Implications of Inflammatory and Oxidative Stress Markers in the Attenuation of Nocturnal Blood Pressure Dipping
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Parameters of 24-h ABPM Collection
2.3. Clinical Baseline Variables
2.4. Laboratory Variables. Assessment of Inflammatory Markers
2.5. Evaluation of Redox Status: Divalent Metals, Antioxidant Vitamins, and Lipid and Protein Oxidation. Assessment of Thiobarbituric Acid Reactive Substances (TBARS) and Reduced Thiols
2.6. Ethics Statement
2.7. Statistical Analysis
3. Results
3.1. Univariate Analysis. Comparison of Baseline Variables between Dipper and Non-Dipper Hypertensive Patients
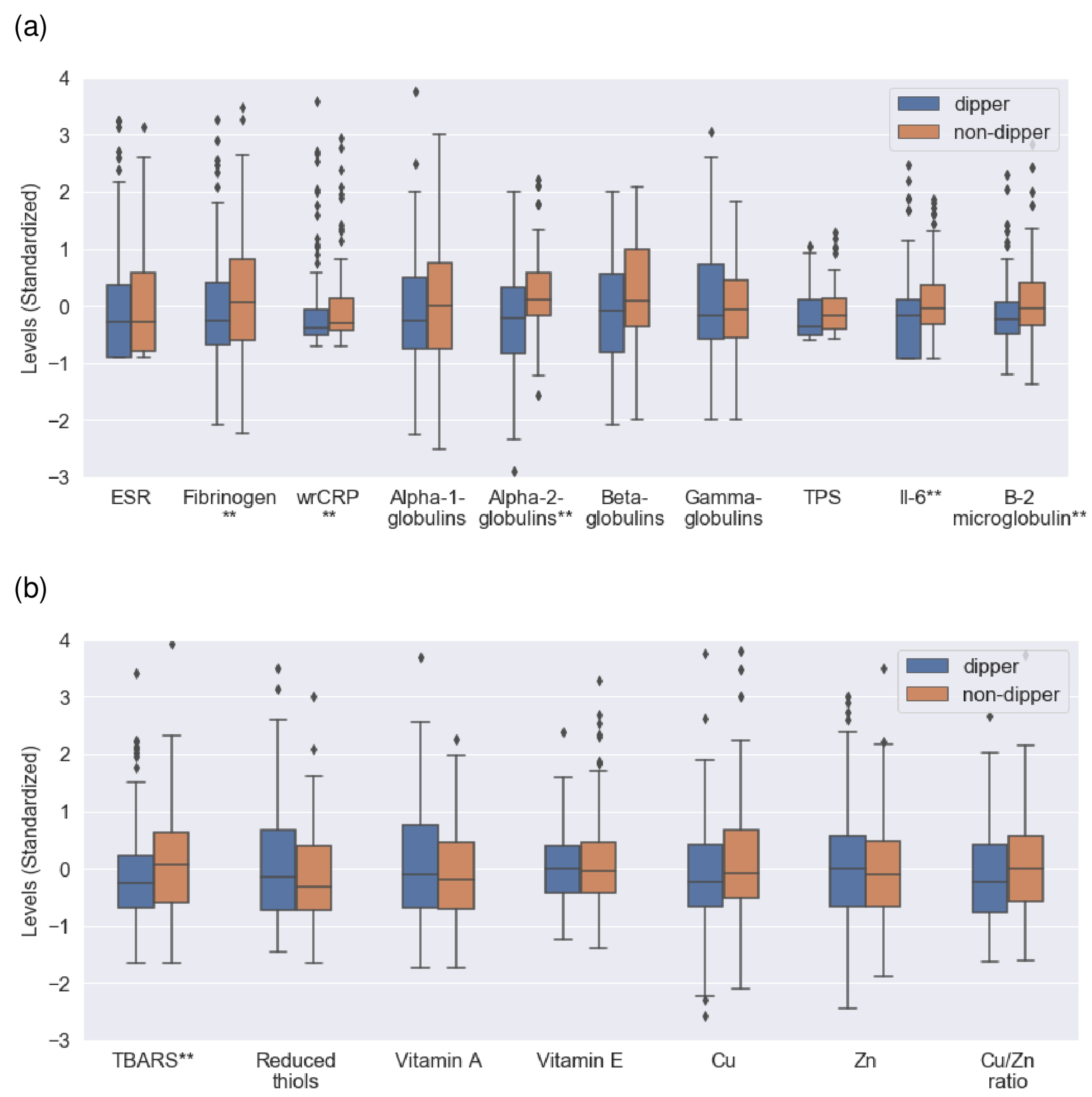
3.2. Univariate Analysis. Comparison of Inflammatory and Redox Markers between Dipper and Non-Dipper Patients
3.3. Multivariate Analysis of the Risk of a Non-Dipper Pattern
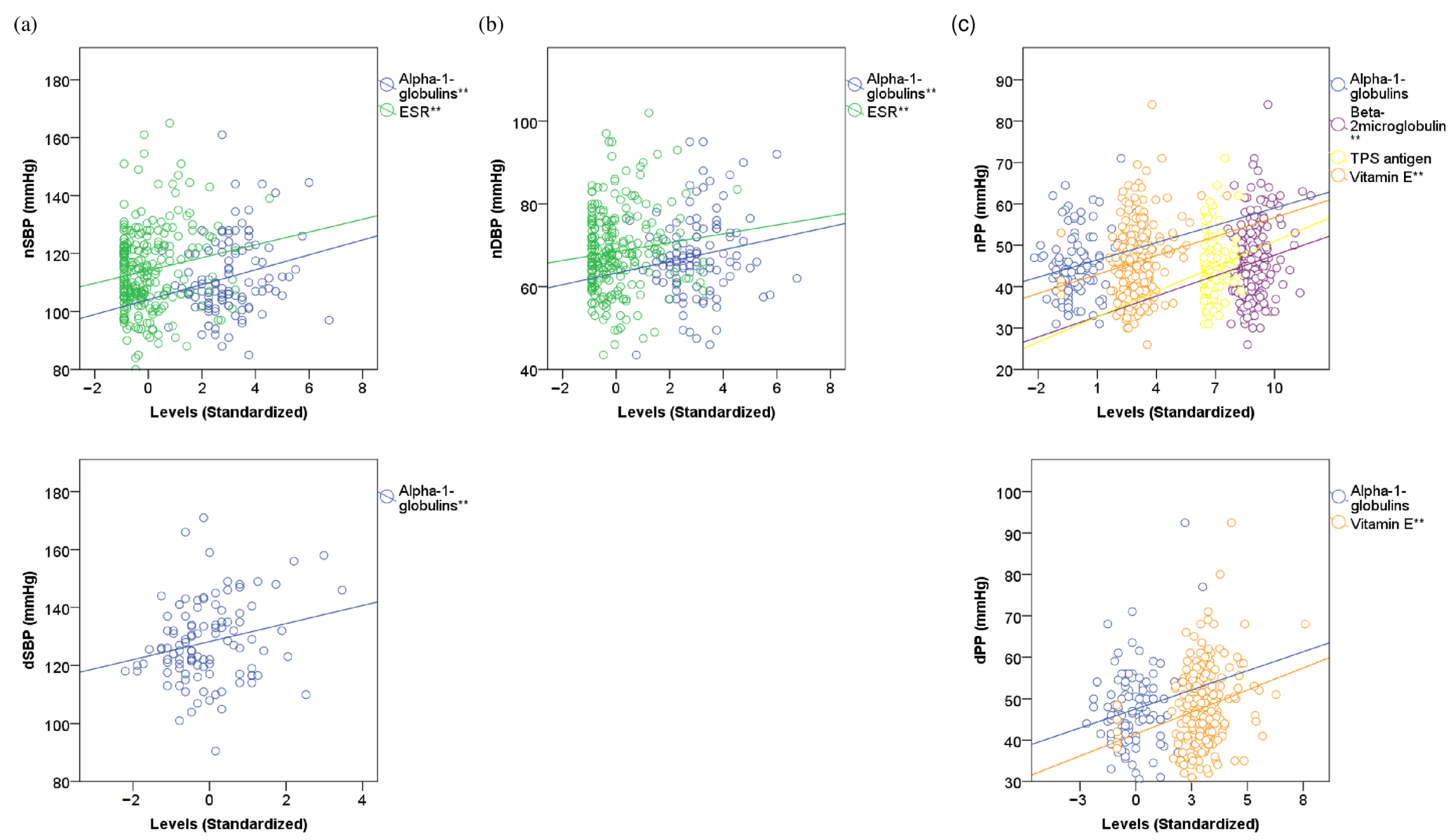
3.4. Linear Correlations of 24-h ABPM Indices with Inflammatory and Redox Markers
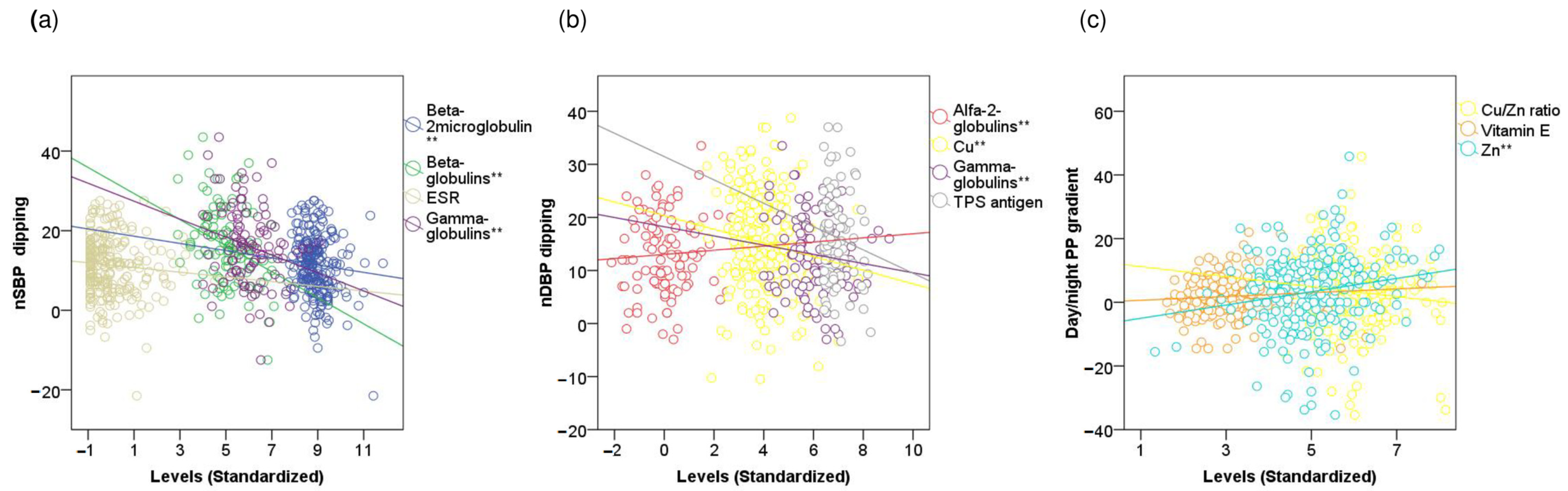
3.5. Linear Correlations of 24-h Day-to-Night BP Indices with Inflammatory and Redox Markers
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaborieau, V.; Delarche, N.; Gosse, P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: Correlation with target organ damage. J. Hypertens. 2008, 26, 1919–1927. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.; Sheridan, J.; O’Malley, K. Dippers and Non-Dippers. Lancet 1988, 332, 397. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.-H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Pré, J. Lipid peroxidation. Pathol. Biol. 1991, 39, 716–736. [Google Scholar]
- Stadtman, E.R.; Berlett, B.S. Reactive Oxygen-Mediated Protein Oxidation in Aging and Disease. Drug Metab. Rev. 1998, 30, 225–243. [Google Scholar] [CrossRef]
- Koekkoek, W.A.C.; van Zanten, A.R.H. Antioxidant Vitamins and Trace Elements in Critical Illness. Nutr. Clin. Pr. 2016, 31, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Mehdi, M.M.; Maurya, P.K.; Rizvi, S.I. Plasma protein oxidation and its correlation with antioxidant potential during human aging. Dis. Markers 2010, 29, 31–36. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.