One-Year Review in Cardiac Arrest: The 2022 Randomized Controlled Trials
Abstract
1. Introduction
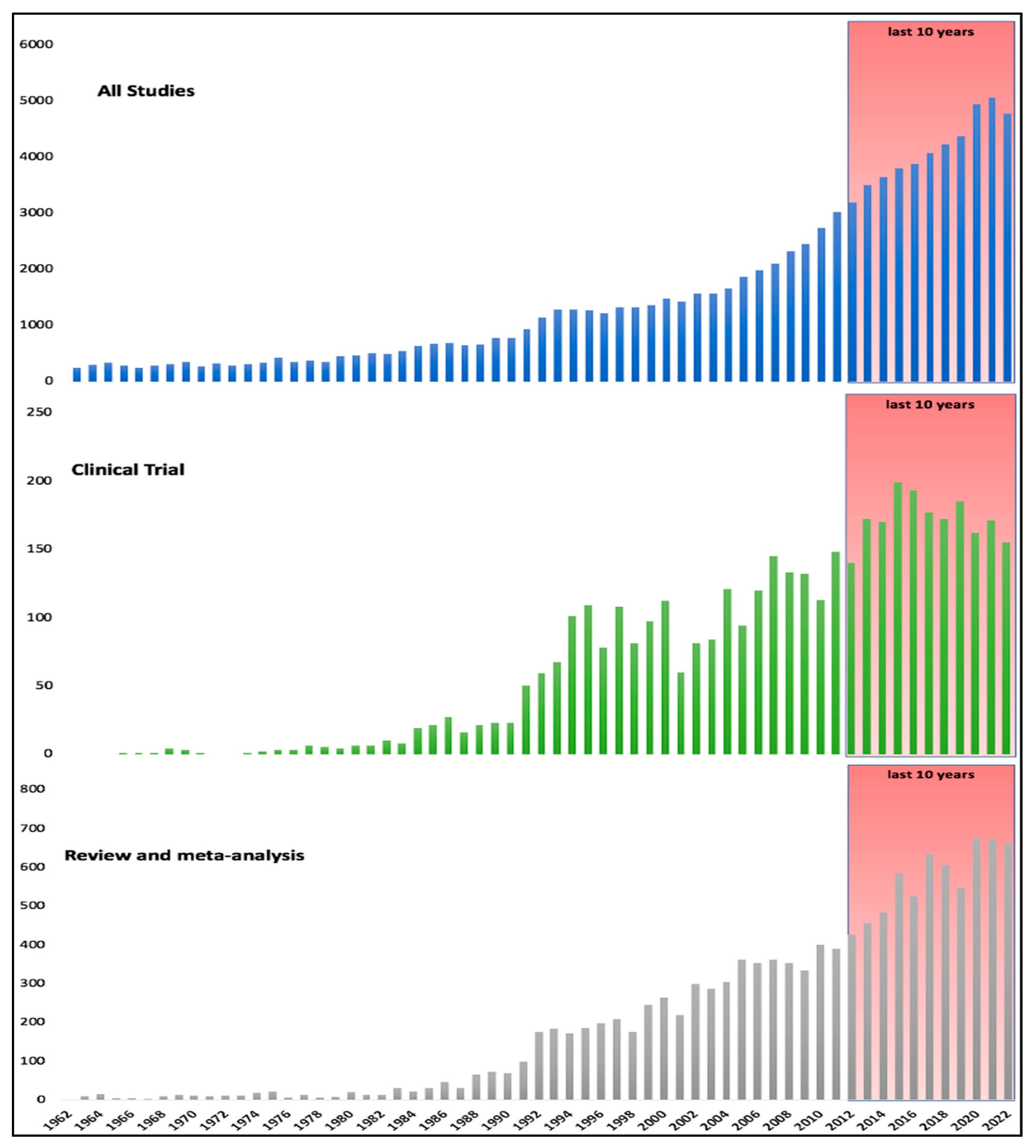
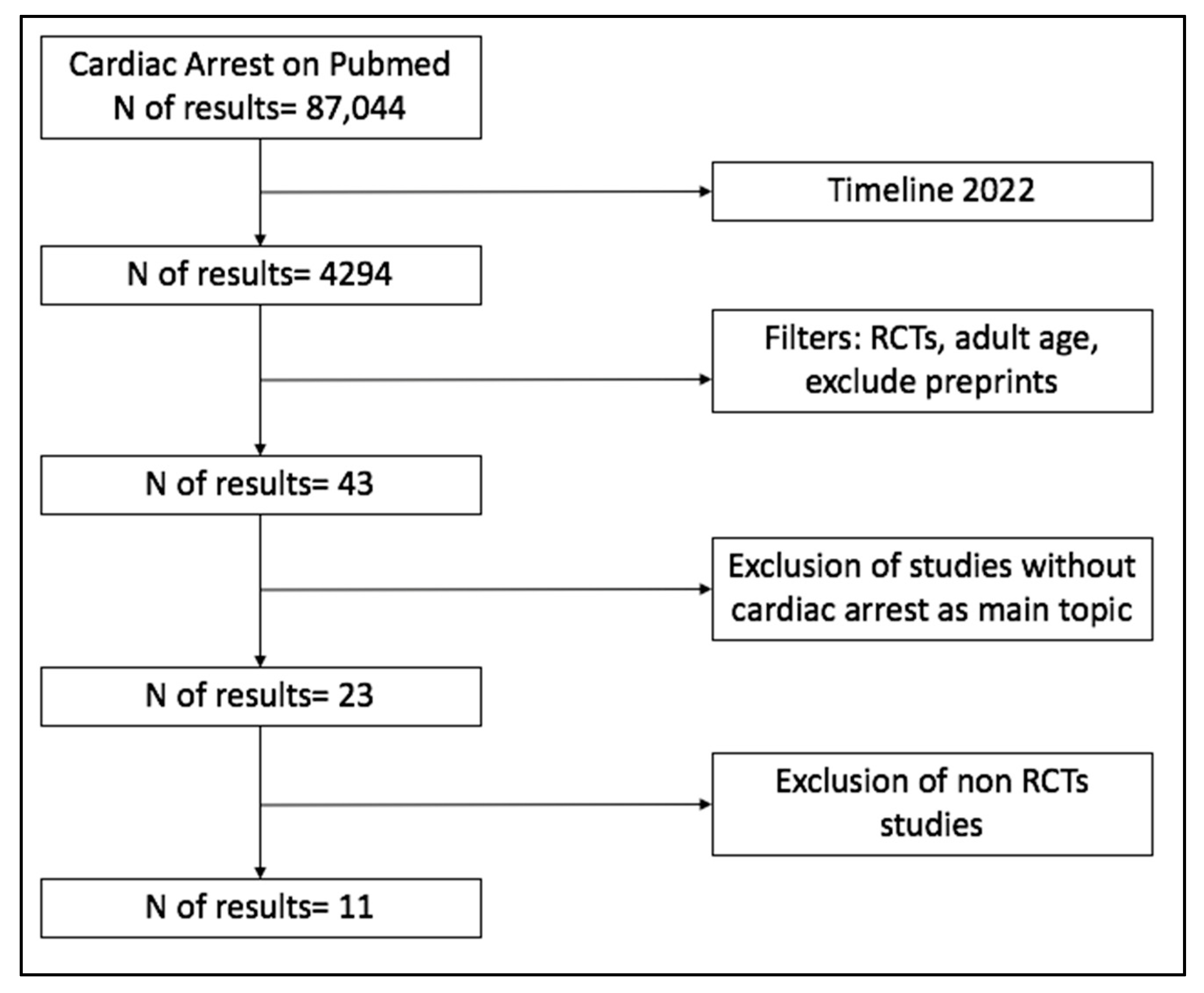
2. Materials and Methods
3. Results and Discussion of 2022 RCTs
3.1. Out-of-Hospital Cardiac Arrest
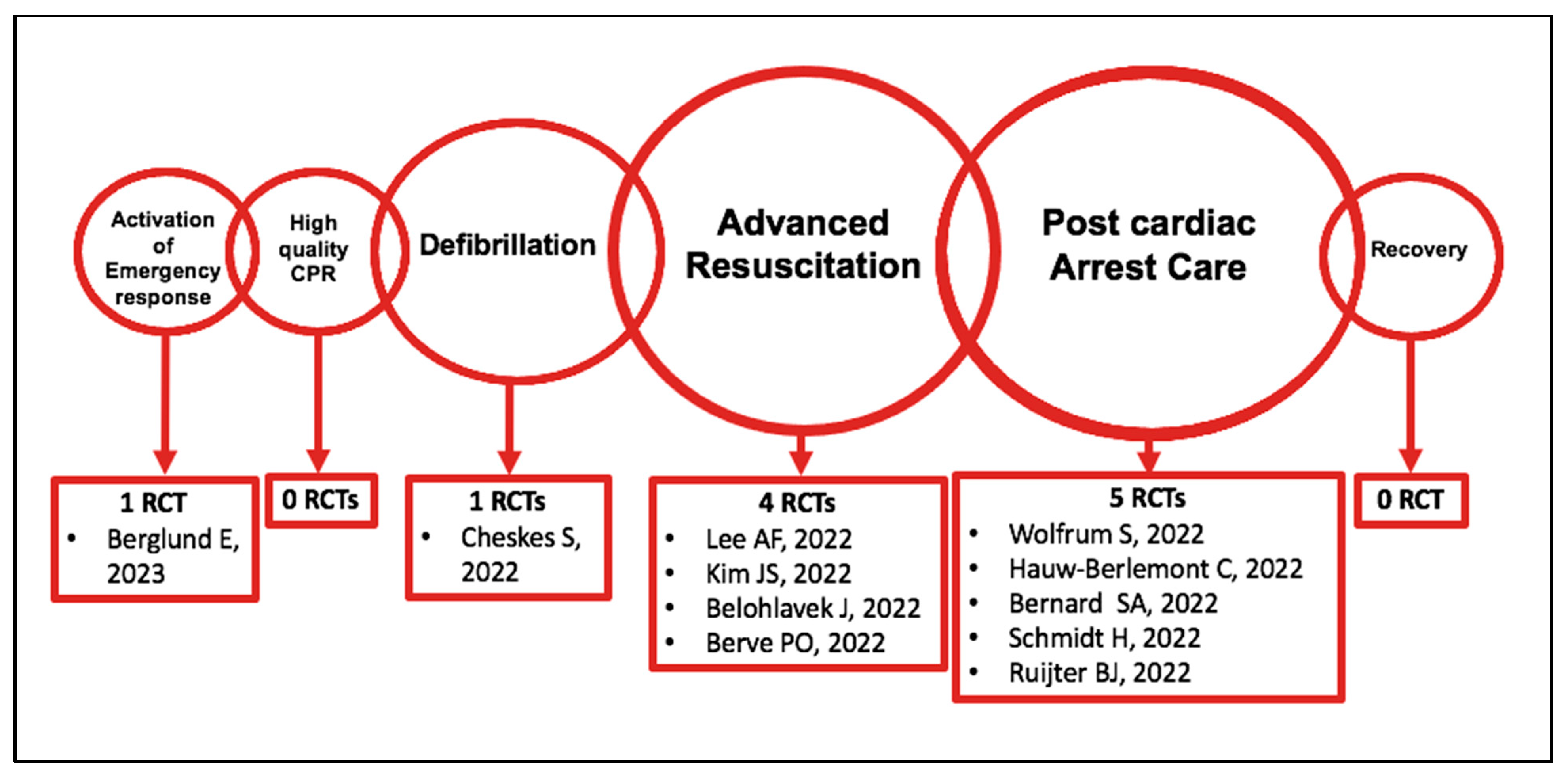
3.1.1. Activation of the Emergency Response
3.1.2. Defibrillation
3.1.3. Advanced Life Support
Airway Management
Pharmacological Interventions
Extracorporeal CPR (ECPR)
Adjunct Treatments during CPR
3.2. In-Hospital Cardiac Arrest
3.3. Post-Cardiac Arrest Care
3.3.1. Early vs. Delayed Percutaneous Coronary Intervention
3.3.2. Oxygen Target
3.3.3. Hemodynamic Targets
3.3.4. Prevention of Fever and TTM
3.3.5. Seizure Treatment
| RCT | Population | Intervention | Number of Patients | Primary Outcome | Secondary Outcomes (Main) |
|---|---|---|---|---|---|
| SAMBA [9] NCT02992873 | OHCA | Intervention: volunteer responders dispatched to the nearest public AED on the way to the OHCA Control: volunteer responders dispatched to perform CPR | 947: - 461, intervention - 486, control | Bystander AED attachment: - 13.2%, intervention - 9.5%, control RR (95%CI): 1.40 (0.97–2.01) | Bystander CPR: - 69.0%, intervention - 71.6%, control RR (95%CI): 0.96 (0.89–1.05) Bystander defibrillation: - 3.7%, intervention - 3.9%, control RR (95%CI): 0.94 (0.50–1.79) |
| DOSE VF [15] NCT04080986 | OHCA (Refractory VF) | Standard defibrillation (std) Double Sequential External Defibrillation (DSED) Vector Change defibrillation (VC) | 405: - 136, standard - 144, VC - 125, DSED | Survival to hospital discharge: - 13.3%, standard - 21.7%, VC RR: 1.71 (1.01–2.88) vs. std - 30.4%, DSED RR: 2.21 (1.33–3.67) vs. std | Termination of VF: - 67.6%, standard - 79.9%, VC RR 1.18 (1.03–1.36) vs. std - 84%, DSED RR 1.25 (1.09–1.44) vs. std ROSC: - 26.5%, standard - 35.4%, VC RR 1.39 (0.97–1.99) vs. std - 46.4%, DSED RR 1.72 (1.22–2.42) vs. std Good neurological recovery at hospital discharge: - 11.2%, SD - 16.2%, VC RR 1.48 (0.81–2.71) - 27.4%, DSED RR 2.21 (1.26–3.88) |
| SAVE [17] NCT02967952 | OHCA | Endotracheal intubation (ETI) Supraglottic airway (SGA) | 936: - 517, ETI - 419, SGA | Sustained ROSC: - 26.9%, ETI - 25.8%, SGA OR 1.02 (0.98–1.06) | Pre-hospital ROSC: - 10.6%, ETI - 6.4%, SGA OR 1.04 (1.02–1.07) Survival to hospital discharge: - 8.5%, ETI - 8.4%, SGA OR 1.00 (0.94–1.06) Good neurological recovery: - 3.9%, ETI - 4.8%, SGA OR 0.99 (0.94–1.03) |
| AMCPR [22] NCT03191240 | OHCA | Intervention: vasopressin + epinephrine Control: epinephrine | 148: - 74, intervention - 74, control | Sustained ROSC: - 36.5%, intervention - 32.4% control RR 0.94 (0.74–1.19) | Survival to hospital discharge: - 8.1%, intervention - 8.1%, control RR 1.00 (0.91–1.10) Good neurological recovery: - 0%, intervention - 0%, control RR 1.00 (1.00–1.00) |
| Prague OHCA [4] NCT01511666 | OHCA | Invasive strategy: mechanical compression + intra-arrest transport + ECPR + immediate invasive assessment and treatment. Standard strategy: ACLS | 256: - 124, invasive - 132, standard | Survival with good neurological recovery at 180 days: - 31.5%, invasive - 22%, standard OR 1.63 (0.93–2.85) | 30-day survival with good neurological recovery: - 30.6%, invasive - 18.2%, standard OR 1.99 (1.11–3.57) 30-day cardiac recovery: - 43.5%, invasive - 34.1% standard OR 1.49 (0.91–2.47) |
