Point of Care Ultrasonography for the Septic Patient in the Emergency Department: A Literature Review
Abstract
1. Introduction
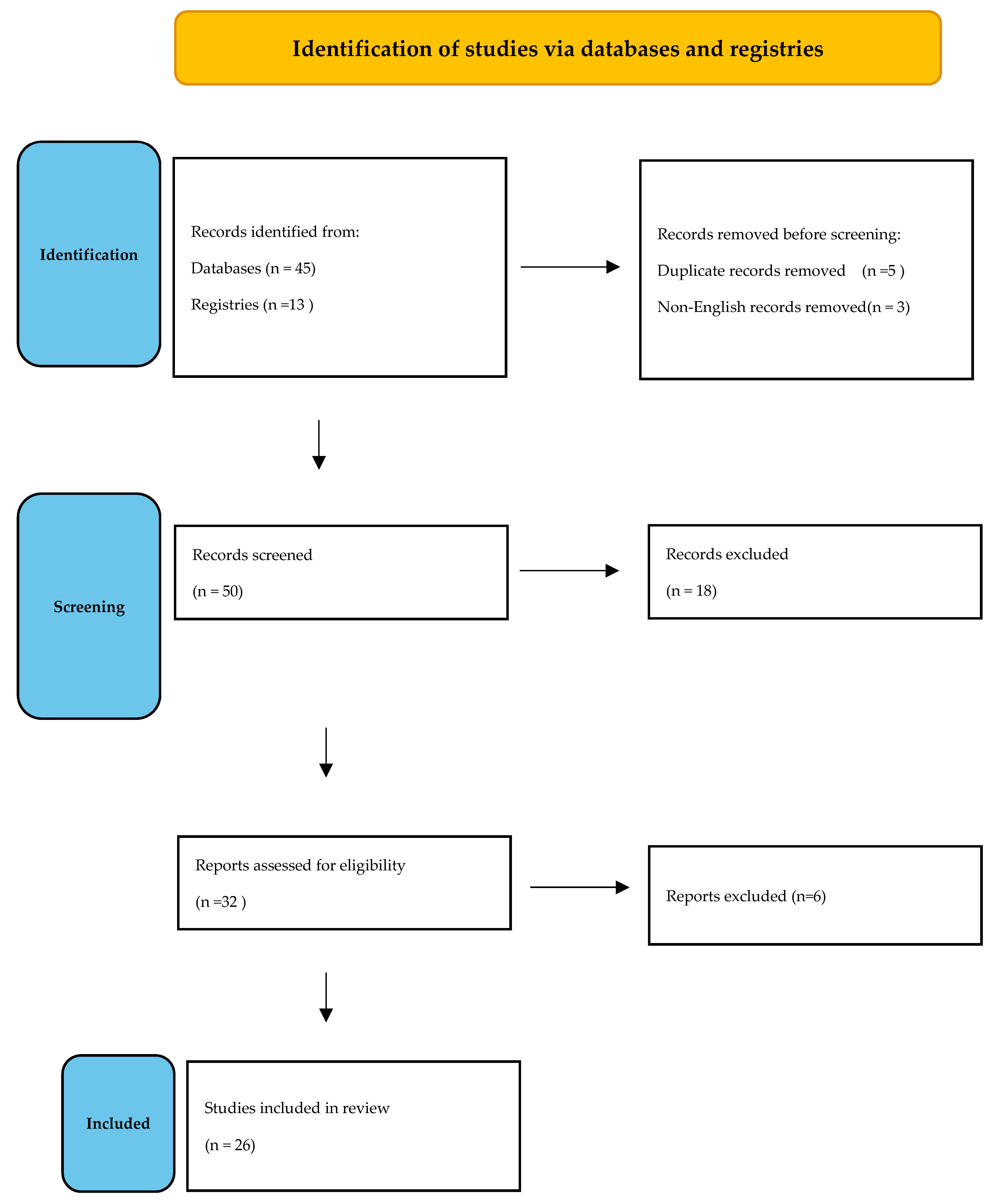
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and Septic Shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, S.P.; Williams, J.M.; Shetty, A.; Bellomo, R.; Finfer, S.; Shapiro, N.; Keijzers, G. Review Article: Sepsis in the Emergency Department—Part 1: Definitions and Outcomes. Emerg. Med. Australas. 2017, 29, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Whitson, M.R.; Mayo, P.H. Ultrasonography in the Emergency Department. Crit Care 2016, 20, 227. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.A.; Brewer, J.; Speaks, T.; Choi, C.; Lahsaei, P.; Romito, B.T. The POCUS Consult: How Point of Care Ultrasound Helps Guide Medical Decision Making. Int. J. Gen. Med. 2021, 14, 9789–9806. [Google Scholar] [CrossRef]
- Ultrasound Guidelines: Emergency, Point-of-Care and Clinical Ultrasound Guidelines in Medicine. Ann. Emerg. Med. 2017, 69, e27–e54. [CrossRef]
- Perera, P.; Mailhot, T.; Riley, D.; Mandavia, D. The RUSH Exam: Rapid Ultrasound in SHock in the Evaluation of the Critically Lll. Emerg. Med. Clin. N. Am. 2010, 28, 29–56. [Google Scholar] [CrossRef]
- Ahn, J.H.; Jeon, J.; Toh, H.-C.; Noble, V.E.; Kim, J.S.; Kim, Y.S.; Do, H.H.; Ha, Y.R. SEARCH 8Es: A Novel Point of Care Ultrasound Protocol for Patients with Chest Pain, Dyspnea or Symptomatic Hypotension in the Emergency Department. PLoS ONE 2017, 12, e0174581. [Google Scholar] [CrossRef]
- Atkinson, P.R.; Milne, J.; Diegelmann, L.; Lamprecht, H.; Stander, M.; Lussier, D.; Pham, C.; Henneberry, R.; Fraser, J.M.; Howlett, M.K.; et al. Does Point-of-Care Ultrasonography Improve Clinical Outcomes in Emergency Department Patients With Undifferentiated Hypotension? An International Randomized Controlled Trial From the SHoC-ED Investigators. Ann. Emerg. Med. 2018, 72, 478–489. [Google Scholar] [CrossRef]
- Bagheri-Hariri, S.; Yekesadat, M.; Farahmand, S.; Arbab, M.; Sedaghat, M.; Shahlafar, N.; Takzare, A.; Seyedhossieni-Davarani, S.; Nejati, A. The Impact of Using RUSH Protocol for Diagnosing the Type of Unknown Shock in the Emergency Department. Emerg. Radiol. 2015, 22, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Ghane, M.R.; Gharib, M.; Ebrahimi, A.; Saeedi, M.; Akbari-Kamrani, M.; Rezaee, M.; Rasouli, H. Accuracy of Early Rapid Ultrasound in Shock (RUSH) Examination Performed by Emergency Physician for Diagnosis of Shock Etiology in Critically Ill Patients. J. Emergencies Trauma Shock 2015, 8, 5–10. [Google Scholar] [CrossRef]