-G. Crosstalk of reactive oxygen species and NF-kappaκB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Hörber, S.; Lehmann, R.; Stefan, N.; Machann, J.; Birkenfeld, A.L.; Wagner, R.; Heni, M.; Häring, H.-U.; Fritsche, A.; Peter, A. Hemostatic alterations linked to body fat distribution, fatty liver, and insulin resistance. Mol. Metab. 2021, 53, 101262. [Google Scholar] [CrossRef]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef]
- Falk, E. Pathogenesis of Atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Fernando, S.; Schwarz, N.; Tan, J.; Bursill, C.; Psaltis, P. Inflammation as a Therapeutic Target in Atherosclerosis. J. Clin. Med. 2019, 8, 1109. [Google Scholar] [CrossRef] [PubMed]
- Konukoglu, D.; Uzun, H. Endothelial Dysfunction and Hypertension. Adv. Exp. Med. Biol. 2017, 956, 511–540. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, G.S.; Palatini, P.; Parati, G.; O’Brien, E.; Januszewicz, A.; Lurbe, E.; Persu, A.; Mancia, G.; Kreutz, R. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J. Hypertens. 2021, 39, 1293–1302. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2022, 46, S19–S40. [Google Scholar] [CrossRef]
- Stevens, P.E.; Levin, A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef]
- Flegal, K.M. Body-mass index and all-cause mortality. Lancet 2017, 389, 2284–2285. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- Ben, J.; Neumann, C.R.; Mengue, S.S. The Brief Medication Questionnaire and Morisky-Green test to evaluate medication adherence. Rev. Saude. Publica. 2012, 46, 279–289. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef]
- Cidoncha Gallego, A.; Pérez Lucena, E.; Vinuesa López, A.; Zaro Bastanzuri, M.J.; Zafra Mezcua, A.; Valencia Roldán, C. El proteinograma en la práctica clínica. Med. Integr. 2001, 38, 127–132. [Google Scholar]
- Alende-Castro, V.; Alonso-Sampedro, M.; Fernández-Merino, C.; Sánchez-Castro, J.; Sopeña, B.; Gude, F.; Gonzalez-Quintela, A. C-Reactive Protein versus Erythrocyte Sedimentation Rate: Implications Among Patients with No Known Inflammatory Conditions. J. Am. Board Fam. Med. 2021, 34, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Stang, L.J.; Mitchell, L.G. Fibrinogen. Methods Mol. Biol. 2013, 992, 181–192. [Google Scholar] [CrossRef]