| ACD-CPR [30] NCT02479152 | OHCA | Mechanical active compression-decompression (ACD) Conventional mechanical compression | 210: - 101, ACD - 109, conventional | Maximal end-tidal CO2: - 29 mmHg, ACD - 29 mmHg, conventional | Arterial blood pressure: - 111/21 mmHg, ACD - 101/18 mmHg, conventional Cerebral saturation: - 111/21 mmHg, ACD - 101/18 mmHg, conventional CPR-related injuries: - 54.8%, ACD - 57.3%, conventional ROSC: - 47%, ACD - 50%, conventional Survival: - 8%, ACD - 6%, conventional |
| HACAinhospital [38] NCT00457431 | IHCA | Hypothermic temperature control (32–34 °C) Normothermia | 249: - 126, hypothermia - 123, normothermia | 180-day mortality: - 73%, hypothermia - 71%, normothermia RR 1.03 (0.79–1.40) | In-hospital death: - 63%, hypothermia - 58%, normothermia RR 1.11 (0.86–1.46) 180-day good neurological recovery: - 22.5%, hypothermia - 23.7%, normothermia RR 1.04 (0.78–1.44) |
| EMERGE [45] NCT02876458 | OHCA | Emergency CAG Delayed CAG (48–96 h) | 279 - 141, emergency CAG - 138, delayed CAG | 180-day survival with good neurological recovery: - 34.1%, emergency CAG - 30.7%, delayed CAG HR 0.87 (0.65–1.15) | 180-day survival: - 36.2%, emergency CAG - 33.3%, delayed CAG HR 0.86 (0.64–1.15) Occurrence of shock during first 48 h - 38.8%, emergency CAG - 39.8, delayed CAG HR 1.03 (0.76–1.39) 90-day good neurological recovery: - 28.4%, emergency CAG - 24.6%, delayed CAG HR 0.86 (0.64–1.14) 180-day LVEF: - 60%, emergency CAG - 57.5%, delayed CAG Hospital length stay: - 7 d, emergency CAG - 5 d, delayed CAG |
| EXACT [52] NCT03138005 | Post-cardiac arrest (OHCA) | Intervention: spO2 90–94% Control: spO2 98%–100% | 425: - 214, intervention - 211, standard | Survival to hospital discharge: - 38.3%, intervention - 47.9%, standard OR 0.68 (0.46–1.00) | Hypoxic episode prior to ICU: - 31.3%, intervention - 16.1%, standard OR 2.37 (1.49–3.79) Pre-ICU re-arrest: - 12.7%, intervention - 10%, standard OR 1.30 (0.71–2.38) |
| BOX [51,60,63] NCT03141099 | Post-cardiac arrest (OHCA) | Blood pressure target: - 63 mmHg, low - 77 mmHg, High PaO2 target: - 68–75 mmHg, restrictive - 98–105 mmHg, liberal Device-based temperature control: - 36 h - 72 h | 789: Blood pressure target: - 396, low - 393, high Oxygen target: - 394, restrictive - 395, liberal Temperature control: - 393, 36 h - 396, 72 h | Death or severe disability or coma: Blood pressure target: - 34%, high - 32%, low HR 1.08 (0.84–1.37) Oxygen target: - 33.9%, liberal - 32%, restrictive HR 0.95 (0.75–1.21) Temperature control: - 32.3%, 36 h - 33.6%, 72 h HR 0.99 (0.77–1.26) | 90-day death 90-day neurological recovery 48-h neuron-specific enolase level No significant difference in any secondary outcomes in any target groups |
| TELSTAR [65] NCT02056236 | Post-cardiac arrest (OHCA) | Intervention: stepwise strategy of antiseizure medications Control: standard care | 172: - 88, intervention - 84, control | Poor neurological recovery at 3 months: - 90%, intervention - 92%, control Risk difference 2 (−7 to 11) | Death at 3 months: - 80%, treatment - 82%, control Rik difference 3 (−9 to 14) Length of stay in ICU: - 8.7 d, intervention - 7.5 d, control Duration of mechanical ventilation: - 7.8 d, intervention - 6.6 d, control |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gräsner, J.T.; Herlitz, J.; Tjelmeland, I.B.M.; Wnent, J.; Masterson, S.; Lilja, G.; Bein, B.; Böttiger, B.W.; Rosell-Ortiz, F.; Nolan, J.P.; et al. European Resuscitation Council Guidelines 2021: Epidemiology of cardiac arrest in Europe. Resuscitation 2021, 161, 61–79. [Google Scholar] [CrossRef]