- Javali, R.H.; Loganathan, A.; Srinivasarangan, M.; Patil, A.; Siddappa, G.B.; Satyanarayana, N.; Bheemanna, A.S.; Jagadeesh, S.; Betkerur, S. Reliability of Emergency Department Diagnosis in Identifying the Etiology of Nontraumatic Undifferentiated Hypotension. Indian J. Crit. Care Med. 2020, 24, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Mosier, J.M.; Stolz, U.; Milligan, R.; Roy-Chaudhury, A.; Lutrick, K.; Hypes, C.D.; Billheimer, D.; Cairns, C.B. Impact of Point-of-Care Ultrasound in the Emergency Department on Care Processes and Outcomes in Critically Ill Nontraumatic Patients. Crit. Care Explor. 2019, 1, e0019. [Google Scholar] [CrossRef]
- Sasmaz, M.I.; Gungor, F.; Guven, R.; Akyol, K.C.; Kozaci, N.; Kesapli, M. Effect of Focused Bedside Ultrasonography in Hypotensive Patients on the Clinical Decision of Emergency Physicians. Emerg. Med. Int. 2017, 2017, 6248687. [Google Scholar] [CrossRef] [PubMed]
- Shokoohi, H.; Boniface, K.S.; Pourmand, A.; Liu, Y.T.; Davison, D.L.; Hawkins, K.D.; Buhumaid, R.E.; Salimian, M.; Yadav, K. Bedside Ultrasound Reduces Diagnostic Uncertainty and Guides Resuscitation in Patients With Undifferentiated Hypotension. Crit. Care Med. 2015, 43, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Lamorte, A.; Tullio, M.; Cardinale, L.; Giraudo, M.; Stefanone, V.; Boero, E.; Nazerian, P.; Pozzi, R.; Frascisco, M.F. Point-of-Care Multiorgan Ultrasonography for the Evaluation of Undifferentiated Hypotension in the Emergency Department. Intensive Care Med. 2013, 39, 1290–1298. [Google Scholar] [CrossRef]
- Cortellaro, F.; Ferrari, L.; Molteni, F.; Aseni, P.; Velati, M.; Guarnieri, L.; Cazzola, K.B.; Colombo, S.; Coen, D. Accuracy of Point of Care Ultrasound to Identify the Source of Infection in Septic Patients: A Prospective Study. Intern. Emerg. Med. 2017, 12, 371–378. [Google Scholar] [CrossRef]
- Devia Jaramillo, G.; Menendez Ramirez, S. USER Protocol as a Guide to Resuscitation of the Patient with Septic Shock in the Emergency Department. Open Access Emerg. Med. 2021, 13, 33–43. [Google Scholar] [CrossRef]
- Haydar, S.A.; Moore, E.T.; Higgins, G.L.; Irish, C.B.; Owens, W.B.; Strout, T.D. Effect of Bedside Ultrasonography on the Certainty of Physician Clinical Decisionmaking for Septic Patients in the Emergency Department. Ann. Emerg. Med. 2012, 60, 346–358.e4. [Google Scholar] [CrossRef]
