The Use of Ultrasonic Bone Scalpel (UBS) in Unilateral Biportal Endoscopic Spine Surgery (UBESS): Technical Notes and Outcomes
Abstract
1. Introduction
2. Methods
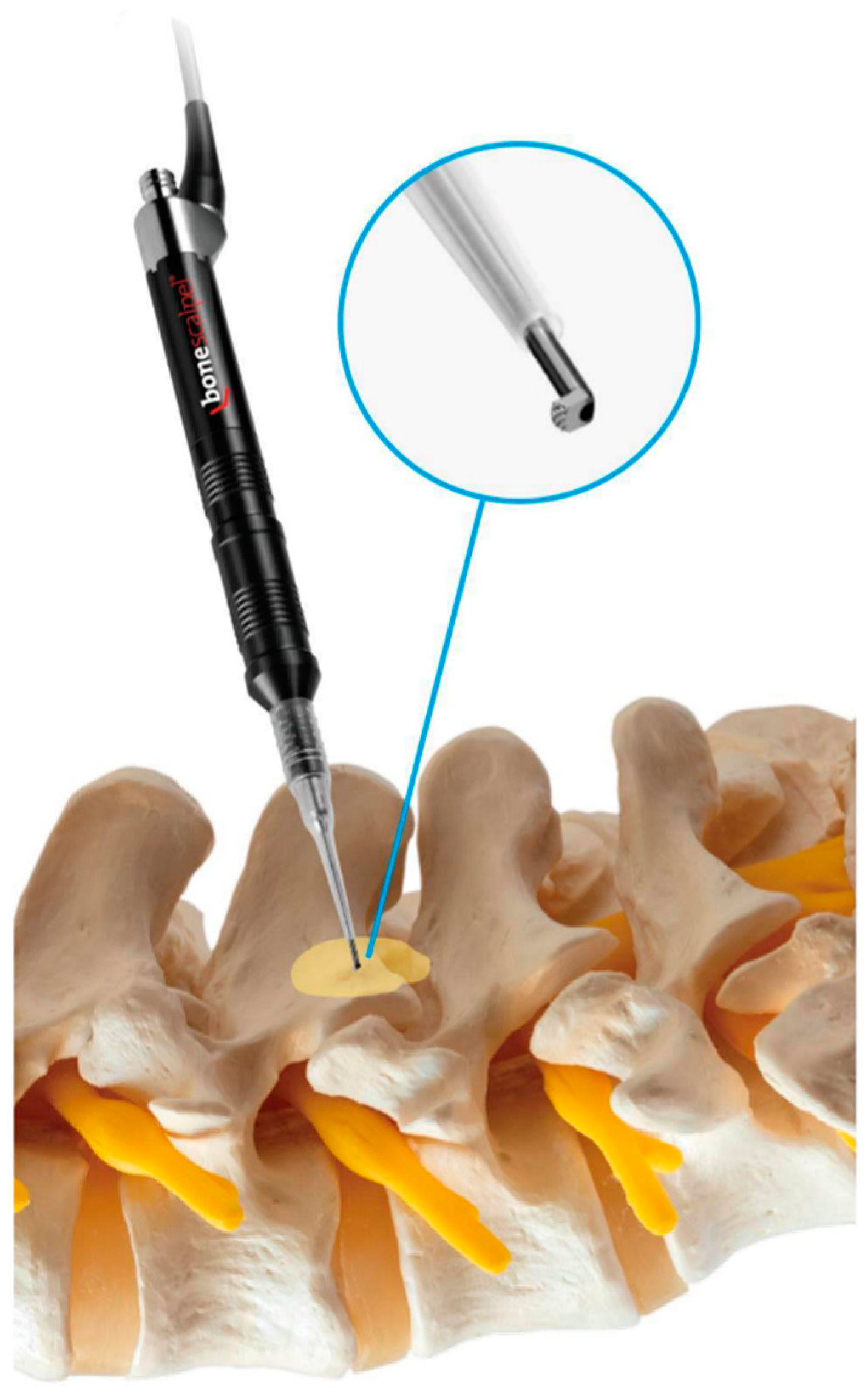
2.1. Technical Note
2.2. Surgical Procedure
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deyo, R.A.; Gray, D.T.; Kreuter, W.; Mirza, S.; Martin, B.I. United States trends in lumbar fusion surgery for degenerative condi-tions. Spine 2005, 30, 1441–1445; Discussion 1446–1447. [Google Scholar] [CrossRef] [PubMed]
- Mobbs, R.J.; Li, J.; Sivabalan, P.; Raley, D.; Rao, P.J. Outcomes after decompressive laminectomy for lumbar spinal stenosis: Com-parison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: Clinical ar-ticle. J. Neurosurg. Spine 2014, 21, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Försth, P.; Ólafsson, G.; Carlsson, T.; Frost, A.; Borgström, F.; Fritzell, P.; Sandén, B. A Randomized, Controlled Trial of Fusion Surgery for Lumbar Spinal Stenosis. N. Engl. J. Med. 2016, 374, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Alvi, M.A.; Kerezoudis, P.; Wahood, W.; Goyal, A.; Bydon, M. Operative Approaches for Lumbar Disc Herniation: A Systematic Review and Multiple Treatment Meta-Analysis of Conventional and Minimally Invasive Surgeries. World Neurosurg. 2018, 114, 391–407.e2. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-C.; Zhong, C.-F.; Deng, G.-B.; Liang, R.-W.; Huang, C.-M. Full-Endoscopic Procedures Versus Traditional Discectomy Surgery for Discectomy: A Systematic Review and Meta-analysis of Current Global Clinical Trials. Pain Physician 2016, 19, 103–118. [Google Scholar]
- Pairuchvej, S.; Muljadi, J.A.; Ho, J.-C.; Arirachakaran, A.; Kongtharvonskul, J. Full-endoscopic (bi-portal or uni-portal) versus microscopic lumbar decompression laminectomy in patients with spinal stenosis: Systematic review and meta-analysis. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 595–611. [Google Scholar] [CrossRef]
- Park, S.-M.; Park, J.; Jang, H.S.; Heo, Y.W.; Han, H.; Kim, H.-J.; Chang, B.-S.; Lee, C.-K.; Yeom, J.S. Biportal endoscopic versus microscopic lumbar decompressive laminectomy in patients with spinal stenosis: A randomized controlled trial. Spine J. 2020, 20, 156–165. [Google Scholar] [CrossRef]
- Kim, C.H.; Chung, C.K.; Choi, Y.; Kuo, C.C.; Lee, U.; Yang, S.H.; Park, H.P. The Efficacy of Ultrasonic Bone Scalpel for Unilateral Cervical Open-Door Laminoplasty: A Randomized Controlled Trial. Neurosurgery 2020, 86, 825–834. [Google Scholar] [CrossRef]
- Nakagawa, H.; Kim, S.-D.; Mizuno, J.; Ohara, Y.; Ito, K. Technical advantages of an ultrasonic bone curette in spinal surgery. J. Neurosurg. Spine 2005, 2, 431–435. [Google Scholar] [CrossRef]