- Semeraro, F.; Greif, R.; Böttiger, B.W.; Burkart, R.; Cimpoesu, D.; Georgiou, M.; Yeung, J.; Lippert, F.; Lockey, A.S.; Olasveengen, T.M.; et al. European Resuscitation Council Guidelines 2021: Systems saving lives. Resuscitation 2021, 161, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Yannopoulos, D.; Bartos, J.; Raveendran, G.; Walser, E.; Connett, J.; Murray, T.A.; Collins, G.; Zhang, L.; Kalra, R.; Kosmopoulos, M.; et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): A phase 2, single centre, open-label, randomised controlled trial. Lancet 2020, 396, 1807–1816. [Google Scholar] [CrossRef]
- Belohlavek, J.; Smalcova, J.; Rob, D.; Franek, O.; Smid, O.; Pokorna, M.; Horák, J.; Mrazek, V.; Kovarnik, T.; Zemanek, D.; et al. Prague OHCA Study Group. Effect of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment on Functional Neurologic Outcome in Refractory out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA 2022, 327, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Gräsner, J.T.; Wnent, J.; Herlitz, J.; Perkins, G.D.; Lefering, R.; Tjelmeland, I.; Koster, R.W.; Masterson, S.; Rossell-Ortiz, F.; Maurer, H.; et al. Survival after out-of-hospital cardiac arrest in Europe—Results of the EuReCa TWO study. Resuscitation 2020, 148, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Arctaedius, I.; Cronberg, T.; Levin, H.; Nielsen, N.; Friberg, H.; Lybeck, A. In-hospital versus out-of-hospital cardiac arrest: Characteristics and outcomes in patients admitted to intensive care after return of spontaneous circulation. Resuscitation 2022, 176, 1–8. [Google Scholar] [CrossRef]
- Cummins, R.O. Emergency medical services and sudden cardiac arrest: The “chain of survival” concept. Annu. Rev. Public Health 1993, 14, 313–333. [Google Scholar] [CrossRef]
- Berg, R.A.; Hemphill, R.; Abella, B.S.; Aufderheide, T.P.; Cave, D.M.; Hazinski, M.F.; Lerner, E.B.; Rea, T.D.; Sayre, M.R.; Swor, R.A. Part 5: Adult basic life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010, 122 (Suppl. 3), S685–S705. [Google Scholar] [CrossRef]
- Berglund, E.; Hollenberg, J.; Jonsson, M.; Svensson, L.; Claesson, A.; Nord, A.; Nordberg, P.; Forsberg, S.; Rosenqvist, M.; Lundgren, P.; et al. Effect of Smartphone Dispatch of Volunteer Responders on Automated External Defibrillators and Out-of-Hospital Cardiac Arrests: The SAMBA Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 81–88. [Google Scholar] [CrossRef]
- Valeriano, A.; Van Heer, S.; de Champlain, F.; Brooks, C.S. Crowdsourcing to save lives: A scoping review of bystander alert technologies for out-of-hospital cardiac arrest. Resuscitation 2021, 158, 94–121. [Google Scholar] [CrossRef]
- Nakashima, T.; Noguchi, T.; Tahara, Y.; Nishimura, K.; Yasuda, S.; Onozuka, D.; Iwami, T.; Yonemoto, N.; Nagao, K.; Nonogi, H.; et al. Japanese Circulation Society with Resuscitation Science Study Group. Public-access defibrillation and neurological outcomes in patients with out-of-hospital cardiac arrest in Japan: A population-based cohort study. Lancet 2019, 394, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Olasveengen, T.M.; Semeraro, F.; Ristagno, G.; Castren, M.; Handley, A.; Kuzovlev, A.; Monsieurs, K.G.; Raffay, V.; Smyth, M.; Soar, J.; et