051. [Google Scholar] [CrossRef]
- Ludwig, M.; Wienke, C.; Betts, M.J.; Zaehle, T.; Hämmerer, D. Current Challenges in Reliably Targeting the Noradrenergic Locus Coeruleus Using Transcutaneous Auricular Vagus Nerve Stimulation (TaVNS). Auton. Neurosci. 2021, 236, 102900. [Google Scholar] [CrossRef] [PubMed]
- Konjusha, A.; Colzato, L.; Ghin, F.; Stock, A.-K.; Beste, C. Auricular Transcutaneous Vagus Nerve Stimulation for Alcohol Use Disorder: A Chance to Improve Treatment? Addict. Biol. 2022, 27, e13202. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G.; Shipley, M.T.; Chouvet, G.; Ennis, M.; van Bockstaele, E.; Pieribone, V.; Shiekhattar, R.; Akaoka, H.; Drolet, G.; Astier, B. Afferent Regulation of Locus Coeruleus Neurons: Anatomy, Physiology and Pharmacology. Prog. Brain Res. 1991, 88, 47–75. [Google Scholar] [PubMed]
- Cutsforth-Gregory, J.K.; Benarroch, E.E. Nucleus of the Solitary Tract, Medullary Reflexes, and Clinical Implications. Neurology 2017, 88, 1187–1196. [Google Scholar] [CrossRef]
- Butt, M.F.; Albusoda, A.; Farmer, A.D.; Aziz, Q. The Anatomical Basis for Transcutaneous Auricular Vagus Nerve Stimulation. J. Anat. 2020, 236, 588–611. [Google Scholar] [CrossRef]
- Peuker, E.T.; Filler, T.J. The Nerve Supply of the Human Auricle. Clin. Anat. 2002, 15, 35–37. [Google Scholar] [CrossRef]
- Hulsey, D.R.; Riley, J.R.; Loerwald, K.W.; Rennaker, R.L.; Kilgard, M.P.; Hays, S.A. Parametric Characterization of Neural Activity in the Locus Coeruleus in Response to Vagus Nerve Stimulation. Exp. Neurol. 2017, 289, 21–30. [Google Scholar] [CrossRef]
- D’Agostini, M.; Burger, A.M.; Franssen, M.; Perkovic, A.; Claes, S.; von Leupoldt, A.; Murphy, P.R.; Van Diest, I. Short Bursts of Transcutaneous Auricular Vagus Nerve Stimulation Enhance Evoked Pupil Dilation as a Function of Stimulation Parameters. Cortex 2022, 159, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.B.; Naritoku, D.K.; Smith, D.C.; Browning, R.A.; Jensen, R.A. Enhanced Recognition Memory Following Vagus Nerve Stimulation in Human Subjects. Nat. Neurosci. 1999, 2, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Evensen, K.; Jørgensen, M.B.; Sabers, A.; Martiny, K. Transcutaneous Vagal Nerve Stimulation in Treatment-Resistant Depression: A Feasibility Study. Neuromodulation 2022, 25, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Glynne, P.; Tahmasebi, N.; Gant, V.; Gupta, R. Long COVID Following Mild SARS-CoV-2 Infection: Characteristic T Cell Alterations and Response to Antihistamines. J. Investig. Med. 2022, 70, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Brain and Cognition Discovery Foundation. Randomized, Double-Blinded, Placebo-Controlled Study Evaluating Vortioxetine for Cognitive Deficits in Persons with Post-COVID-19 Condition. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05047952 (accessed on 28 September 2022).
- Wang, C.; Yu, C.; Jing, H.; Wu, X.; Novakovic, V.A.; Xie, R.; Shi, J. Long COVID: The Nature of Thrombotic Sequelae Determines the Necessity of Early Anticoagulation. Front. Cell. Infect. Microbiol. 2022, 12, 861703. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Goyal, N.; Nagaraja, R.; Kumar, R. Systemic Corticosteroids for Management of “Long-COVID”: An Evaluation after 3 Months of Treatment. Monaldi Arch. Chest Dis. 2021, 92, 1981. [Google Scholar] [CrossRef]
- Salvà Lacombe, P.; García Vicente, J.A.; Costa Pagès, J.; Lucio Morselli, P. Causes and Problems of Nonresponse or Poor Response to Drugs. Drugs 1996, 51, 552–570. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Vanderhasselt, M.-A. Working Memory Improvement with Non-Invasive Brain Stimulation of the Dorsolateral Prefrontal Cortex: A Systematic Review and Meta-Analysis. Brain Cogn. 2014, 86, 1–9. [Google Scholar] [CrossRef]
- Beste, C.; Steenbergen, L.; Sellaro, R.; Grigoriadou, S.; Zhang, R.; Chmielewski, W.; Stock, A.-K.; Colzato, L. Effects of Concomitant Stimulation of the GABAergic and Norepinephrine System on Inhibitory Control–A Study Using Transcutaneous Vagus Nerve Stimulation. Brain Stimul. 2016, 9, 811–818. [Google Scholar] [CrossRef]
- Konjusha, A.; Colzato, L.; Mückschel, M.; Beste, C. Auricular Transcutaneous Vagus Nerve Stimulation Diminishes Alpha-Band Related Inhibitory Gating Processes during Conflict Monitoring in Frontal Cortices. Int. J. Neuropsychopharmacol. 2022, 25, 457–467. [Google Scholar] [CrossRef]
- Redgrave, J.; Day, D.; Leung, H.; Laud, P.J.; Ali, A.; Lindert, R.; Majid, A. Safety and Tolerability of Transcutaneous Vagus Nerve Stimulation in Humans; a Systematic Review. Brain Stimul. 2018, 11, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Anguera, J.A.; Boccanfuso, J.; Rintoul, J.L.; Al-Hashimi, O.; Faraji, F.; Janowich, J.; Kong, E.; Larraburo, Y.; Rolle, C.; Johnston, E.; et al. Video Game Training Enhances Cognitive Control in Older Adults. Nature 2013, 501, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, L.E.; Ilieva, I.P.; Hamilton, R.H.; Farah, M.J. Does Transcranial Direct Current Stimulation Improve Healthy Working Memory?: A Meta-Analytic Review. J. Cogn. Neurosci. 2016, 28, 1063–1089. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colzato, L.S.; Elmers, J.; Beste, C.; Hommel, B. A Prospect to Ameliorate Affective Symptoms and to Enhance Cognition in Long COVID Using Auricular Transcutaneous Vagus Nerve Stimulation. J. Clin. Med. 2023, 12, 1198. https://doi.org/10.3390/jcm12031198
Colzato LS, Elmers J, Beste C, Hommel B. A Prospect to Ameliorate Affective Symptoms and to Enhance Cognition in Long COVID Using Auricular Transcutaneous Vagus Nerve Stimulation. Journal of Clinical Medicine. 2023; 12(3):1198. https://doi.org/10.3390/jcm12031198
Chicago/Turabian StyleColzato, Lorenza S., Julia Elmers, Christian Beste, and Bernhard Hommel. 2023. "A Prospect to Ameliorate Affective Symptoms and to Enhance Cognition in Long COVID Using Auricular Transcutaneous Vagus Nerve Stimulation" Journal of Clinical Medicine 12, no. 3: 1198. https://doi.org/10.3390/jcm12031198
APA StyleColzato, L. S., Elmers, J., Beste, C., & Hommel, B. (2023). A Prospect to Ameliorate Affective Symptoms and to Enhance Cognition in Long COVID Using Auricular Transcutaneous Vagus Nerve Stimulation. Journal of Clinical Medicine, 12(3), 1198. https://doi.org/10.3390/jcm12031198