- Viedma, J.A.; Pacheco, S.; Albaladejo, M.D. Determination of beta 2-microglobulin in serum by a microparticle-enhanced nephelometric immunoassay. Clin. Chem. 1992, 38, 2464–2468. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-Y.; Lan, J.-L.; Lin, F.-J.; Hsieh, T.-Y. Proinflammatory cytokine profiles in sera and pathological tissues of patients with active untreated adult onset Still’s disease. J. Rheumatol. 2004, 31, 2189–2198. [Google Scholar]
- Gonzalez-Quintela, A.; Tome, S.; Fernandez-Merino, C.; Rey, J.; Meijide, L.; Gude, F. Synergistic Effect of Alcohol Consumption and Body Mass on Serum Concentrations of Cytokeratin-18. Alcohol. Clin. Exp. Res. 2011, 35, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M.; De Toni, S.; Sanzari, M.C.; Bernardi, D.; Stockreiter, E. The TEST 1 automated system: A new method for measuring the erythrocyte sedimentation rate. Am. J. Clin. Pathol. 1998, 110, 334–340. [Google Scholar] [CrossRef]
- Arnaud, J.; Chappuis, P.; Zawislak, R.; Jaudon, M.C.; Bellanger, J. [Determination of trace elements by an assay using flameless atomic absorption spectrometry]. Ann. de Biol. Clin. 1989, 47, 583–595. [Google Scholar]
- Márquez, M.; Yépez, C.E.; Sútil-Naranjo, R.; Rincón, M. Aspectos básicos y determinación de las vitaminas antioxidantes E. Investig. Clínica. 2002, 43, 191–204. [Google Scholar]
- Wasowicz, W.; Nève, J.; Peretz, A. Optimized steps in fluorometric determination of thiobarbituric acid-reactive substances in serum: Importance of extraction pH and influence of sample preservation and storage. Clin. Chem. 1993, 39, 2522–2526. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Hoving, E.B.; Laing, C.; Rutgers, H.M.; Teggeler, M.; Van Doormaal, J.J.; Muskiet, F.A. Optimized determination of malondialdehyde in plasma lipid extracts using 1,3-diethyl-2-thiobarbituric acid: Influence of detection method and relations with lipids and fatty acids in plasma from healthy adults. Clin. Chim. Acta 1992, 208, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, V.B.; Pavićević, I.D.; Takić, M.M.; Penezić-Romanjuk, A.Z.; Aćimović, J.M.; Mandić, L.M. The influence of fatty acids on determination of human serum albumin thiol group. Anal. Biochem. 