al. European Resuscitation Council Guidelines 2021: Basic Life Support. Resuscitation 2021, 161, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Ristagno, G.; Mauri, T.; Cesana, G.; Li, Y.; Finzi, A.; Fumagalli, F.; Rossi, G.; Grieco, N.; Migliori, M.; Andreassi, A.; et al. Azienda Regionale Emergenza Urgenza Research Group. Amplitude spectrum area to guide defibrillation: A validation on 1617 patients with ventricular fibrillation. Circulation 2015, 131, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Soar, J.; Böttiger, B.W.; Carli, P.; Couper, K.; Deakin, C.D.; Djärv, T.; Lott, C.; Olasveengen, T.; Paal, P.; Pellis, T.; et al. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation 2021, 161, 115–151. [Google Scholar] [CrossRef]
- Cheskes, S.; Verbeek, P.R.; Drennan, I.R.; McLeod, S.L.; Turner, L.; Pinto, R.; Feldman, M.; Davis, M.; Vaillancourt, C.; Morrison, L.J.; et al. Defibrillation Strategies for Refractory Ventricular Fibrillation. N. Engl. J. Med. 2022, 387, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Koster, R.W.; Walker, R.G.; Chapman, F.W. Recurrent ventricular fibrillation during advanced life support care of patients with prehospital cardiac arrest. Resuscitation 2008, 78, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.F.; Chien, Y.C.; Lee, B.C.; Yang, W.S.; Wang, Y.C.; Lin, H.Y.; Huang, E.P.; Chong, K.M.; Sun, J.T.; Huei-Ming, M.; et al. Effect of Placement of a Supraglottic Airway Device vs Endotracheal Intubation on Return of Spontaneous Circulation in Adults with Out-of-Hospital Cardiac Arrest in Taipei, Taiwan: A Cluster Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2148871. [Google Scholar] [CrossRef]
- Wang, H.E.; Schmicker, R.H.; Daya, M.R.; Stephens, S.W.; Idris, A.H.; Carlson, J.N.; Colella, M.R.; Herren, H.; Hansen, M.; Richmond, N.J.; et al. Effect of a Strategy of Initial Laryngeal Tube Insertion vs. Endotracheal Intubation on 72-Hour Survival in Adults with Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA 2018, 320, 769–778. [Google Scholar] [CrossRef]
- Benoit, J.L.; McMullan, J.T.; Wang, H.E.; Xie, C.; Xu, P.; Hart, K.W.; Stolz, U.; Lindsell, C.J. Timing of Advanced Airway Placement after Witnessed out-of-Hospital Cardiac Arrest. Prehosp. Emerg. Care 2019, 23, 838–846. [Google Scholar] [CrossRef]
- Perkins, G.D.; Ji, C.; Deakin, C.D.; Quinn, T.; Nolan, J.P.; Scomparin, C.; Regan, S.; Long, J.; Slowther, A.; Pocock, H.; et al. PARAMEDIC2 Collaborators. A Randomized Trial of Epinephrine in out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2018, 379, 711–721. [Google Scholar] [CrossRef]
- Callaway, C.W.; Hostler, D.; Doshi, A.A.; Pinchalk, M.; Roth, R.N.; Lubin, J.; Newman, D.H.; Kelly, L.J. Usefulness of vasopressin administered with epinephrine during out-of-hospital cardiac arrest. Am. J. Cardiol. 2006, 98, 1316–1321. [Google Scholar] [CrossRef]
- Kim, J.S.; Ryoo, S.M.; Kim, Y.J.; Sohn, C.H.; Ahn, S.; Seo, D.W.; Hong, S.I.; Kim, S.M.; Chae, B.; Kim, W.Y. Augmented-Medication CardioPulmonary Resuscitation Trials in out-of-hospital cardiac arrest: A pilot randomized controlled trial. Crit. Care 2022, 26, 378. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, M.J.; Granfeldt, A.; Mentzelopoulos, S.D.; Andersen, L.W. Vasopressin and glucocorticoids for in-hospital cardiac arrest: A systematic review and meta-analysis of individual participant data. Resuscitation 2022, 171, 48–56. [Google Scholar] [CrossRef]
