Initial In-Hospital Visit-to-Visit Heart Rate Variability Is Associated with Higher Risk of Atrial Fibrillation in Patients with Acute Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
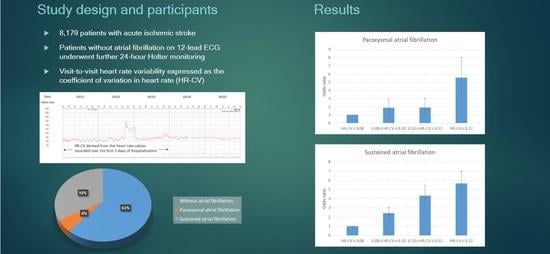
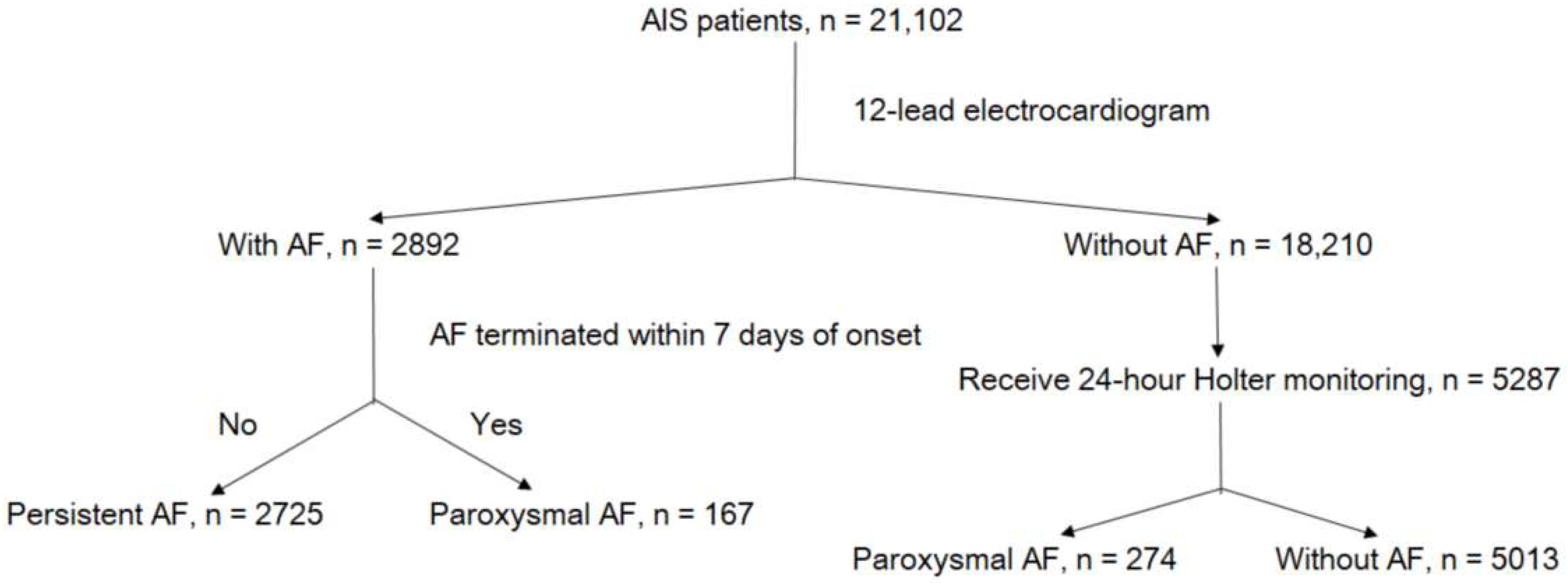
2.1. Study Population
2.2. Ascertainment of AF Types
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
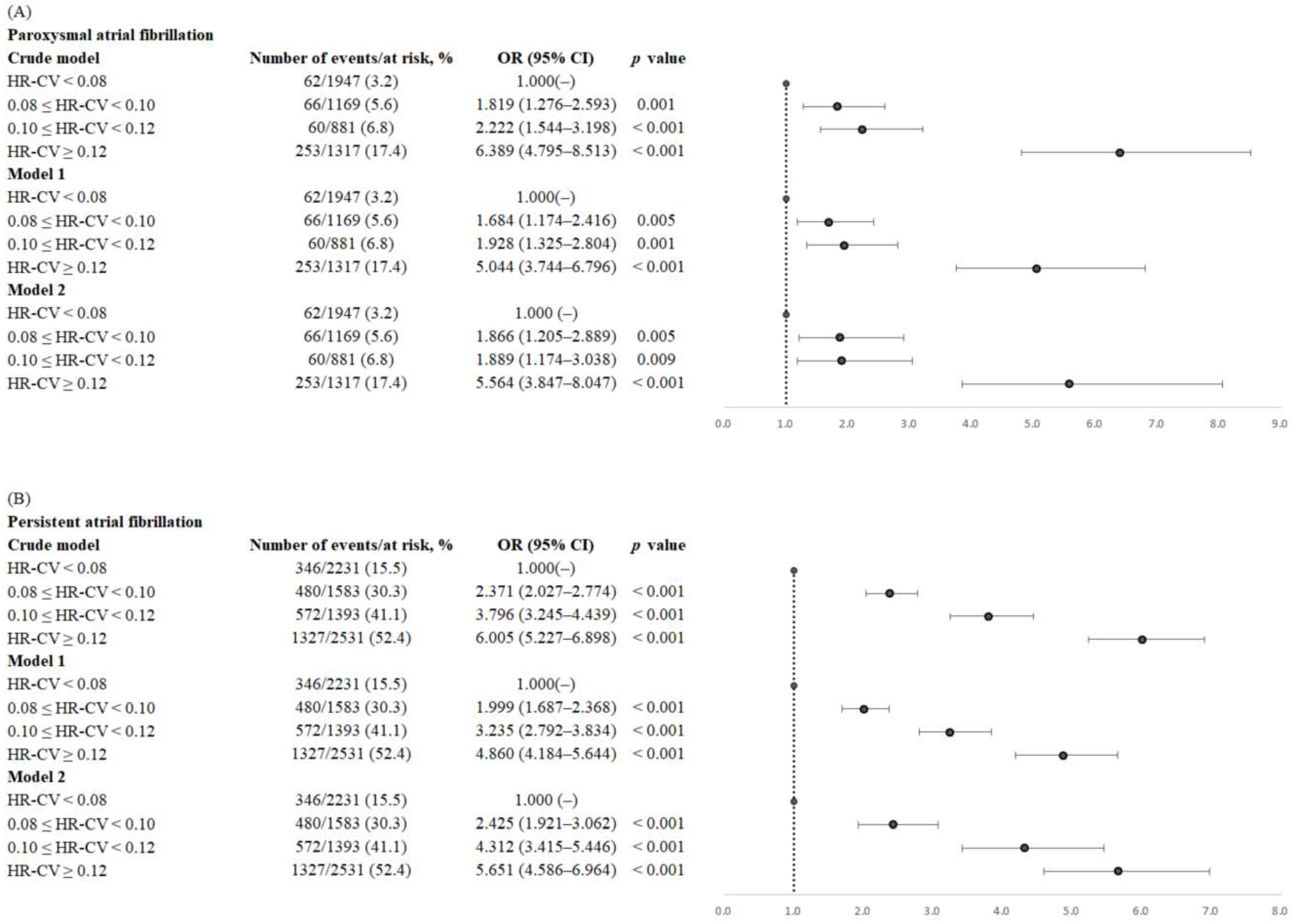
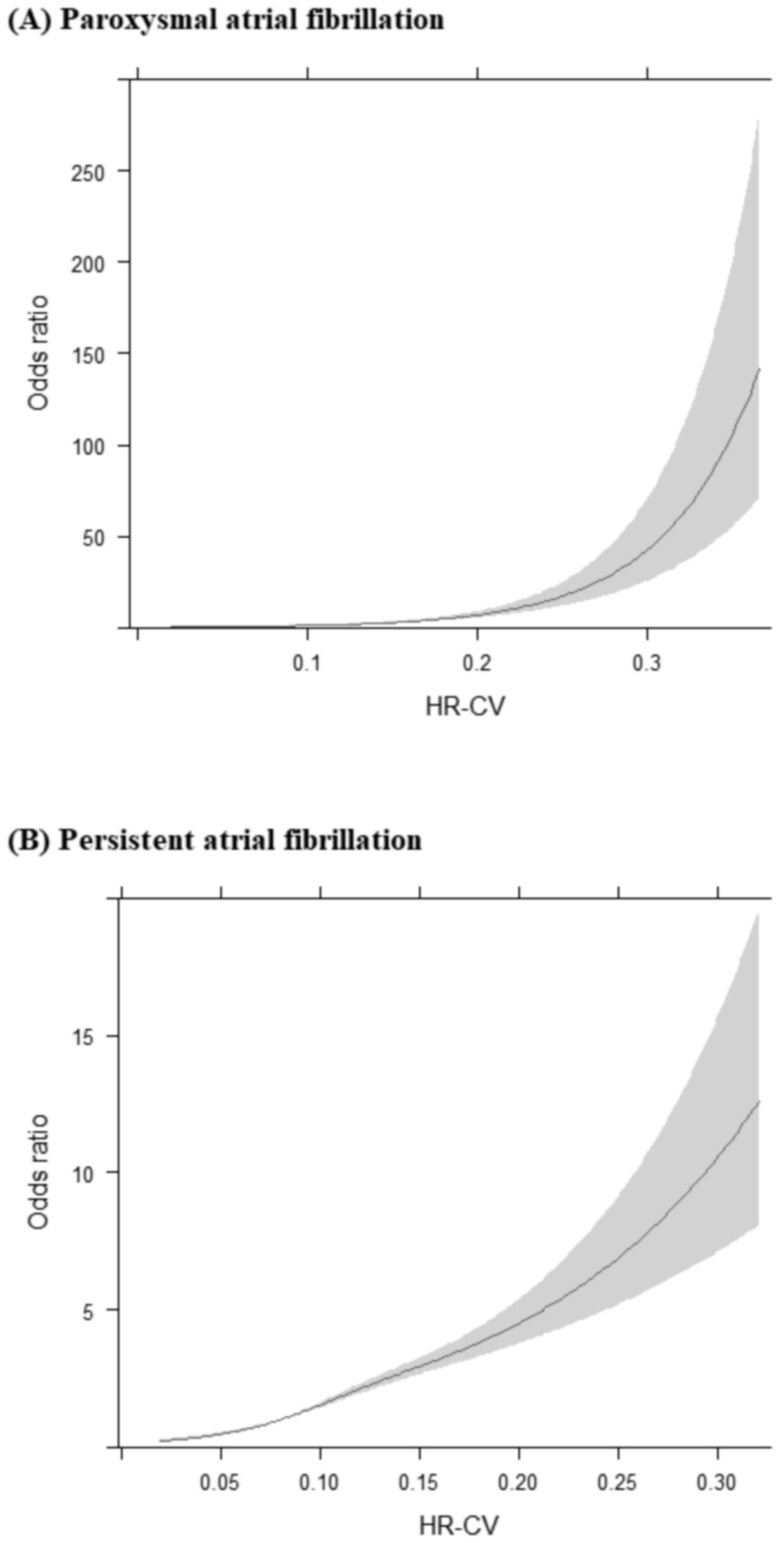
3.2. Association of HR-CV Level with AF
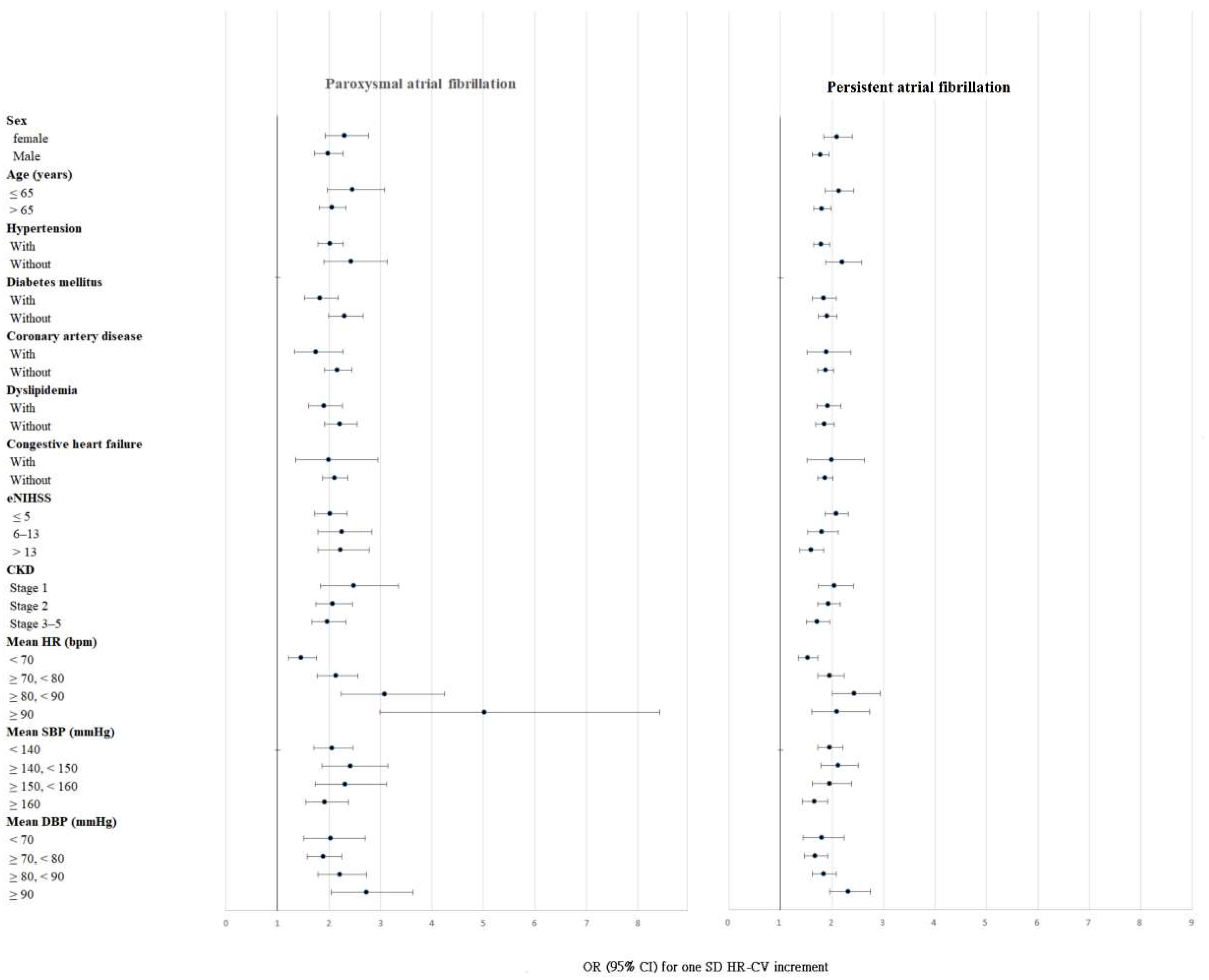
3.3. Subgroup Analysis
3.4. Interaction of Mean HR and HR-CV
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Acronyms
References
- Marini, C.; De Santis, F.; Sacco, S.; Russo, T.; Olivieri, L.; Totaro, R.; Carolei, A. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: Results from a population-based study. Stroke 2005, 36, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Pajitnev, D.; Pogue, J.; Healey, J.S.; Pfeffer, M.A.; Yusuf, S.; Connolly, S.J.; Investigators, A.W. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: An ACTIVE W Substudy. J. Am. Coll. Cardiol. 2007, 50, 2156–2161. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G.; Pearce, L.A.; Rothbart, R.M.; McAnulty, J.H.; Asinger, R.W.; Halperin, J.L. Stroke with intermittent atrial fibrillation: Incidence and predictors during aspirin therapy. Stroke Prevention in Atrial Fibrillation Investigators. J. Am. Coll. Cardiol. 2000, 35, 183–187. [Google Scholar] [CrossRef]
- Sposato, L.A.; Cipriano, L.E.; Saposnik, G.; Ruiz Vargas, E.; Riccio, P.M.; Hachinski, V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Schaer, B.A.; Zellweger, M.J.; Cron, T.A.; Kaiser, C.A.; Osswald, S. Value of routine holter monitoring for the detection of paroxysmal atrial fibrillation in patients with cerebral ischemic events. Stroke 2004, 35, e68–e70. [Google Scholar] [CrossRef]