- Bydon, M.; Xu, R.; Papademetriou, K.; Sciubba, D.M.; Wolinsky, J.-P.; Witham, T.F.; Gokaslan, Z.L.; Jallo, G.; Bydon, A. Safety of spinal decompression using an ultrasonic bone curette compared with a high-speed drill: Outcomes in 337 patients. J. Neurosurg. Spine 2013, 18, 627–633. [Google Scholar] [CrossRef]
- Bydon, M.; Macki, M.; Xu, R.; Ain, M.C.; Ahn, E.S.; Jallo, G.I. Spinal decompression in achondroplastic patients using high-speed drill versus ultrasonic bone curette: Technical note and outcomes in 30 cases. J. Pediatr. Orthop. 2014, 34, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ohnmeiss, D.D.; Lieberman, I.H. Use of an ultrasonic osteotome device in spine surgery: Experience from the first 128 patients. Eur. Spine J. 2013, 22, 2845–2849. [Google Scholar] [CrossRef] [PubMed]
- Suetsuna, F.; Harata, S.; Yoshimura, N. Influence of the Ultrasonic Surgical Aspirator on the Dura and Spinal Cord. Spine 1991, 16, 503–508. [Google Scholar] [CrossRef]
- Kumar, V.; Neradi, D.; Salaria, A.K.; Dagar, A.; Dhatt, S.S.; Jindal, K. Role of Ultrasonic Bone Scalpel in Spine Surgery: A Review Article. SN Compr. Clin. Med. 2020, 2, 1883–1889. [Google Scholar] [CrossRef]
- Moon, R.D.; Srikandarajah, N.; Clark, S.; Wilby, M.J.; Pigott, T.D. Primary lumbar decompression using ultrasonic bone curette compared to conventional technique. Br. J. Neurosurg. 2021, 35, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Fairbank, J.C.; Pynsent, P.B. The Oswestry Disability Index. Spine 2000, 25, 2940–2952; Discussion 2952. [Google Scholar] [CrossRef]
- Macnab, I. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J. Bone Jt. Surg. 1971, 53, 891–903. [Google Scholar] [CrossRef]
- Schizas, C.; Theumann, N.; Burn, A.; Tansey, R.; Wardlaw, D.; Smith, F.W.; Kulik, G. Qualitative Grading of Severity of Lumbar Spinal Stenosis Based on the Morphology of the Dural Sac on Magnetic Resonance Images. Spine 2010, 35, 1919–1924. [Google Scholar] [CrossRef]
- Hwa Eum, J.; Hwa Heo, D.; Son, S.K.; Park, C.K. Percutaneous biportal endoscopic decompression for lumbar spinal stenosis: A technical note and preliminary clinical results. J. Neurosurg. Spine 2016, 24, 602–607. [Google Scholar] [CrossRef]
- Minamide, A.; Yoshida, M.; Yamada, H.; Nakagawa, Y.; Kawai, M.; Maio, K.; Hashizume, H.; Iwasaki, H.; Tsutsui, S. Endoscope-assisted spinal decompression surgery for lumbar spinal stenosis. J. Neurosurg. Spine 2013, 19, 664–671. [Google Scholar] [CrossRef]
- Pakzaban, P. Bonescalpeltm Ultrasonic Bone Dissector: Applications in Spine Surgery and Surgical Technique Guide; Misonix: New York, NY, USA, 2014. [Google Scholar]