2014, 448, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Geovanini, G.R.; Libby, P. Atherosclerosis and inflammation: Overview and updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Winther, S.V.; Ahmed, D.; Al-Shuweli, S.; Landt, E.M.; Nordestgaard, B.G.; Seersholm, N.; Dahl, M. Severe α1-antitrypsin deficiency associated with lower blood pressure and reduced risk of ischemic heart disease: A cohort study of 91,540 individuals and a meta-analysis. Respir. Res. 2022, 23, 1–8. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, H.; Shi, F.; Li, M.; Liu, X.; Ji, C.; Han, Y. Serum Ceruloplasmin Is the Candidate Predictive Biomarker for Acute Aortic Dissection and Is Related to Thrombosed False Lumen: A Propensity Score–Matched Observational Case–Control Study. Biol. Trace Element Res. 2020, 199, 895–911. [Google Scholar] [CrossRef] [PubMed]
- Rickli, C.; Borato, D.C.K.; Silva, A.J.D.M.E.; Schuinski, A.F.M.; Vilela, G.H.F.; Vellosa, J.C.R. Serum Myeloperoxidase, C-reactive Protein and α1-acid Glycoprotein: Insights about Cardiovascular Risk in End-stage Renal Disease. Exp. Clin. Endocrinol. Diabetes 2019, 128, 731–736. [Google Scholar] [CrossRef]
- Si, Q.-J.; Wang, Y.-T.; Wang, H.-J.; Shi, Y.; Guo, Y.; Li, Y. The prognostic values of beta-2 microglobulin for risks of cardiovascular events and mortality in the elderly patients with isolated systolic hypertension. J. Res. Med Sci. 2018, 23, 82. [Google Scholar] [CrossRef]
- Keefe, J.A.; Hwang, S.-J.; Huan, T.; Mendelson, M.; Yao, C.; Courchesne, P.; Saleh, M.A.; Madhur, M.S.; Levy, D. Evidence for a Causal Role of the SH2B3 -β 2 M Axis in Blood Pressure Regulation. Hypertension 2019, 73, 497–503. [Google Scholar] [CrossRef]
- Vazquez-Agra, N.; Marques-Afonso, A.-T.; Cruces-Sande, A.; Novo-Veleiro, I.; Lopez-Paz, J.-E.; Pose-Reino, A.; Hermida-Ameijeiras, A. Are Differences in Inflammatory Markers between Patients with and without Hypertension-Mediated Organ Damage Influenced by Circadian Blood Pressure Abnormalities? J. Clin. Med. 2022, 11, 1252. [Google Scholar] [CrossRef]
- Surma, S.; Banach, M. Fibrinogen and Atherosclerotic Cardiovascular Diseases—Review of the Literature and Clinical Studies. Int. J. Mol. Sci. 2021, 23, 193. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.R.; Vinh, A.; Guzik, T.J.; Sobey, C.G. Immune mechanisms of hypertension. Nat. Rev. Immunol. 