- Suissa, L.; Lachaud, S.; Mahagne, M.H. Optimal timing and duration of continuous electrocardiographic monitoring for detecting atrial fibrillation in stroke patients. J. Stroke Cerebrovasc. Dis. 2013, 22, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Wang, Z.; Cheng, W.; Yang, K. Visit-to-Visit Heart Rate Variability Is Positively Associated with the Risk of Adverse Cardiovascular Outcomes. Front. Cardiovasc. Med. 2022, 9, 850223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, M.; Sun, Y.; Hou, Z.; Wang, C.; Yun, C.; Li, Y.; Li, Z.; Wang, M.; Wu, S.; et al. Frequency of Visit-to-Visit Variability of Resting Heart Rate and the Risk of New-Onset Atrial Fibrillation in the General Population. Am. J. Cardiol. 2021, 155, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Shiroto, H.; Tomita, H.; Hagii, J.; Metoki, N.; Fujita, A.; Kamada, T.; Takahashi, K.; Saito, S.; Sasaki, S.; Hitomi, H.; et al. Impact of Atrial Natriuretic Peptide Value for Predicting Paroxysmal Atrial Fibrillation in Ischemic Stroke Patients. J. Stroke Cerebrovasc. Dis. 2017, 26, 772–778. [Google Scholar] [CrossRef]
- Baturova, M.A.; Sheldon, S.H.; Carlson, J.; Brady, P.A.; Lin, G.; Rabinstein, A.A.; Friedman, P.A.; Platonov, P.G. Electrocardiographic and Echocardiographic predictors of paroxysmal atrial fibrillation detected after ischemic stroke. BMC Cardiovasc. Disord. 2016, 16, 209. [Google Scholar] [CrossRef]
- Seo, W.K.; Kang, S.H.; Jung, J.M.; Choi, J.Y.; Oh, K. Novel composite score to predict atrial Fibrillation in acute stroke patients: AF predicting score in acute stroke. Int. J. Cardiol. 2016, 209, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Lin, M.H.; Lee, C.P.; Yang, Y.H.; Chen, W.C.; Chang, G.H.; Tsai, Y.T.; Chen, P.C.; Tsai, Y.H. Chang Gung Research Database: A multi-institutional database consisting of original medical records. Biomed. J. 2017, 40, 263–269. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014, 64, e1–e76. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Heart Rhythm. 2017, 14, e445–e494. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.F.; Hsieh, C.Y.; Lin, H.J.; Chen, Y.W.; Chen, C.H.; Kao Yang, Y.H.; Hu, Y.H. Validity of a stroke severity index for administrative claims data research: A retrospective cohort study. BMC Health Serv. Res. 2016, 16, 509. [Google Scholar] [CrossRef] [PubMed]
