The Belgian Diabetes in Pregnancy Follow-Up Study (BEDIP-FUS): A Multi-Centric Prospective Cohort Study on the Long-Term Metabolic Risk across Different Degrees of Gestational Glucose Intolerance: Methodology and Design
Abstract
1. Introduction
2. Aim and Objectives of the BEDIP-FUS Study
- The prevalence of glucose intolerance (T2D and prediabetes);
- The prevalence of overweight and obesity;
- The degree of adiposity;
- The prevalence of metabolic syndrome;
- The degree of insulin resistance;
- The degree of β-cell dysfunction;
- Glycated CD59 as a predictor of metabolic dysfunction;
- Sex differences in metabolic profile in the offspring.
3. Methods and Analysis
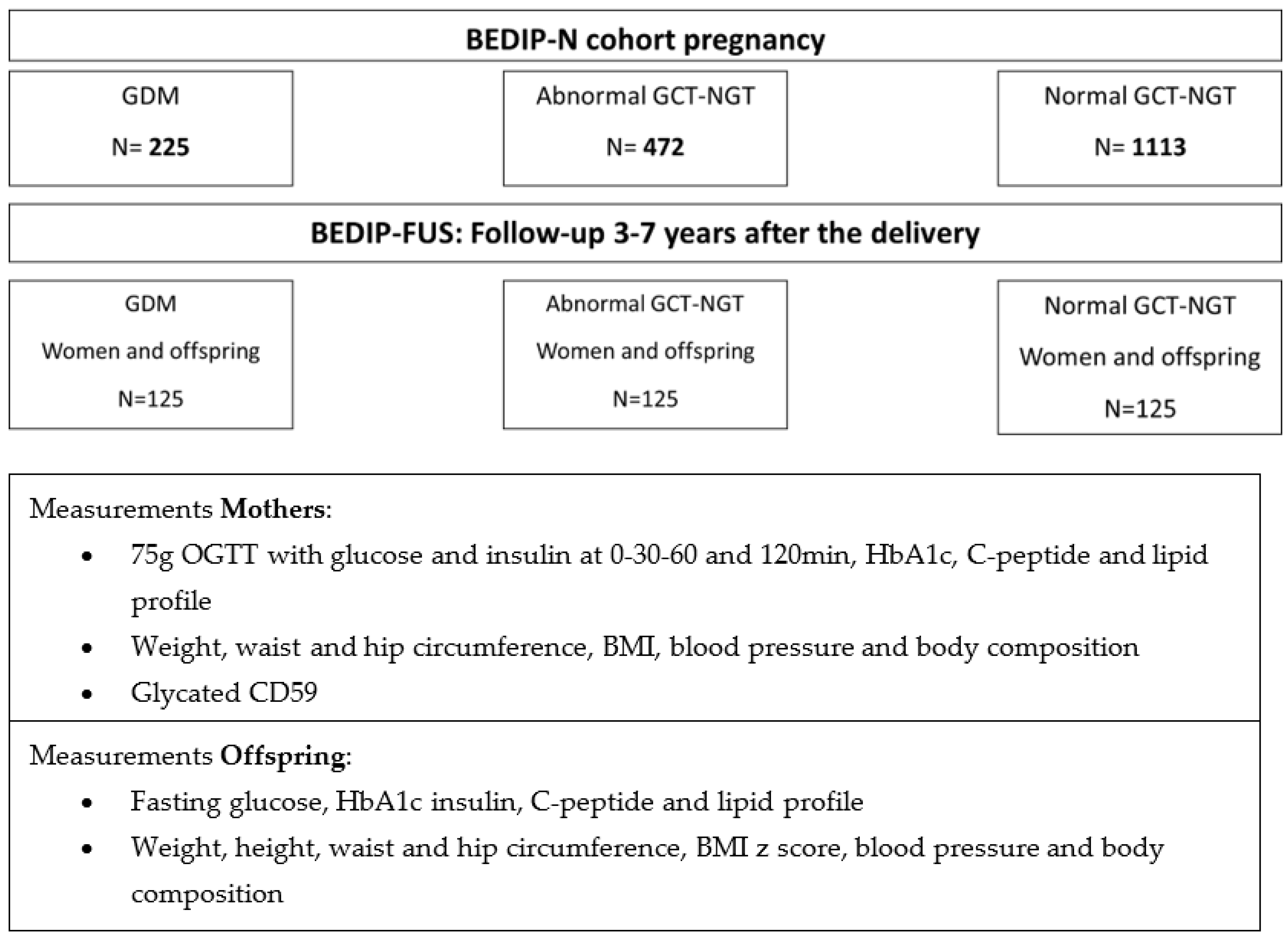
3.1. Study Design and Setting
3.2. Recruitment and Eligibility
- Age ≥ 18 years;
- Participated in the completed BEDIP-N study and received both the GCT and the OGTT during pregnancy;
- Born at the time of participation in the BEDIP-N study.