2019, 19, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Li, H.; Gu, Y.; Song, Y.; Zhang, Q.; Liu, L.; Meng, G.; Yao, Z.; Wu, H.; et al. Relationship of serum immunoglobulin levels to blood pressure and hypertension in an adult population. J. Hum. Hypertens. 2018, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Dhas, Y.; Banerjee, J.; Mishra, N. Blood Viscosity, Glycemic Markers and Blood Pressure: A Study in Middle-Aged Normotensive and Hypertensive Type 2 Diabetics. Indian J. Clin. Biochem. 2018, 35, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Conca, P.; Coppola, A.; Vecchione, R.; Di Minno, G. Serum concentrations of the tissue polypeptide specific antigen in patients suffering from non-alcoholic steatohepatitis. Eur. J. Clin. Investig. 2006, 37, 48–53. [Google Scholar] [CrossRef]
- Tofas, T.; Fatouros, I.; Draganidis, D.; Deli, C.; Chatzinikolaou, A.; Tziortzis, C.; Panayiotou, G.; Koutedakis, Y.; Jamurtas, A. Effects of Cardiovascular, Resistance and Combined Exercise Training on Cardiovascular, Performance and Blood Redox Parameters in Coronary Artery Disease Patients: An 8-Month Training-Detraining Randomized Intervention. Antioxidants 2021, 10, 409. [Google Scholar] [CrossRef]
- Pawluk, H.; Pawluk, R.; Robaczewska, J.; Kędziora-Kornatowska, K.; Kędziora, J. Biomarkers of antioxidant status and lipid peroxidation in elderly patients with hypertension. Redox Rep. 2017, 22, 542–546. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Xu, X.; Li, L.; Liang, N.; Zhang, L.; Lv, J.; Wu, Y.-C.; Yin, H. Ferroptosis and cardiovascular disease: Role of free radical-induced lipid peroxidation. Free. Radic. Res. 2021, 55, 405–415. [Google Scholar] [CrossRef]
- Brewer, G.J. Iron and copper toxicity in diseases of aging, particularly atherosclerosis and Alzheimer’s disease. Exp. Biol. Med. 2007, 232, 323–335. [Google Scholar]
- Núñez, C.T.; Pérez, E.F.; Álvarez, S.; Rodríguez, M.M. Relation of nocturnal blood pressure dipping to pulse pressure, inflamation and thrombosis. 2006, 127, 676–677. [CrossRef]
- Villalón-García, I.; Álvarez-Córdoba, M.; Povea-Cabello, S.; Talaverón-Rey, M.; Villanueva-Paz, M.; Luzón-Hidalgo, R.; Suárez-Rivero, J.M.; Suárez-Carrillo, A.; Munuera-Cabeza, M.; Salas, J.J.; et al. Vitamin E prevents lipid peroxidation and iron accumulation in PLA2G6-Associated Neurodegeneration. Neurobiol. Dis. 2022, 165, 105649. [Google Scholar] [CrossRef] [PubMed]