- Uphaus, T.; Weber-Kruger, M.; Grond, M.; Toenges, G.; Jahn-Eimermacher, A.; Jauss, M.; Kirchhof, P.; Wachter, R.; Groschel, K. Development and validation of a score to detect paroxysmal atrial fibrillation after stroke. Neurology 2019, 92, e115–e124. [Google Scholar] [CrossRef]
- Bohm, M.; Schumacher, H.; Linz, D.; Reil, J.C.; Ukena, C.; Lonn, E.; Teo, K.; Sliwa, K.; Schmieder, R.E.; Sleight, P.; et al. Low resting heart rates are associated with new-onset atrial fibrillation in patients with vascular disease: Results of the ONTARGET/TRANSCEND studies. J. Intern. Med. 2015, 278, 303–312. [Google Scholar] [CrossRef]
- Bohm, M.; Robertson, M.; Borer, J.; Ford, I.; Komajda, M.; Mahfoud, F.; Ewen, S.; Swedberg, K.; Tavazzi, L. Effect of Visit-to-Visit Variation of Heart Rate and Systolic Blood Pressure on Outcomes in Chronic Systolic Heart Failure: Results from the Systolic Heart Failure Treatment with the If Inhibitor Ivabradine Trial (SHIFT) Trial. J. Am. Heart Assoc. 2016, 5, e002160. [Google Scholar] [CrossRef]
- Zhao, M.; Yao, S.; Li, Y.; Wang, M.; Wang, C.; Yun, C.; Zhang, S.; Sun, Y.; Hou, Z.; Wu, S.; et al. Combined effect of visit-to-visit variations in heart rate and systolic blood pressure on all-cause mortality in hypertensive patients. Hypertens. Res. 2021, 44, 1291–1299. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Norby, F.L.; Whitsel, E.A.; Soliman, E.Z.; Chen, L.Y.; Loehr, L.R.; Fuster, V.; Heiss, G.; Coresh, J.; Alonso, A. Cardiac Autonomic Dysfunction and Incidence of Atrial Fibrillation: Results From 20 Years Follow-Up. J. Am. Coll. Cardiol. 2017, 69, 291–299. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.S.; McManus, D.D.; Newton-Cheh, C.; Lubitz, S.A.; Magnani, J.W.; Ellinor, P.T.; et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015, 386, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Dulli, D.A.; Stanko, H.; Levine, R.L. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology 2003, 22, 118–123. [Google Scholar] [CrossRef]