- Biological father of the child born during participation in the BEDIP-N study.
- Current pregnancy;
- Current treatment that influences glycemic status, such as high dose corticoids or incretin mimetic medication;
- History of bariatric surgery or any gastrointestinal surgery that alters glucose absorption (Billroth II);
- Inability to complete a normal study visit (incompliance, psychiatric problems);
- Diagnosis of type 1 diabetes or the presence of autoimmune antibodies for type 1 diabetes.
- Current treatment that influences glycemic status, such as high dose corticoids;
- Inability to complete a normal study visit (incompliance, psychiatric problems);
- Diagnosis of type 1 diabetes or the presence of autoimmune antibodies for type 1 diabetes.
3.3. Study Visit
3.3.1. Lab Measurements
3.3.2. Clinical Measurements
3.3.3. Self-Administered Questionnaires
- Self-designed questionnaire on general habits (including smoking and alcohol habits) and socioeconomic factors, as used in the BEDIP-N study [22];
- The Frequency Food Questionnaire (FFQ) validated for the Belgian population [24] to assess food and nutrition intake. This is a questionnaire that contains questions on portion and frequency of food and beverage consumption;
- The international questionnaire on physical activity (IPAQ), validated for use in the Belgian population and as used in the BEDIP-N study for the mothers [22,25]. This is a questionnaire measuring different areas of physical activity such as job-related physical activity, transportation, housework and caring for family, recreation and time spent sitting. We added a question on time watching television or playing computer games to better assess sedentary behavior;
- The validated 20-item Center for Epidemiologic Studies Depression Scale (CES-D) questionnaire [26] to assess symptoms of depression in the past 7 days;
- A self-designed questionnaire on breastfeeding and contraception as used in the BEDIP-N study [22];
- The validated Diabetes Risk Perception questionnaire [28];
- The validated Treatment Self-Regulation Questionnaire (TSRQ) to evaluate motivation for lifestyle change [29];
- The validated short version STAI-6 to measure anxiety [30];
- The validated Pittsburgh Sleep Quality Index to evaluate sleep quality as a risk factor for the development of glucose intolerance [31].
- Self-designed questionnaire on general habits (including smoking and alcohol habits) and socioeconomic factors as used in the BEDIP-N study [22] including data on paternal weight and BMI over time.
3.4. Outcomes of the Study
3.4.1. Primary outcome
3.4.2. Secondary Outcomes
- Overweight and obesity defined by BMI z-score according to the WHO guidelines (resp. score >1 and >2) [23];
- Prediabetes and diabetes based on the fasting plasma glucose defined by the ADA criteria [1];
- Metabolic syndrome based on the WHO criteria [35];
- Insulin sensitivity by 1/HOMA-IR [37];
- Beta-cell function by the HOMA-B index [22];
- Adiposity measured by BIA and measured by skin folds [41];
- Weight and growth trajectory;
- Plasma glycated CD59 as a predictor of metabolic risk [11];
- Sex differences in metabolic profile in the offspring.