- Musikatavorn, K.; Plitawanon, P.; Lumlertgul, S.; Narajeenron, K.; Rojanasarntikul, D.; Tarapan, T.; Saoraya, J. Randomized Controlled Trial of Ultrasound-Guided Fluid Resuscitation of Sepsis-Induced Hypoperfusion and Septic Shock. West J. Emerg. Med. 2021, 22, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Lafon, T.; Appert, A.; Hadj, M.; Bigrat, V.; Legarcon, V.; Claveries, P.; Goudelin, M.; Evrard, B.; Padilla, A.C.H.; Baisse, A.; et al. Comparative Early Hemodynamic Profiles in Patients Presenting to the Emergency Department with Septic and Nonseptic Acute Circulatory Failure Using Focused Echocardiography. Shock 2020, 53, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Alhabashy, W.S. Echocardiography guided septic shock management. In Proceedings of the Seventh International Conference and Exhibition on Surgery and the Third International Conference on Anesthesia, Dublin, Ireland, 21–23 June 2018; International Online Medical Council (IOMC): Brussels, Belgium, 2018; Volume 8, p. 70. [Google Scholar]
- Valle Alonso, J.; Lakshmanan, G.; Saleem, Y. Use of POCUS Ultrasound in Sepsis, Bedside Diagnosis of Necrotizing Fasciitis. QJM 2017, 110, 687–688. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.V.; Turpie, J.; Farhad, I.; Ruffino, G. Protocols for Point-of-Care-Ultrasound (POCUS) in a Patient with Sepsis; An Algorithmic Approach. Bull. Emerg. Trauma 2019, 7, 67–71. [Google Scholar] [CrossRef]
- Cohen, S.; Ford, L.; Situ-LaCasse, E.; Tolby, N. Infective Endocarditis Causing Acute Myocardial Infarction. Cureus 2020, 12, e11245. [Google Scholar] [CrossRef]
- Derr, C.; Drake, J.M. Esophageal Rupture Diagnosed with Bedside Ultrasound. Am. J. Emerg. Med. 2012, 30, e1–e3. [Google Scholar] [CrossRef]
- Gibbons, R.; Leonard, N.; Magee, M.; Zanaboni, A.; Patterson, J.; Costantino, T. Xanthogranulomatous Pyelonephritis: A Complicated Febrile Urinary Tract Infection Detected by Point-of-Care Ultrasound in the Emergency Department. J. Emerg. Med. 2018, 55, e1–e4. [Google Scholar] [CrossRef]
- Hill, A.; Guillén, M.; Martin, D.; Dreyfuss, A. Point-of-Care Ultrasound Diagnosis of Pulmonary Hydatid Cyst Disease Causing Shock: A Case Report. Clin. Pract. Cases Emerg. Med. 2021, 5, 403–406. [Google Scholar] [CrossRef]
- Kinas, D.; Dalley, M.; Guidry, K.; Newberry, M.A.; Farcy, D.A. Point-of-Care Ultrasound Identifies Decompensated Heart Failure in a Young Male with Methamphetamine-Associated Cardiomyopathy Presenting in Severe Sepsis to the Emergency Department. Case Rep. Emerg. Med. 2018, 2018, 2859676. [Google Scholar] [CrossRef]
- Kotlarsky, P.; Shavit, I.; Kassis, I.; Eidelman, M. Treatment of Septic Hip in a Pediatric ED: A Retrospective Case Series Analysis. Am. J. Emerg. Med. 2016, 34, 602–605. [Google Scholar] [CrossRef]