- Zoni-Berisso, M.; Lercari, F.; Carazza, T.; Domenicucci, S. Epidemiology of atrial fibrillation: European perspective. Clin. Epidemiol. 2014, 6, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Tanabe, N.; Yagihara, N.; Watanabe, T.; Aizawa, Y.; Kodama, M. Association between lipid profile and risk of atrial fibrillation. Circ. J. 2011, 75, 2767–2774. [Google Scholar] [CrossRef]
- Ruigomez, A.; Johansson, S.; Wallander, M.A.; Garcia Rodriguez, L.A. Predictors and prognosis of paroxysmal atrial fibrillation in general practice in the UK. BMC Cardiovasc. Disord. 2005, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Staerk, L.; Wang, B.; Preis, S.R.; Larson, M.G.; Lubitz, S.A.; Ellinor, P.T.; McManus, D.D.; Ko, D.; Weng, L.C.; Lunetta, K.L.; et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: Cohort study based on longitudinal data from the Framingham Heart Study. BMJ 2018, 361, k1453. [Google Scholar] [CrossRef]
- Camm, A.J.; Accetta, G.; Al Mahmeed, W.; Ambrosio, G.; Goldhaber, S.Z.; Haas, S.; Jansky, P.; Kayani, G.; Misselwitz, F.; Oh, S.; et al. Impact of gender on event rates at 1 year in patients with newly diagnosed non-valvular atrial fibrillation: Contemporary perspective from the GARFIELD-AF registry. BMJ Open 2017, 7, e014579. [Google Scholar] [CrossRef]
- Holroyd-Leduc, J.M.; Kapral, M.K.; Austin, P.C.; Tu, J.V. Sex differences and similarities in the management and outcome of stroke patients. Stroke 2000, 31, 1833–1837. [Google Scholar] [CrossRef]
- Lopez, F.L.; Agarwal, S.K.; Maclehose, R.F.; Soliman, E.Z.; Sharrett, A.R.; Huxley, R.R.; Konety, S.; Ballantyne, C.M.; Alonso, A. Blood lipid levels, lipid-lowering medications, and the incidence of atrial fibrillation: The atherosclerosis risk in communities study. Circ. Arrhythm. Electrophysiol. 2012, 5, 155–162. [Google Scholar] [CrossRef]
- Mora, S.; Akinkuolie, A.O.; Sandhu, R.K.; Conen, D.; Albert, C.M. Paradoxical association of lipoprotein measures with incident atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2014, 7, 612–619. [Google Scholar] [CrossRef]