3.5. Collection of Data from the Medical Electronical Records
3.6. Power Calculation and Statistical Analyses
3.6.1. Sample Size
3.6.2. Statistical Analyses
3.7. Quality Control Procedures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013, 36, S11–S66. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Benhalima, K.; Lens, K.; Bosteels, J.; Chantal, M. The Risk for Glucose Intolerance after Gestational Diabetes Mellitus since the Introduction of the IADPSG Criteria: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1431. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Swaminathan, B.; Hanley, A.J.; Connelly, P.W.; Sermer, M.; Zinman, B.; Retnakaran, R. Each degree of glucose intolerance in pregnancy predicts distinct trajectories of beta-cell function, insulin sensitivity, and glycemia in the first 3 years postpartum. Diabetes Care 2014, 37, 3262–3269. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Santaguida, P.; Raina, P.; Morrison, K.M.; Balion, C.; Hunt, D.; Yazdi, H.; Booker, L. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: A systematic overview and meta-analysis of prospective studies. Diabetes Res. Clin. Pract. 2007, 78, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shen, S.; Sun, L.; Yang, H.; Jin, B.; Cao, X. Metabolic syndrome risk after gestational diabetes: A systematic review and meta-analysis. PLoS ONE 2014, 9, e87863. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Shah, B.R. Role of Type 2 Diabetes in Determining Retinal, Renal, and Cardiovascular Outcomes in Women with Previous Gestational Diabetes Mellitus. Diabetes Care 2017, 40, 101–108. [Google Scholar] [CrossRef]
- O’Reilly, M.W.; Avalos, G.; Dennedy, M.C.; O’Sullivan, E.P.; Dunne, F. Atlantic DIP: High prevalence of abnormal glucose tolerance post partum is reduced by breast-feeding in women with prior gestational diabetes mellitus. Eur. J. Endocrinol. 2011, 165, 953–959. [Google Scholar] [CrossRef]
- Noctor, E.; Crowe, C.; Carmody, L.A.; Avalos, G.M.; Kirwan, B.; Infanti, J.J.; O’Dea, A.; Gillespie, P.; Newell, J.; McGuire, B.; et al. ATLANTIC DIP: Simplifying the follow-up of women with previous gestational diabetes. Eur. J. Endocrinol. 2013, 169, 681–687. [Google Scholar] [CrossRef]
- Benhalima, K.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E.; De Block, C.; et al. A modified two-step screening strategy for gestational diabetes mellitus based on the 2013 WHO criteria by combining the glucose challenge test and clinical risk factors. J. Clin. Med. 2018, 7, 351. [Google Scholar] [CrossRef]
- Ghosh, P.; Vaidya, A.; Sahoo, R.; Goldfine, A.; Herring, N.; Bry, L.; Chorev, M.; Halperin, J.A. Glycation of the complement regulatory protein CD59 is a novel biomarker for glucose handling in humans. J. Clin. Endocrinol. Metab. 2014, 99, E999–E1006. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Luque-Fernandez, M.A.; Vaidya, A.; Ma, D.; Sahoo, R.; Chorev, M.; Zera, C.; McElrath, T.F.; Williams, M.A.; Seely, E.W.; et al. Plasma Glycated CD59, a Novel Biomarker for Detection of Pregnancy-Induced Glucose Intolerance. Diabetes Care 2017, 40, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Benhalima, K.; Ma, D.D.; Laenen, A.; Mathieu, C.; Halperin, J.A. Plasma glycated CD59 predicts postpartum glucose intolerance after gestational diabetes. Eur. J. Endocrinol. 2021, 185, 755–763. [Google Scholar] [CrossRef]
- Nijs, H.; Benhalima, K. Gestational Diabetes Mellitus and the Long-Term Risk for Glucose Intolerance and Overweight in the Offspring: A Narrative Review. J. Clin. Med. 2020, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Grunnet, L.G.; Hansen, S.; Hjort, L.; Madsen, C.M.; Kampmann, F.B.; Thuesen, A.C.B.; Granstrømi, C.; Strøm, M.; Maslova, E.; Frikke-Schmidt, R.; et al. Adiposity, Dysmetabolic Traits, and Earlier Onset of Female Puberty in Adolescent Offspring of Women with Gestational Diabetes Mellitus: A Clinical Study within the Danish National Birth Cohort. Diabetes Care 2017, 40, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Donovan, L.E.; Cundy, T. Does exposure to hyperglycaemia in utero increase the risk of obesity and diabetes in the offspring? A critical reappraisal. Diabet. Med. J. Br. Diabet. Assoc. 2015, 32, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Philipps, L.H.; Santhakumaran, S.; Gale, C.; Prior, E.; Logan, K.M.; Hyde, M.J.; Modi, N. The diabetic pregnancy and offspring BMI in childhood: A systematic review and meta-analysis. Diabetologia 2011, 54, 1957–1966. [Google Scholar] [CrossRef]