- Perez, J.; Sorensen, S.; Rosselli, M. Utilisation of Musculoskeletal Ultrasonography for the Evaluation of Septic Arthritis in a Patient Presenting to the Emergency Department with Fever during the Era of COVID-19. BMJ Case Rep. 2021, 14, e242370. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Jelic, T.; Chenkin, J. Simple Pneumonia or Something More?: A Case Report and Discussion of Unexpected Empyema Identified by Point-of-Care Ultrasound. CJEM 2016, 18, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.L.; Fernandes, R.M.; Melão, M.L.; Moreno, J.; Granja, C. Shedding Light on a Hidden Source of Septic Shock with POCUS. POCUS 2019, 4, 15–16. [Google Scholar] [CrossRef]
- Sweeney, D.A.; Wiley, B.M. Integrated Multiorgan Bedside Ultrasound for the Diagnosis and Management of Sepsis and Septic Shock. Semin. Respir. Crit. Care Med. 2021, 42, 641–649. [Google Scholar] [CrossRef]
| 1st Author, Year, Country, Design, Setting | POCUS Protocol (If Any) | Aim | Patient Number/Age | Main Results |
|---|---|---|---|---|
| Ahn et al., 2017, Korea, POS, single- center ED [9] | SEARCH 8E’s | “SEARCH 8E’s” protocol vs. final diagnosis | 308/>18 yo |
|
| Atkinson et al., 2018, international (N. America & S. Africa), RCT, multicenter (n = 6) [10] | Μulti-organ POCUS based on ACES & RUSH protocols | POCUS protocol vs. standard care without POCUS | 273/>19 yo |
|
| Bagheri-Hariri et al., 2015, Iran, POS pilot, single-center ED [11] | RUSH | RUSH-based shock type diagnosis vs. final diagnosis | 25/N/A |
|
| Ghane et al., 2015, Iran, POS, single- center ED [12] | RUSH | Accuracy of early RUSH protocol performed by emergency physicians to predict shock type in critically ill patients | 52/>18 yo |
|
| Javali et al., 2020, India, POS, single—center ED, 18-month period [13] | Multi-organ POCUS protocol | Multi-organ POCUS to improve accuracy, narrow differential diagnosis, test effectiveness of EGDT | 100/>18 yo |
|
| Mosier et al., 2019, USA, ROS (cohort), 2-center EDs [14] | Impact of POCUS on care processes and outcomes in critically ill nontraumatic patients Method: 3 patient cohorts: no POCUS (cohort 1 = 4165), POCUS prior to key intervention (cohort 2 = 614), and POCUS after key intervention (cohort 3 = 662). Primary outcome: in-hospital mortality | 5441/> 18 yo |
| |
| Sasmaz et al., 2017, Turkey, POS, single-center ED [15] | RUSH | Effect of POCUS on clinical decision, by comparing diagnosis before and after POCUS with the definitive diagnosis | 180/>18 yo |
|
| Shokoohi et al., 2015, USA, POS, single-center ED, 32-month period [16] | US hypotensionprotocol (FOCUS, RV, IVC, abdominal & transthoracic scans) | Impact of protocol on diagnostic certainty & ability, treatment, and resource utilization | 118/>18 yo |
|
| Volpicelli et al., 2013, Italy, POS, single-center ED [17] | Multi-organ POCUS protocol | Efficacy of protocol, for diagnostic process of symptomatic, hypotensive patients in the ED Assessment of decisive role of included lung scan | 108/N/A |
