Clinically Manifest Infections Do Not Increase the Relapse Risk in People with Multiple Sclerosis Treated with Disease-Modifying Therapies: A Prospective Study †
Abstract
1. Introduction
2. Materials and Methods
Statistical Methods
3. Results
3.1. Population
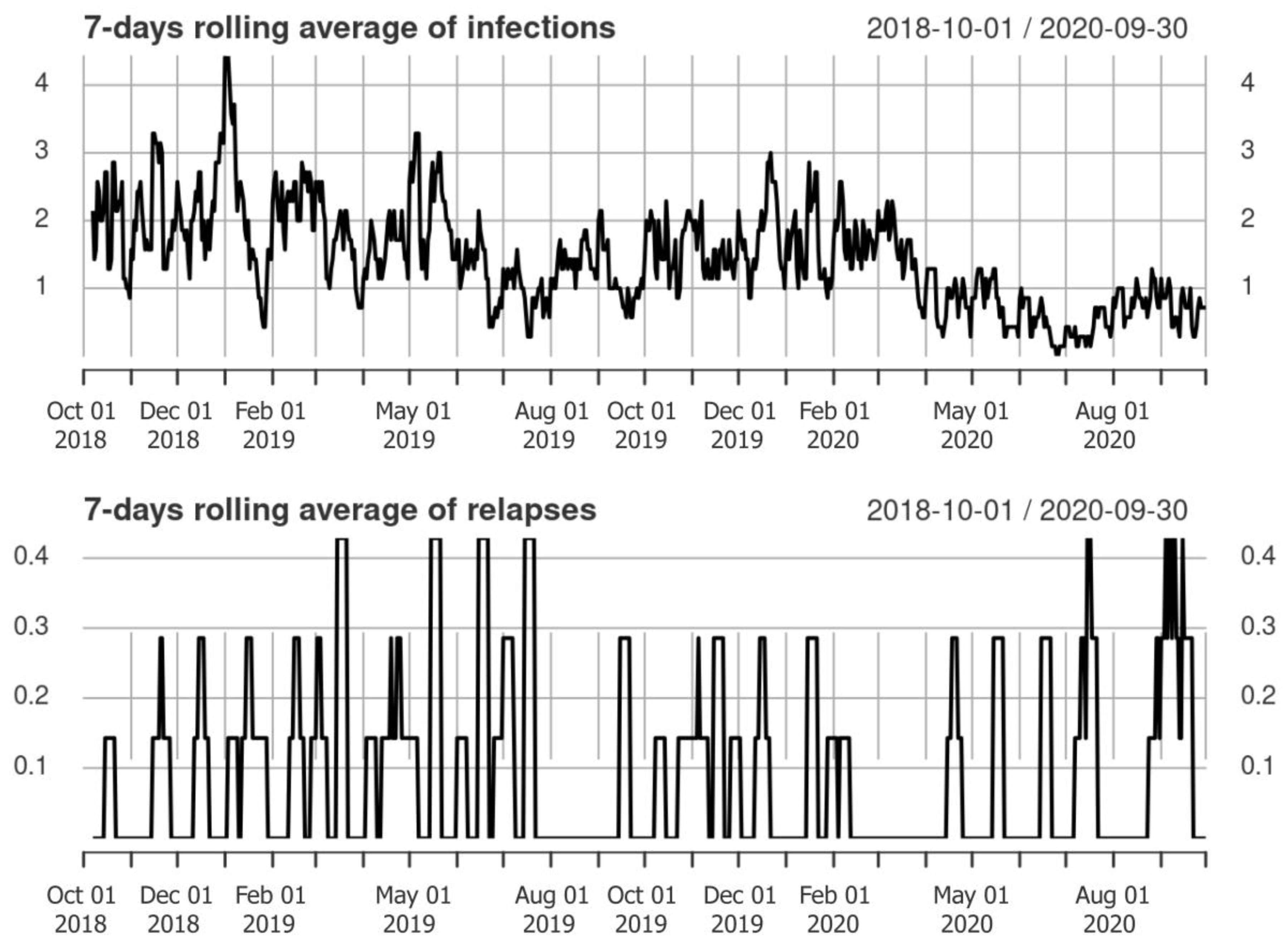
3.2. Clinical Relapses and Infections
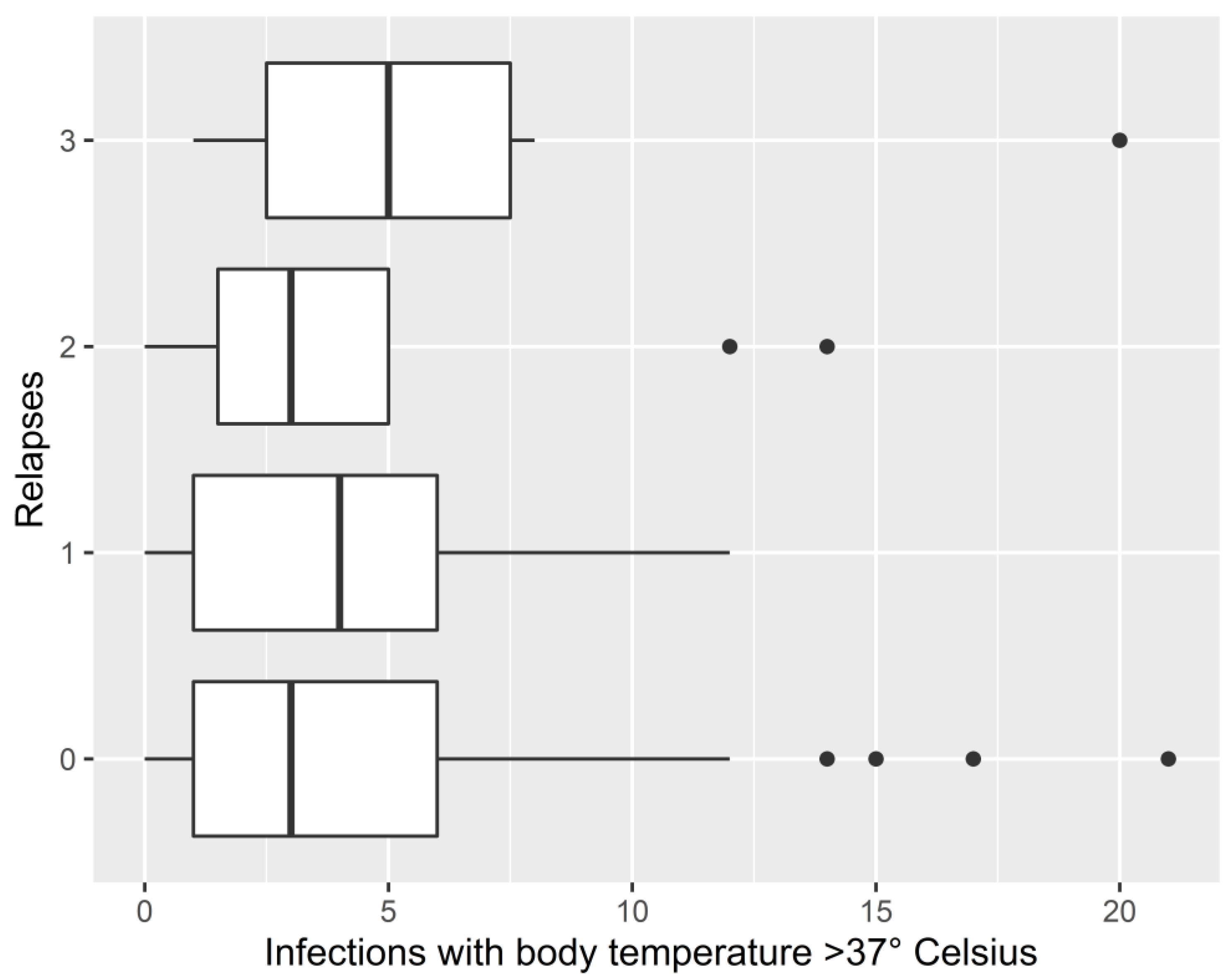
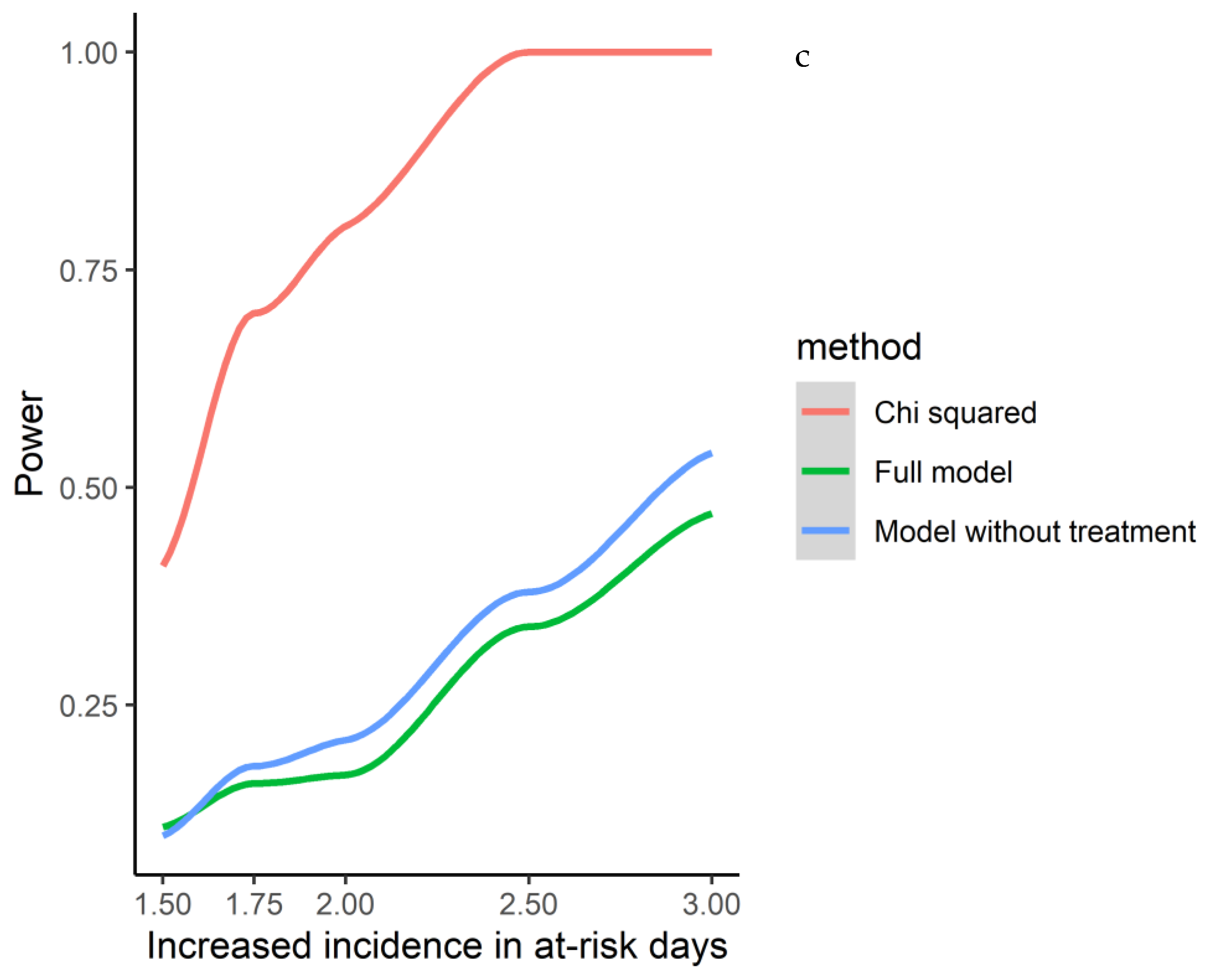
3.3. Associations between Infections and Relapse Occurrence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weinstock-Guttman, B.; Jacobs, L.D. What is new in the treatment of multiple sclerosis? Drugs 2000, 59, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2017, 7, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Mohr, D.C.; Hart, S.L.; Julian, L.; Cox, D.; Pelletier, D. Association between stressful life events and exacerbation in multiple sclerosis: A meta-analysis. BMJ 2004, 328, 731. [Google Scholar] [CrossRef] [PubMed]
- Sparaco, M.; Miele, G.; Lavorgna, L.; Abbadessa, G.; Bonavita, S. Association between relapses, stress, and depression in people with multiple sclerosis during the COVID-19 pandemic. Neurol. Sci. 2022, 43, 2935–2942. [Google Scholar] [CrossRef] [PubMed]