- Lowe, W.L., Jr.; Scholtens, D.M.; Lowe, L.P.; Kuang, A.; Nodzenski, M.; Talbot, O.; Catalano, P.M.; Linder, B.; Brickman, W.J.; Clayton, P.; et al. Association of Gestational Diabetes with Maternal Disorders of Glucose Metabolism and Childhood Adiposity. JAMA 2018, 320, 1005–1016. [Google Scholar] [CrossRef]
- Scholtens, D.M.; Kuang, A.; Lowe, L.P.; Hamilton, J.; Lawrence, J.M.; Lebenthal, Y.; Brickman, W.J.; Clayton, P.; Ma, R.C.; McCance, D.; et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Glycemia and Childhood Glucose Metabolism. Diabetes Care 2019, 42, 381–392. [Google Scholar] [CrossRef]
- Benhalima, K.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E.; De Block, C.; et al. The Sensitivity and Specificity of the Glucose Challenge Test in a Universal Two-Step Screening Strategy for Gestational Diabetes Mellitus Using the 2013 World Health Organization Criteria. Diabetes Care 2018, 41, e111–e112. [Google Scholar] [CrossRef]
- Benhalima, K.; Jegers, K.; Devlieger, R.; Verhaeghe, J.; Mathieu, C. Glucose Intolerance after a Recent History of Gestational Diabetes Based on the 2013 WHO Criteria. PLoS ONE 2016, 11, e0157272. [Google Scholar] [CrossRef] [PubMed]
- Benhalima, K.; Van Crombrugge, P.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Dufraimont, E.; De Block, C.; Jacquemyn, Y.; Mekahli, F.; et al. The Belgian Diabetes in Pregnancy Study (BEDIP-N), a multi-centric prospective cohort study on screening for diabetes in pregnancy and gestational diabetes: Methodology and design. BMC Pregnancy Childbirth 2014, 14, 226. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef]
- Matthys, C.; Meulemans, A.; Van Der Schueren, B. Development and validation of general FFQ for use in clinical practice. Ann. Nutr. Metab. 2015, 67, 239. [Google Scholar]
- Harrison, C.L.; Thompson, R.G.; Teede, H.J.; Lombard, C.B. Measuring physical activity during pregnancy. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 19. [Google Scholar] [CrossRef]
- Dalfra, M.G.; Nicolucci, A.; Bisson, T.; Bonsembiante, B.; Lapolla, A. Quality of life in pregnancy and post-partum: A study in diabetic patients. Qual. Life Res. 2012, 21, 291–298. [Google Scholar] [CrossRef]
- Petrou, S.; Morrell, J.; Spiby, H. Assessing the empirical validity of alternative multi-attribute utility measures in the maternity context. Health Qual. Life Outcomes 2009, 7, 40. [Google Scholar] [CrossRef]
- Kim, C.; McEwen, L.N.; Piette, J.D.; Goewey, J.; Ferrara, A.; Walker, E.A. Risk perception for diabetes among women with histories of gestational diabetes mellitus. Diabetes Care 2007, 30, 2281–2286. [Google Scholar] [CrossRef]
- Shigaki, C.; Kruse, R.L.; Mehr, D.; Sheldon, K.M.; Bin, G.; Moore, C.; Lemaster, J. Motivation and diabetes self-management. Chronic Illn. 2010, 6, 202–214. [Google Scholar] [CrossRef]
- Bogaerts, A.; Ameye, L.; Bijlholt, M.; Amuli, K.; Heynickx, D.; Devlieger, R. INTER-ACT: Prevention of pregnancy complications through an e-health driven interpregnancy lifestyle intervention—Study protocol of a multicentre randomised controlled trial. BMC Pregnancy Childbirth 2017, 17, 154. [Google Scholar] [CrossRef]
- Ferrari, U.; Kunzel, H.; Trondle, K.; Rottenkolber, M.; Kohn, D.; Fugmann, M.; Banning, F.; Weise, M.; Sacco, V.; Hasbargen, U.; et al. Poor sleep quality is associated with impaired glucose tolerance in women after gestational diabetes. J. Psychiatr. Res. 2015, 65, 166–171. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, S.D.B.G.; De Henauw, S.; Huybrechts, I.; Kornitzer, K.; Leveque, A.; Moreau, M.; Van Oyen, H. The Belgian food consumption survey: Aims, design and methods. Arch. Public Health 2005, 63, 1–16. [Google Scholar]