|
| 1st Author, Year, Country, Design, Setting | POCUS Protocol (If Any) | Aim | Patient Number/Age and Main Inclusion Criteria | Main Results |
|---|---|---|---|---|
| Cortellaro et al., 2017, Italy, POS, single-center ED [18] | Comparison of standard diagnostic work-up vs. early POCUS use regarding speed of diagnosis and accuracy in identification of the infectious source | 200/>18 yo |
| |
| Devia Jaramillo et al., 2021, Colombia, POS cohort, single-center ED [19] | USER | US-based protocol for fluid administration and initiation of vasopressors in septic shock. | 83/>18 yo in septic shock |
|
| Haydar et al., 2012, USA, POS, single-center ED [20] | Protocol consisting of 3 main POCUS measures | Effect of 3 POCUS measures on clinical decision-making | 74/>18 yo |
|
| Musikatavorn et al., 2020, RCT, single-center ED [21] | IVC assessment | Effect of UGFM strategy on 30-d mortality in patients with septic shock or sepsis-indued hypoperfusion vs. standard care. | 202/>18 yo |
|
| Lafon et al., 2020, France, POS, single-center ED [22] | FOCUS | FOCUS-based evaluation of early hemodynamic profile in patients presenting with ACF | 100/>18 yo presenting with ACF |
Sepsis cohort: 55 patients, Non-Sepsis: 45 patients. FOCUS was performed after administration of 500 mL of crystalloids Patients with sepsis had qSOFA score ≥ 2 points on ED admission and:
|
| 1st Author, Year, Country | Patient’ s Symptoms/Clinical Status on ED Presentation | Management and POCUS Findings | Final Diagnosis |
|---|---|---|---|
| Alhabashy, 2018, Egypt [23] | 63 yo female with CAP |
| AHFREF with severe aortic stenosis and mitral regurgitation |
| Alonso et al., 2017, UK [24] | 60-yo female, 3-day left leg pain, treated for suspected cellulitis |
| Necrotizing fasciitis |
| Alonso et al., 2019, UK [25] | 70-yo female with diarrhea, vomiting for 1 week |
| Obstructive stone causing moderate right-sided hydronephrosis |
| Cohen et al., 2020, USA [26] | 26-yo female, intravenous drug user, agitated |
| Myocardial infarction caused by endocarditis-related septic embolization |
| Derr et al., 2012, USA [27] | 69-yo male, hematemesis |
| Esophageal perforation |
| Gibbons et al., 2018, USA [28] | 40-yo female in severe sepsis, flank pain |
| Xanthogranulomatous pyelonephritis |
| Hill et al., 2021, USA [29] | 5-yo male, 2 days febrile, cough, rhinorrhea, pruritus, decreased appetite |
| Ruptured pulmonary hydatid cyst |
| Kinas et al., 2018, USA [30] | 34-yo male, after smoking crystal methamphetamine Symptoms: palpitations, dyspnea, cough with one episode of hemoptysis |
| Methamphetamine-associated cardiomyopathy |