- Li, X.; Gao, L.; Wang, Z.; Guan, B.; Guan, X.; Wang, B.; Han, X.; Xiao, X.; Waleed, K.B.; Chandran, C.; et al. Lipid profile and incidence of atrial fibrillation: A prospective cohort study in China. Clin. Cardiol. 2018, 41, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Fauchier, L.; Clementy, N.; Pelade, C.; Collignon, C.; Nicolle, E.; Lip, G.Y. Patients with Ischemic Stroke and Incident Atrial Fibrillation: A Nationwide Cohort Study. Stroke 2015, 46, 2432–2437. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Munoz-Venturelli, P.; Billot, L.; Wang, X.; Song, L.; Arima, H.; Lavados, P.M.; Hackett, M.L.; Olavarria, V.V.; Brunser, A.; et al. Low blood pressure and adverse outcomes in acute stroke: HeadPoST study explanations. J. Hypertens. 2021, 39, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Steger, C.; Pratter, A.; Martinek-Bregel, M.; Avanzini, M.; Valentin, A.; Slany, J.; Stollberger, C. Stroke patients with atrial fibrillation have a worse prognosis than patients without: Data from the Austrian Stroke registry. Eur. Heart J. 2004, 25, 1734–1740. [Google Scholar] [CrossRef]
- Temu, T.M.; Lane, K.A.; Shen, C.; Ng’ang’a, L.; Akwanalo, C.O.; Chen, P.S.; Emonyi, W.; Heckbert, S.R.; Koech, M.M.; Manji, I.; et al. Clinical characteristics and 12-month outcomes of patients with valvular and non-valvular atrial fibrillation in Kenya. PLoS One 2017, 12, e0185204. [Google Scholar] [CrossRef]
- Ruperti Repilado, F.J.; Doerig, L.; Blum, S.; Aeschbacher, S.; Krisai, P.; Ammann, P.; Erne, P.; Moschovitis, G.; di Valentino, M.; Shah, D.; et al. Prevalence and predictors of atrial fibrillation type among individuals with recent onset of atrial fibrillation. Swiss. Med. Wkly. 2018, 148, w14652. [Google Scholar] [CrossRef]
- Meinertz, T.; Kirch, W.; Rosin, L.; Pittrow, D.; Willich, S.N.; Kirchhof, P.; investigators, A. Management of atrial fibrillation by primary care physicians in Germany: Baseline results of the ATRIUM registry. Clin. Res. Cardiol. 2011, 100, 897–905. [Google Scholar] [CrossRef]
| Acute Ischemic Stroke | p Value | ||||