- Bervoets, L.; Van Noten, C.; Van Roosbroeck, S.; Hansen, D.; Van Hoorenbeeck, K.; Verheyen, E.; Van Hal, G.; Vankerckhoven, V. Reliability and Validity of the Dutch Physical Activity Questionnaires for Children (PAQ-C) and Adolescents (PAQ-A). Arch. Public Health 2014, 72, 47. [Google Scholar] [CrossRef]
- Raat, H.; Bonsel, G.J.; Essink-Bot, M.L.; Landgraf, J.M.; Gemke, R.J. Reliability and validity of comprehensive health status measures in children: The Child Health Questionnaire in relation to the Health Utilities Index. J. Clin. Epidemiol. 2002, 55, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C. Definition of metabolic syndrome: Report of the National Heart, Lung and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Kahn, S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003, 46, 3–19. [Google Scholar] [CrossRef]
- Retnakaran, R.; Qi, Y.; Goran, M.I.; Hamilton, J.K. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet. Med. J. Br. Diabet. Assoc. 2009, 26, 1198–1203. [Google Scholar] [CrossRef]
- Stumvoll, M.; Van Haeften, T.; Fritsche, A.; Gerich, J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care 2001, 24, 796–797. [Google Scholar] [CrossRef]
- Nightingale, C.M.; Rudnicka, A.R.; Owen, C.G.; Donin, A.S.; Newton, S.L.; Furness, C.A.; Howard, E.L.; Gillings, R.D.; Wells, J.C.K.; Cook, D.; et al. Are ethnic and gender specific equations needed to derive fat free mass from bioelectrical impedance in children of South Asian, black African-Caribbean and white European origin? Results of the assessment of body composition in children study. PLoS ONE 2013, 8, e76426. [Google Scholar] [CrossRef] [PubMed]
- Gillman, M.W.; Oakey, H.; Baghurst, P.A.; Volkmer, R.E.; Robinson, J.S.; Crowther, C.A. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care 2010, 33, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Landon, M.B.; Rice, M.M.; Varner, M.W.; Casey, B.M.; Reddy, U.M.; Wapner, R.J.; Rouse, D.J.; Biggio, J.J.R.; Thorp, J.M.; Chien, E.K.; et al. Mild gestational diabetes mellitus and long-term child health. Diabetes Care 2015, 38, 445–452. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raets, L.; Van Hoorenbeeck, K.; Maes, T.; Vercammen, C.; De Block, C.; Dirinck, E.; Van Pottelbergh, I.; Wierckx, K.; Laenen, A.; Bogaerts, A.; et al. The Belgian Diabetes in Pregnancy Follow-Up Study (BEDIP-FUS): A Multi-Centric Prospective Cohort Study on the Long-Term Metabolic Risk across Different Degrees of Gestational Glucose Intolerance: Methodology and Design. J. Clin. Med. 2023, 12, 1025. https://doi.org/10.3390/jcm12031025
Raets L, Van Hoorenbeeck K, Maes T, Vercammen C, De Block C, Dirinck E, Van Pottelbergh I, Wierckx K, Laenen A, Bogaerts A, et al. The Belgian Diabetes in Pregnancy Follow-Up Study (BEDIP-FUS): A Multi-Centric Prospective Cohort Study on the Long-Term Metabolic Risk across Different Degrees of Gestational Glucose Intolerance: Methodology and Design. Journal of Clinical Medicine. 2023; 12(3):1025. https://doi.org/10.3390/jcm12031025
Chicago/Turabian StyleRaets, Lore, Kim Van Hoorenbeeck, Toon Maes, Chris Vercammen, Christophe De Block, Eveline Dirinck, Inge Van Pottelbergh, Katrien Wierckx, Annouschka Laenen, Annick Bogaerts, and et al. 2023. "The Belgian Diabetes in Pregnancy Follow-Up Study (BEDIP-FUS): A Multi-Centric Prospective Cohort Study on the Long-Term Metabolic Risk across Different Degrees of Gestational Glucose Intolerance: Methodology and Design" Journal of Clinical Medicine 12, no. 3: 1025. https://doi.org/10.3390/jcm12031025
APA StyleRaets, L., Van Hoorenbeeck, K., Maes, T., Vercammen, C., De Block, C., Dirinck, E., Van Pottelbergh, I., Wierckx, K., Laenen, A., Bogaerts, A., Mathieu, C., & Benhalima, K. (2023). The Belgian Diabetes in Pregnancy Follow-Up Study (BEDIP-FUS): A Multi-Centric Prospective Cohort Study on the Long-Term Metabolic Risk across Different Degrees of Gestational Glucose Intolerance: Methodology and Design. Journal of Clinical Medicine, 12(3), 1025. https://doi.org/10.3390/jcm12031025