- Wyckoff, M.H.; Greif, R.; Morley, P.T.; Ng, K.C.; Olasveengen, T.M.; Singletary, E.M.; Soar, J.; Cheng, A.; Drennan, I.R.; Liley, H.G.; et al. 2022 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations: Summary From the Basic Life Support; Advanced Life Support; Pediatric Life Support; Neonatal Life Support; Education, Implementation, and Teams; and First Aid Task Forces. Circulation 2022, 146, e483–e557. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: Post-resuscitation care. Intensive Care Med. 2021, 47, 369–421. [Google Scholar] [CrossRef] [PubMed]
- Schmitzberger, F.F.; Haas, N.L.; Coute, R.A.; Bartos, J.; Hackmann, A.; Haft, J.W.; Hsu, C.H.; Hutin, A.; Lamhaut, L.; Marinaro, J.; et al. ECPR2: Expert Consensus on PeRcutaneous Cannulation for Extracorporeal CardioPulmonary Resuscitation. Resuscitation 2022, 179, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Funada, A.; Goto, Y. Relationship Between the Duration of Cardiopulmonary Resuscitation and Favorable Neurological Outcomes After Out-of-Hospital Cardiac Arrest: A Prospective, Nationwide, Population-Based Cohort Study. J. Am. Heart Assoc. 2016, 5, e002819. [Google Scholar] [CrossRef]
- Havranek, S.; Fingrova, Z.; Rob, D.; Smalcova, J.; Kavalkova, P.; Franek, O.; Smid, O.; Huptych, M.; Dusik, M.; Linhart, A.; et al. Initial rhythm and survival in refractory out-of-hospital cardiac arrest. Post-hoc analysis of the Prague OHCA randomized trial. Resuscitation 2022, 181, 289–296. [Google Scholar] [CrossRef]
- Rob, D.; Smalcova, J.; Smid, O.; Kral, A.; Kovarnik, T.; Zemanek, D.; Kavalkova, P.; Huptych, M.; Komarek, A.; Franek, O.; et al. Extracorporeal versus conventional cardiopulmonary resuscitation for refractory out-of-hospital cardiac arrest: A secondary analysis of the Prague OHCA trial. Crit. Care 2022, 26, 330. [Google Scholar] [CrossRef]
- Berve, P.O.; Hardig, B.M.; Skålhegg, T.; Kongsgaard, H.; Kramer-Johansen, J.; Wik, L. Mechanical active compression-decompression versus standard mechanical cardiopulmonary resuscitation: A randomised haemodynamic out-of-hospital cardiac arrest study. Resuscitation 2022, 170, 1–10. [Google Scholar] [CrossRef]
- Lindner, K.H.; Pfenninger, E.G.; Lurie, K.G.; Schürmann, W.; Lindner, I.M.; Ahnefeld, F.W. Effects of active compression-decompression resuscitation on myocardial and cerebral blood flow in pigs. Circulation 1993, 88, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Stiell, I.G.; Hébert, P.C.; Wells, G.A.; Laupacis, A.; Vandemheen, K.; Dreyer, J.F.; Eisenhauer, M.A.; Gibson, J.; Higginson, L.A.; Kirby, A.S.; et al. The Ontario trial of active compression-decompression cardiopulmonary resuscitation for in-hospital and prehospital cardiac arrest. JAMA 1996, 275, 1417–1423. [Google Scholar] [CrossRef]
- Aufderheide, T.P.; Frascone, R.J.; Wayne, M.A.; Mahoney, B.D.; Swor, R.A.; Domeier, R.M.; Olinger, M.L.; Holcomb, R.G.; Tupper, D.E.; Yannopoulos, D.; et al. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: A randomised trial. Lancet 2011, 377, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Lafuente-Lafuente, C.; Melero-Bascones, M. Active chest compression-decompression for cardiopulmonary resuscitation. Cochrane Database Syst. Rev. 2013, 2013, CD002751. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.; Wetterslev, J.; Cronberg, T.; Erlinge, D.; Gasche, Y.; Hassager, C.; Horn, J.; Hovdenes, J.; Kjaergaard, J.; Kuiper, M.; et al. TTM Trial Investigators. Targeted temperature management at 33 °C versus 36 °C after cardiac arrest. N. Engl. J. Med. 2013, 369, 2197–2206. [Google Scholar] [CrossRef]