|---|---|---|---|---|---|
| Total (N = 8179) | Without AF (N = 5013) | With Paroxysmal AF (N = 441) | With Persistent AF (N = 2725) | ||
| Age, years | <0.001 | ||||
| Mean (SD) | 68.05 (13.56) | 64.30 (13.67) | 74.73 (10.38) | 73.86 (11.15) | |
| Median (Q1, Q3) | 69.00 (59.00, 78.00) | 65.00 (55.00, 75.00) | 76.00 (68.00, 82.00) | 75.00 (66.00, 82.00) | |
| Male | 4997 (61.1) | 3340 (66.6) | 240 (54.4) | 1417 (52.0) | <0.001 |
| eNIHSS | <0.001 | ||||
| Mean (SD) | 8.34 (6.64) | 6.58 (4.96) | 9.78 (7.26) | 11.34 (7.98) | |
| Median (Q1, Q3) | 4.06 (4.06, 10.90) | 4.06 (4.06, 6.00) | 5.66 (4.06, 16.27) | 8.95 (4.06, 20.04) | |
| Hypertension | 5931 (72.5) | 3662 (73.1) | 336 (76.2) | 1933 (70.9) | 0.028 |
| Diabetes mellitus | 2837 (34.7) | 1874 (37.4) | 129 (29.3) | 834 (30.6) | <0.001 |
| Dyslipidemia | 3600 (44.0) | 2592 (51.7) | 139 (31.5) | 869 (31.9) | <0.001 |
| Congestive heart failure | 687 (8.4) | 212 (4.2) | 55 (12.5) | 420 (15.4) | <0.001 |
| Coronary artery disease | 871 (10.6) | 445 (8.9) | 79 (17.9) | 347 (12.7) | <0.001 |
| Current smoker | 2180 (26.7) | 1630 (32.5) | 97 (22.0) | 453 (16.6) | <0.001 |
| History of cancer | 478 (5.8) | 279 (5.6) | 29 (6.6) | 170 (6.2) | 0.385 |
| Body mass index, kg/m2 | <0.001 | ||||
| Mean (SD) | 24.76 (4.26) | 25.05 (4.32) | 24.01 (3.96) | 24.35 (4.17) | |
| Median (Q1, Q3) | 24.40 (21.91, 27.12) | 24.66 (22.20, 27.34) | 23.92 (21.23, 26.67) | 24.07 (21.45, 26.69) | |
| Total cholesterol, mmol/L | <0.001 | ||||
| Mean (SD) | 4.55 (1.09) | 4.69 (1.13) | 4.36 (1.06) | 4.32 (0.99) | |
| Median (Q1, Q3) | 4.45 (3.83, 5.15) | 4.58 (3.96, 5.31) | 4.27 (3.66, 4.91) | 4.25 (3.68, 4.90) | |
| Triglycerides, mmol/L | <0.001 | ||||
| Mean (SD) | 1.41 (1.05) | 1.56 (1.08) | 1.26 (0.85) | 1.15 (0.97) | |
| Median (Q1, Q3) | 1.18 (0.84, 1.67) | 1.31 (0.94, 1.86) | 1.05 (0.79, 1.46) | 0.97 (0.72, 1.33) | |
| Creatinine, μmol/L | <0.001 | ||||
| Mean (SD) | 112.09 (115.82) | 111.25 (120.01) | 126.77 (138.48) | 111.26 (103.13) | |
| Median (Q1, Q3) | 85.75 (69.84, 109.62) | 83.98 (68.95, 106.96) | 91.94 (73.37, 118.90) | 88.40 (70.72, 114.04) | |