| Kotlarsky et al., 2016, Israel [31] | ROS included pediatric patients with septic arthritis of the hip joint |
| Septic arthritis of the hip joint |
| Perez et al., 2021, USA [32] | 79-yo male, with a medical history of DM, hypertension, CAD, febrile, mild dyspnea, chills, myalgias, arthralgias for the past 2 days. |
| Glenohumeral joint septic arthritis and subdeltoid septic bursitis |
| Romano et al., 2016, Canada [33] | 61-yo female with rheumatoid arthritis, Sjogren syndrome, presented with confusion, decreased LOC, 2 weeks of productive cough, fatigue, mild dyspnea in the last 24 h |
| (Unsuspected) empyema in a patient being treated for CAP |
| Varela et al., 2019, Portugal [34] | 77-yo male suffering from acute dyspnea, 1 week of malaise, nausea, vomiting |
| Liver abscess |
| 1st Author (Year) | Shock Type | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Kappa | p |
|---|---|---|---|---|---|---|---|
| Ahn et al. (2017) [9] | Sepsis (distributive shock) | 63.6 | 99.7 | 87.5 | 98.7 | 0.729 | <0.001 |
| Bagheri-Hariri et al. (2015) [11] | Distributive | 75 | 100 | 100 | 95.5 | 0.83 | 0.002 |
| Hypovolemic Distributive | 100 | 100 | 100 | 100 | 1.00 | 0.003 | |
| Ghane et al. (2015) [12] | Distributive (RUSH Protocol) | 75 | 100 | 100 | 94.9 | 0.83 | 0.000 |
| Javali et al. (2020) [13] | Distributive (POCUS alone) | 15 | 100 | 100 | 71.5 | N/A | N/A |
| Distributive (combined clinical and POCUS evaluation) | 73.68 | 100 | 100 | 86.11 | 0.717 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verras, C.; Ventoulis, I.; Bezati, S.; Matsiras, D.; Parissis, J.; Polyzogopoulou, E. Point of Care Ultrasonography for the Septic Patient in the Emergency Department: A Literature Review. J. Clin. Med. 2023, 12, 1105. https://doi.org/10.3390/jcm12031105
Verras C, Ventoulis I, Bezati S, Matsiras D, Parissis J, Polyzogopoulou E. Point of Care Ultrasonography for the Septic Patient in the Emergency Department: A Literature Review. Journal of Clinical Medicine. 2023; 12(3):1105. https://doi.org/10.3390/jcm12031105
Chicago/Turabian StyleVerras, Christos, Ioannis Ventoulis, Sofia Bezati, Dionysis Matsiras, John Parissis, and Effie Polyzogopoulou. 2023. "Point of Care Ultrasonography for the Septic Patient in the Emergency Department: A Literature Review" Journal of Clinical Medicine 12, no. 3: 1105. https://doi.org/10.3390/jcm12031105
APA StyleVerras, C., Ventoulis, I., Bezati, S., Matsiras, D., Parissis, J., & Polyzogopoulou, E. (2023). Point of Care Ultrasonography for the Septic Patient in the Emergency Department: A Literature Review. Journal of Clinical Medicine, 12(3), 1105. https://doi.org/10.3390/jcm12031105