- Dankiewicz, J.; Cronberg, T.; Lilja, G.; Jakobsen, J.C.; Levin, H.; Ullén, S.; Rylander, C.; Wise, M.P.; Oddo, M.; Cariou, A.; et al. TTM2 Trial Investigators. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2021, 384, 2283–2294. [Google Scholar] [CrossRef]
- Sandroni, C.; Nolan, J.P.; Andersen, L.W.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Lilja, G.; Morley, P.T.; et al. ERC-ESICM guidelines on temperature control after cardiac arrest in adults. Intensive Care Med. 2022, 48, 261–269. [Google Scholar] [CrossRef]
- Wolfrum, S.; Roedl, K.; Hanebutte, A.; Pfeifer, R.; Kurowski, V.; Riessen, R.; Daubmann, A.; Braune, S.; Söffker, G.; Bibiza-Freiwald, E.; et al. Hypothermia After In-Hospital Cardiac Arrest Study Group. Temperature Control after in-Hospital Cardiac Arrest: A Randomized Clinical Trial. Circulation 2022, 146, 1357–1366. [Google Scholar] [CrossRef]
- Blanc, A.; Colin, G.; Cariou, A.; Merdji, H.; Grillet, G.; Girardie, P.; Coupez, E.; Dequin, P.F.; Boulain, T.; Frat, J.P.; et al. Targeted Temperature Management After In-Hospital Cardiac Arrest: An Ancillary Analysis of Targeted Temperature Management for Cardiac Arrest with Nonshockable Rhythm Trial Data. Chest 2022, 62, 356–366. [Google Scholar] [CrossRef]
- Yang, B.Y.; Bulger, N.; Chocron, R.; Counts, C.R.; Drucker, C.; Yin, L.; Parayil, M.; Johnson, N.J.; Sotoodehenia, N.; Kudenchuk, P.J.; et al. Analysis of Epinephrine Dose, Targeted Temperature Management, and Neurologic and Survival Outcomes among Adults with out-of-Hospital Cardiac Arrest. JAMA Netw. Open 2022, 5, e2226191. [Google Scholar] [CrossRef]
- Callaway, C.W.; Coppler, P.J.; Faro, J.; Puyana, J.S.; Solanki, P.; Dezfulian, C.; Doshi, A.A.; Elmer, J.; Frisch, A.; Guyette, F.X.; et al. Association of Initial Illness Severity and Outcomes after Cardiac Arrest with Targeted Temperature Management at 36 °C or 33 °C. JAMA Netw. Open 2020, 3, e208215. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Neumar, R.W.; Adrie, C.; Aibiki, M.; Berg, R.A.; Böttiger, B.W.; Callaway, C.; Clark, R.S.; Geocadin, R.G.; Jauch, E.C.; et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 2008, 79, 350–379. [Google Scholar] [CrossRef] [PubMed]
- Dumas, F.; Cariou, A.; Manzo-Silberman, S.; Grimaldi, D.; Vivien, B.; Rosencher, J.; Empana, J.P.; Carli, P.; Mira, J.P.; Jouven, X.; et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: Insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ. Cardiovasc Interv. 2010, 3, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Hauw-Berlemont, C.; Lamhaut, L.; Diehl, J.L.; Andreotti, C.; Varenne, O.; Leroux, P.; Lascarrou, J.B.; Guerin, P.; Loeb, T.; Roupie, E.; et al. EMERGE Investigators. Emergency vs Delayed Coronary Angiogram in Survivors of Out-of-Hospital Cardiac Arrest: Results of the Randomized, Multicentric EMERGE Trial. JAMA Cardiol. 2022, 7, 700–707. [Google Scholar] [CrossRef]
- Lemkes, J.S.; Janssens, G.N.; van der Hoeven, N.W.; Jewbali, L.S.D.; Dubois, E.A.; Meuwissen, M.; Rijpstra, T.A.; Bosker, H.A.; Blans, M.J.; Bleeker, G.B.; et al. Coronary Angiography after Cardiac Arrest without ST-Segment Elevation. N. Engl. J. Med. 2019, 380, 1397–1407. [Google Scholar] [CrossRef]