- Amar, J.; Chamontin, B. Cardiovascular Risk Factors, Atherosclerosis and Pulse Pressure. Adv. Cardiol. 2006, 44, 212–222. [Google Scholar] [CrossRef]
- Walter, M.F.; Jacob, R.F.; Jeffers, B.; Ghadanfar, M.M.; Preston, G.M.; Buch, J.; Mason, R.P. Serum levels of thiobarbituric acid reactive substances predict cardiovascular events in patients with stable coronary artery disease: A longitudinal analysis of the PREVENT study. J. Am. Coll. Cardiol. 2004, 44, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
| Variables | Groups a | p-Value | |
|---|---|---|---|
| Dipper (n = 135) | Non-Dipper (n = 112) | ||
| Age (years) † | 54 (46–61) | 58 (49–66) | <0.001 |
| Sex (women) ‡ | 81 (60) | 57 (51) | 0.191 |
| Alcohol intake b‡ | 18 (13) | 28 (25) | 0.029 |
| Former smokers ‡ | 54 (40) | 38 (34) | 0.395 |
| Physical activity ‡ | 58 (43) | 46 (41) | 0.796 |
| BMI (kg/m2) † | 28 (25–31) | 30 (26–34) | 0.018 |
| WC (cm) † | 100 (91–108) | 105 (97–112) | 0.024 |
| RAAS blockers ‡ | 72 (53) | 68 (61) | 0.300 |
| Diuretics ‡ | 27 (20) | 41 (37) | 0.006 |
| CCBs ‡ | 51(40) | 49 (45) | 0.515 |
| B-blockers ‡ | 21 (16) | 19 (17) | 0.900 |
| Compliant patients ‡ | 109 (81) | 90 (80) | 0.999 |
| FPG (mg/dL) † | 98 (89–106) | 101 (92–109) | 0.097 |
| Creatinine (mg/dL) † | 0.80 (0.65–0.95) | 0.82 (0.72–0.92) | 0.259 |
| Uric acid (mg/dL) † | 4.8 (3.6–5.9) | 5.2 (4.1–6.2) | 0.106 |
| Total proteins (g/dL) † | 7.2 (6.9–7.5) | 7.2 (6.9–7.5) | 0.294 |
| TG (mg/dL) † | 92 (58–125) | 92 (60–124) | 0.170 |
| TC (mg/dL) † | 188 (163–212) | 186 (165–207) | 0.623 |
| Variables | Groups a | p-Value | |
|---|---|---|---|
| Dipper (n = 135) | Non-Dipper (n = 112) | ||
| 24-hSBP (mmHg) † | 125 (115–135) | 125 (116–133) | 0.978 |
| dSBP (mmHg) † | 129 (118–139) | 127 (118–135) | 0.171 |
| nSBP (mmHg) † | 107 (99–115) | 120 (111–128) | <0.001 |
| 24-hDBP (mmHg) † | 80 (73–86) | 75 (68–82) | 0.024 |
| dDBP (mmHg) † | 83 (76–90) | 77 (70–84) | <0.001 |
| nDBP (mmHg) † | 66 (60–71) | 70 (63–76) | <0.001 |
| 24-hPP (mmHg) † | 45 (40–50) | 48 (41–54) | 0.005 |
| dPP (mmHg) † | 46 (40–52) | 48 (41–54) | 0.163 |
| nPP (mmHg) † | 43 (38–47) | 49 (42–55) | <0.001 |
| nSBP dipping (mmHg) † | 20 (14–25) | 8 (4–11) | <0.001 |
| nSBP dipping (%) † | 15 (11–18) | 6 (3–9) | <0.001 |