| Alanine aminotransferase, U/L | <0.001 | ||||
| Mean (SD) | 26.42 (38.14) | 26.86 (25.90) | 23.78 (19.01) | 26.05 (55.33) | |
| Median (Q1, Q3) | 21.00 (16.00, 29.00) | 21.00 (16.00, 29.00) | 20.00 (14.00, 27.00) | 20.00 (15.00, 28.00) | |
| Mean SBP, mmHg | <0.001 | ||||
| Mean (SD) | 148.36 (19.35) | 150.51 (19.73) | 145.29 (18.78) | 144.90 (18.15) | |
| Median (Q1, Q3) | 147.20 (134.50, 161.60) | 149.43 (136.39, 164.06) | 144.50 (132.63, 156.78) | 144.01 (132.12, 157.24) | |
| Mean DBP, mmHg | <0.001 | ||||
| Mean (SD) | 83.85 (11.26) | 84.76 (11.17) | 79.49 (11.46) | 82.90 (11.15) | |
| Median (Q1, Q3) | 83.14 (76.37, 90.91) | 84.00 (77.14, 91.76) | 79.00 (72.61, 86.06) | 82.30 (75.44, 90.19) | |
| Mean heart rate, bpm | <0.001 | ||||
| Mean (SD) | 75.84 (12.01) | 73.19 (10.71) | 77.68 (13.41) | 80.42 (12.59) | |
| Median (Q1, Q3) | 74.86 (67.62, 82.83) | 72.74 (65.92, 79.36) | 75.89 (68.76, 85.46) | 79.88 (12.59, 89.19) | |
| CV in heart rate | <0.001 | ||||
| Mean (SD) | 0.109 (0.048) | 0.097 (0.039) | 0.148 (0.078) | 0.126 (0.047) | |
| Median (Q1, Q3) | 0.101 (0.077, 0.133) | 0.090 (0.069, 0.118) | 0.132 (0.095, 0.181) | 0.118 (0.093, 0.149) | |
| Paroxysmal Atrial Fibrillation | Persistent Atrial Fibrillation | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| HR-CV (per 1 SD) | 2.082 (1.867–2.322) | <0.001 | 1.887 (1.748–2.036) | <0.001 |
| Male | 0.652 (0.484–0.879) | 0.005 | 0.612 (0.519–0.721) | <0.001 |
| Age | 1.057 (1.044–1.071) | <0.001 | 1.067 (1.060–1.075) | <0.001 |
| Hypertension | 1.048 (0.759–1.446) | 0.776 | 0.965 (0.811–1.149) | 0.693 |
| Diabetes mellitus | 0.838 (0.628–1.117) | 0.228 | 0.997 (0.852–1.167) | 0.972 |
| Current smoker | 1.039 (0.736–1.467) | 0.829 | 0.835 (0.694–1.004) | 0.055 |
| Coronary artery disease | 1.823 (1.260–2.635) | 0.001 | 1.128 (0.895–1.421) | 0.308 |
| Dyslipidemia | 0.559 (0.420–0.744) | <0.001 | 0.630 (0.540–0.735) | <0.001 |
| Congestive heart failure | 1.543 (0.975–2.443) | 0.064 | 2.515 (1.938–3.265) | <0.001 |