- Kern, K.B.; Radsel, P.; Jentzer, J.C.; Seder, D.B.; Lee, K.S.; Lotun, K.; Janardhanan, R.; Stub, D.; Hsu, C.H.; Noc, M. Randomized Pilot Clinical Trial of Early Coronary Angiography Versus No Early Coronary Angiography after Cardiac Arrest without ST-Segment Elevation: The PEARL Study. Circulation 2020, 142, 2002–2012. [Google Scholar] [CrossRef]
- Desch, S.; Freund, A.; Akin, I.; Behnes, M.; Preusch, M.R.; Zelniker, T.A.; Skurk, C.; Landmesser, U.; Graf, T.; Eitel, I.; et al. TOMAHAWK Investigators. Angiography after out-of-Hospital Cardiac Arrest without ST-Segment Elevation. N. Engl. J. Med. 2021, 385, 2544–2553. [Google Scholar] [CrossRef]
- Al Lawati, K.; Forestell, B.; Binbraik, Y.; Sharif, S.; Ainsworth, C.; Mathew, R.; Amin, F.; Al Fawaz, M.; Pinilla-Echeverri, N.; Belley-Côté, E.; et al. Early Versus Delayed Coronary Angiography after out-of-Hospital Cardiac Arrest without ST-Segment Elevation-A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Care Explor. 2023, 5, e0874. [Google Scholar] [CrossRef]
- Skrifvars, M.B.; Olasveengen, T.M.; Ristagno, G. Oxygen and carbon dioxide targets during and after resuscitation of cardiac arrest patients. Intensive Care Med. 2019, 45, 284–286. [Google Scholar] [CrossRef]
- Schmidt, H.; Kjaergaard, J.; Hassager, C.; Mølstrøm, S.; Grand, J.; Borregaard, B.; Roelsgaard Obling, L.E.; Venø, S.; Sarkisian, L.; Mamaev, D.; et al. Oxygen Targets in Comatose Survivors of Cardiac Arrest. N. Engl. J. Med. 2022, 387, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.A.; Bray, J.E.; Smith, K.; Stephenson, M.; Finn, J.; Grantham, H.; Hein, C.; Masters, S.; Stub, D.; Perkins, G.D.; et al. EXACT Investigators. Effect of Lower vs Higher Oxygen Saturation Targets on Survival to Hospital Discharge among Patients Resuscitated after out-of-Hospital Cardiac Arrest: The EXACT Randomized Clinical Trial. JAMA 2022, 328, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, S.; Stride, N.; Bro-Jeppesen, J.; Holmberg, M.J.; Kjærgaard, J.; Larsen, S.; Donnino, M.W.; Hassager, C.; Dela, F. Mitochondrial dysfunction in adults after out-of-hospital cardiac arrest. Eur. Heart J. Acute Cardiovasc Care 2020, 9 (Suppl. 4), S138–S144. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, J.; Weatherall, M.; Shirtcliffe, P.; Bellomo, R.; Young, P.; Beasley, R. The effect of hyperoxia following cardiac arrest—A systematic review and meta-analysis of animal trials. Resuscitation 2012, 83, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Young, P.J.; Bailey, M.; Bellomo, R.; Bernard, S.; Bray, J.; Jakkula, P.; Kuisma, M.; Mackle, D.; Martin, D.; Nolan, J.P.; et al. Conservative or liberal oxygen therapy in adults after cardiac arrest: An individual-level patient data meta-analysis of randomised controlled trials. Resuscitation 2020, 157, 15–22. [Google Scholar] [CrossRef]
- Ristagno, G.; Tang, W.; Sun, S.; Weil, M.H. Cerebral cortical microvascular flow during and following cardiopulmonary resuscitation after short duration of cardiac arrest. Resuscitation 2008, 77, 229–234. [Google Scholar] [CrossRef]
- Sundgreen, C.; Larsen, F.S.; Herzog, T.M.; Knudsen, G.M.; Boesgaard, S.; Aldershvile, J. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke 2001, 32, 128–132. [Google Scholar] [CrossRef]