- Kalincik, T. Multiple Sclerosis Relapses: Epidemiology, Outcomes and Management. A Systematic Review. Neuroepidemiology 2015, 44, 199–214. [Google Scholar] [CrossRef]
- Miclea, A.; Bagnoud, M.; Chan, A.; Hoepner, R. A Brief Review of the Effects of Vitamin D on Multiple Sclerosis. Front. Immunol. 2020, 11, 781. [Google Scholar] [CrossRef]
- Simpson, S., Jr.; Taylor, B.; Blizzard, L.; Ponsonby, A.L.; Pittas, F.; Tremlett, H.; Dwyer, T.; Gies, P.; van der Mei, I. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann. Neurol. 2010, 68, 193–203. [Google Scholar]
- Mowry, E.M.; Krupp, L.B.; Milazzo, M.; Chabas, D.; Strober, J.B.; Belman, A.L.; McDonald, J.C.; Oksenberg, J.R.; Bacchetti, P.; Waubant, E. Vitamin D status is associated with relapse rate in pediatric-onset multiple sclerosis. Ann. Neurol. 2010, 67, 618–624. [Google Scholar] [CrossRef]
- Laursen, J.H.; Søndergaard, H.B.; Sørensen, P.S.; Sellebjerg, F.; Oturai, A.B. Vitamin D supplementation reduces relapse rate in relapsing-remitting multiple sclerosis patients treated with natalizumab. Mult. Scler. Relat. Disord. 2016, 10, 169–173. [Google Scholar] [CrossRef]