| History of cancer | 0.717 (0.408–1.261) | 0.248 | 0.928 (0.688–1.253) | 0.627 |
| eNIHSS | 1.015 (0.994–1.037) | 0.168 | 1.074 (1.061–1.086) | <0.001 |
| Body mass index | 1.017 (0.983–1.052) | 0.326 | 1.042 (1.024–1.061) | <0.001 |
| Total Cholesterol | 0.985 (0.864–1.123) | 0.822 | 0.899 (0.832–0.970) | 0.006 |
| Triglycerides | 0.974 (0.823–1.152) | 0.757 | 0.756 (0.678–0.844) | <0.001 |
| Alanine aminotransferase | 0.998 (0.993–1.004) | 0.600 | 1.000 (0.999–1.002) | 0.719 |
| Creatinine | 1.001 (1.000–1.002) | 0.216 | 1.000 (0.999–1.001) | 0.816 |
| Mean heart rate | 1.020 (1.008–1.033) | 0.002 | 1.027 (1.020–1.035) | <0.001 |
| Mean systolic blood pressure | 0.986 (0.977–0.996) | 0.006 | 0.961 (0.955–0.966) | <0.001 |
| Mean diastolic blood pressure | 0.995 (0.977–1.013) | 0.587 | 1.065 (1.054–1.076) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-D.; Kuo, Y.-W.; Lee, C.-P.; Huang, Y.-C.; Lee, M.; Lee, T.-H. Initial In-Hospital Visit-to-Visit Heart Rate Variability Is Associated with Higher Risk of Atrial Fibrillation in Patients with Acute Ischemic Stroke. J. Clin. Med. 2023, 12, 1050. https://doi.org/10.3390/jcm12031050
Lee J-D, Kuo Y-W, Lee C-P, Huang Y-C, Lee M, Lee T-H. Initial In-Hospital Visit-to-Visit Heart Rate Variability Is Associated with Higher Risk of Atrial Fibrillation in Patients with Acute Ischemic Stroke. Journal of Clinical Medicine. 2023; 12(3):1050. https://doi.org/10.3390/jcm12031050
Chicago/Turabian StyleLee, Jiann-Der, Ya-Wen Kuo, Chuan-Pin Lee, Yen-Chu Huang, Meng Lee, and Tsong-Hai Lee. 2023. "Initial In-Hospital Visit-to-Visit Heart Rate Variability Is Associated with Higher Risk of Atrial Fibrillation in Patients with Acute Ischemic Stroke" Journal of Clinical Medicine 12, no. 3: 1050. https://doi.org/10.3390/jcm12031050
APA StyleLee, J.-D., Kuo, Y.-W., Lee, C.-P., Huang, Y.-C., Lee, M., & Lee, T.-H. (2023). Initial In-Hospital Visit-to-Visit Heart Rate Variability Is Associated with Higher Risk of Atrial Fibrillation in Patients with Acute Ischemic Stroke. Journal of Clinical Medicine, 12(3), 1050. https://doi.org/10.3390/jcm12031050