- Ameloot, K.; De Deyne, C.; Eertmans, W.; Ferdinande, B.; Dupont, M.; Palmers, P.J.; Petit, T.; Nuyens, P.; Maeremans, J.; Vundelinckx, J.; et al. Early goal-directed haemodynamic optimization of cerebral oxygenation in comatose survivors after cardiac arrest: The Neuroprotect post-cardiac arrest trial. Eur. Heart J. 2019, 40, 1804–1814. [Google Scholar] [CrossRef]
- Jakkula, P.; Pettilä, V.; Skrifvars, M.B.; Hästbacka, J.; Loisa, P.; Tiainen, M.; Wilkman, E.; Toppila, J.; Koskue, T.; Bendel, S.; et al. COMACARE study group. Targeting low-normal or high-normal mean arterial pressure after cardiac arrest and resuscitation: A randomised pilot trial. Intensive Care Med. 2018, 44, 2091–2101. [Google Scholar] [CrossRef]
- Kjaergaard, J.; Møller, J.E.; Schmidt, H.; Grand, J.; Mølstrøm, S.; Borregaard, B.; Venø, S.; Sarkisian, L.; Mamaev, D.; Jensen, L.O.; et al. Blood-Pressure Targets in Comatose Survivors of Cardiac Arrest. N. Engl. J. Med. 2022, 387, 1456–1466. [Google Scholar] [CrossRef]
- Zeiner, A.; Holzer, M.; Sterz, F.; Schörkhuber, W.; Eisenburger, P.; Havel, C.; Kliegel, A.; Laggner, A.N. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch. Intern. Med. 2001, 161, 2007–2012. [Google Scholar] [CrossRef]
- Makker, P.; Kanei, Y.; Misra, D. Clinical Effect of Rebound Hyperthermia after Cooling Postcardiac Arrest: A Meta-Analysis. Ther. Hypothermia Temp Manag. 2017, 7, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Hassager, C.; Schmidt, H.; Møller, J.E.; Grand, J.; Mølstrøm, S.; Beske, R.P.; Boesgaard, S.; Borregaard, B.; Bekker-Jensen, D.; Dahl, J.S.; et al. Duration of Device-Based Fever Prevention after Cardiac Arrest. N. Engl. J. Med. 2022, 388, 888–897. [Google Scholar] [CrossRef]
- Ruijter, B.J.; van Putten, M.J.; Hofmeijer, J. Generalized epileptiform discharges in postanoxic encephalopathy: Quantitative characterization in relation to outcome. Epilepsia 2015, 56, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Ruijter, B.J.; Keijzer, H.M.; Tjepkema-Cloostermans, M.C.; Blans, M.J.; Beishuizen, A.; Tromp, S.C.; Scholten, E.; Horn, J.; van Rootselaar, A.F.; Admiraal, M.M.; et al. TELSTAR Investigators. Treating Rhythmic and Periodic EEG Patterns in Comatose Survivors of Cardiac Arrest. N. Engl. J. Med. 2022, 386, 724–734. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penna, A.; Magliocca, A.; Merigo, G.; Stirparo, G.; Silvestri, I.; Fumagalli, F.; Ristagno, G. One-Year Review in Cardiac Arrest: The 2022 Randomized Controlled Trials. J. Clin. Med. 2023, 12, 2235. https://doi.org/10.3390/jcm12062235
Penna A, Magliocca A, Merigo G, Stirparo G, Silvestri I, Fumagalli F, Ristagno G. One-Year Review in Cardiac Arrest: The 2022 Randomized Controlled Trials. Journal of Clinical Medicine. 2023; 12(6):2235. https://doi.org/10.3390/jcm12062235
Chicago/Turabian StylePenna, Alessio, Aurora Magliocca, Giulia Merigo, Giuseppe Stirparo, Ivan Silvestri, Francesca Fumagalli, and Giuseppe Ristagno. 2023. "One-Year Review in Cardiac Arrest: The 2022 Randomized Controlled Trials" Journal of Clinical Medicine 12, no. 6: 2235. https://doi.org/10.3390/jcm12062235
APA StylePenna, A., Magliocca, A., Merigo, G., Stirparo, G., Silvestri, I., Fumagalli, F., & Ristagno, G. (2023). One-Year Review in Cardiac Arrest: The 2022 Randomized Controlled Trials. Journal of Clinical Medicine, 12(6), 2235. https://doi.org/10.3390/jcm12062235