| nDBP dipping (mmHg) † | 17 (12–21) | 7 (4–10) | <0.001 |
| nDBP dipping (%) † | 20 (15–25) | 9 (5–12) | <0.001 |
| Variables a,b | B | p -Value | Exp(B) | 95%CI | |
|---|---|---|---|---|---|
| Inferior | Superior | ||||
| Age (>63.5 years) c | 0.797 | 0.029 | 2.219 | 1.086 | 4.536 |
| BMI (>33 Kg/m2) c | 1.775 | <0.001 | 5.901 | 2.178 | 15.986 |
| Alcohol consumption (yes) | 0.937 | 0.022 | 2.553 | 1.146 | 5.688 |
| TPS antigen (>183 U/L) c | 1.016 | 0.004 | 2.763 | 1.391 | 5.487 |
| Fibrinogen (>290 mg/dL) c | 0.806 | 0.016 | 2.238 | 1.160 | 4.320 |
| Beta-2-microglobulin (>1.74 mg/dL) c | 0.659 | 0.041 | 1.932 | 1.027 | 3.637 |
| TBARS (>3.2 nmol/mg TC) c | 0.671 | 0.034 | 1.955 | 1.051 | 3.638 |
| Cu/Zn ratio (>1.06) c | 1.274 | 0.001 | 3.576 | 1.743 | 7.339 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermida-Ameijeiras, A.; Vazquez-Agra, N.; Cruces-Sande, A.; Mendez-Alvarez, E.; Soto-Otero, R.; Lopez-Paz, J.-E.; Pose-Reino, A.; Gonzalez-Quintela, A. Implications of Inflammatory and Oxidative Stress Markers in the Attenuation of Nocturnal Blood Pressure Dipping. J. Clin. Med. 2023, 12, 1643. https://doi.org/10.3390/jcm12041643
Hermida-Ameijeiras A, Vazquez-Agra N, Cruces-Sande A, Mendez-Alvarez E, Soto-Otero R, Lopez-Paz J-E, Pose-Reino A, Gonzalez-Quintela A. Implications of Inflammatory and Oxidative Stress Markers in the Attenuation of Nocturnal Blood Pressure Dipping. Journal of Clinical Medicine. 2023; 12(4):1643. https://doi.org/10.3390/jcm12041643
Chicago/Turabian StyleHermida-Ameijeiras, Alvaro, Nestor Vazquez-Agra, Anton Cruces-Sande, Estefania Mendez-Alvarez, Ramon Soto-Otero, Jose-Enrique Lopez-Paz, Antonio Pose-Reino, and Arturo Gonzalez-Quintela. 2023. "Implications of Inflammatory and Oxidative Stress Markers in the Attenuation of Nocturnal Blood Pressure Dipping" Journal of Clinical Medicine 12, no. 4: 1643. https://doi.org/10.3390/jcm12041643
APA StyleHermida-Ameijeiras, A., Vazquez-Agra, N., Cruces-Sande, A., Mendez-Alvarez, E., Soto-Otero, R., Lopez-Paz, J.-E., Pose-Reino, A., & Gonzalez-Quintela, A. (2023). Implications of Inflammatory and Oxidative Stress Markers in the Attenuation of Nocturnal Blood Pressure Dipping. Journal of Clinical Medicine, 12(4), 1643. https://doi.org/10.3390/jcm12041643