- Kim, K.; Isu, T.; Matsumoto, R.; Isobe, M.; Kogure, K. Surgical Pitfalls of an Ultrasonic Bone Curette (Sonopet) in Spinal Surgery. Neurosurgery 2006, 59, ONS390-3; Discussion ONS393. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.H.; Son, S.K.; Eum, J.H.; Park, C.K. Fully endoscopic lumbar interbody fusion using a percutaneous unilateral biportal endoscopic technique: Technical note and preliminary clinical results. Neurosurg. Focus 2017, 43, E8. [Google Scholar] [CrossRef] [PubMed]
- Papavero, L.; Engler, N.; Kothe, R. Incidental durotomy in spine surgery: First aid in ten steps. Eur. Spine J. 2015, 24, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Javid, M.J.; Hadar, E.J. Long-term follow-up review of patients who underwent laminectomy for lumbar stenosis: A prospective study. J. Neurosurg. 1998, 89, 1–7. [Google Scholar] [CrossRef]





| Factors | Value |
|---|---|
| Age (mean, SD) | 66.1 (7.8) |
| Sex (M/F) | 5/15 |
| Level (N) | |
| L4-5 | 15 |
| L3-4 | 5 |
| Follow-up (months) | 13.2 (0.71) |
| Mean operative time (mins) | 74.4 (9.4) |
| Blood loss (mL) | 22.1 (5.3) |
| Length of stay (days) | 2.3 (0.62) |
| Schizas Score (N) * | |
| Grade A | 0 |
| Grade B | 3 |
| Grade C | 15 |
| Grade D | 2 |
| Modified MacNab Criteria (N) | |
| Excellent | 17 |
| Good | 2 |
| Fair | 1 |
| Poor | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, S.H.L.; Chang, C.-W.; Lin, T.-Y.; Wang, Y.-C.; Wong, C.-B.; Ghaith, A.K.; Alvi, M.A.; Fu, T.-S.; Bydon, M. The Use of Ultrasonic Bone Scalpel (UBS) in Unilateral Biportal Endoscopic Spine Surgery (UBESS): Technical Notes and Outcomes. J. Clin. Med. 2023, 12, 1180. https://doi.org/10.3390/jcm12031180
Tsai SHL, Chang C-W, Lin T-Y, Wang Y-C, Wong C-B, Ghaith AK, Alvi MA, Fu T-S, Bydon M. The Use of Ultrasonic Bone Scalpel (UBS) in Unilateral Biportal Endoscopic Spine Surgery (UBESS): Technical Notes and Outcomes. Journal of Clinical Medicine. 2023; 12(3):1180. https://doi.org/10.3390/jcm12031180
Chicago/Turabian StyleTsai, Sung Huang Laurent, Chia-Wei Chang, Tung-Yi Lin, Ying-Chih Wang, Chak-Bor Wong, Abdul Karim Ghaith, Mohammed Ali Alvi, Tsai-Sheng Fu, and Mohamad Bydon. 2023. "The Use of Ultrasonic Bone Scalpel (UBS) in Unilateral Biportal Endoscopic Spine Surgery (UBESS): Technical Notes and Outcomes" Journal of Clinical Medicine 12, no. 3: 1180. https://doi.org/10.3390/jcm12031180
APA StyleTsai, S. H. L., Chang, C.-W., Lin, T.-Y., Wang, Y.-C., Wong, C.-B., Ghaith, A. K., Alvi, M. A., Fu, T.-S., & Bydon, M. (2023). The Use of Ultrasonic Bone Scalpel (UBS) in Unilateral Biportal Endoscopic Spine Surgery (UBESS): Technical Notes and Outcomes. Journal of Clinical Medicine, 12(3), 1180. https://doi.org/10.3390/jcm12031180