- Linden, J.; Granåsen, G.; Salzer, J.; Svenningsson, A.; Sundström, P. Inflammatory activity and vitamin D levels in an MS population treated with rituximab. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 2055217319826598. [Google Scholar] [CrossRef]
- Ferre’, L.; Clarelli, F.; Sferruzza, G.; Rocca, M.A.; Mascia, E.; Radaelli, M.; Sangalli, F.; Costa, G.D.; Moiola, L.; Aboulwafa, M.; et al. Basal vitamin D levels and disease activity in multiple sclerosis patients treated with fingolimod. Neurol. Sci. 2018, 39, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Miele, G.; Abbadessa, G.; Cavalla, P.; Valentino, P.; Marfia, G.A.; Landi, D.; Bosa, C.; Vercellino, M.; De Martino, A.; Ponzano, M.; et al. Association of vitamin D serum levels and vitamin D supplementation with B cell kinetics and disease activity in Multiple Sclerosis patients treated with ocrelizumab: An Italian multi-center study. Mult. Scler. Relat. Disord. 2022, 68, 104395. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.; Lygner, P.-E.; Andersson, M.; Vablne, A. Viral infections trigger multiple sclerosis relapses: A prospective seroepidemiological study. J. Neurol. 1993, 240, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.; Zvartau, M.; Clarke, H.; Irving, W.; Blumhardt, L.D. Clinical relapses and disease activity on magnetic resonance imaging associated with viral upper respiratory tract infections in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1998, 64, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Buljevac, D.; Flach, H.Z.; Hop, W.C.J.; Hijdra, D.; Laman, J.D.; Savelkoul, H.F.J.; Van Der Meché, F.G.A.; Van Doorn, P.A.; Hintzen, R.Q. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain 2002, 125, 952–960. [Google Scholar] [CrossRef]
- Correale, J.; Fiol, M.; Gilmore, W. The risk of relapses in multiple sclerosis during systemic infections. Neurology 2006, 67, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Tremlett, H.; van der Mei, I.A.; Pittas, F.; Blizzard, L.; Paley, G.; Mesaros, D.; Woodbaker, R.; Nunez, M.; Dwyer, T.; Taylor, B.V.; et al. Monthly Ambient Sunlight, Infections and Relapse Rates in Multiple Sclerosis. Neuroepidemiology 2008, 31, 271–279. [Google Scholar] [CrossRef]
- Marrodan, M.; Alessandro, L.; Farez, M.F.; Correale, J. The role of infections in multiple sclerosis. Mult. Scler. J. 2019, 25, 891–901. [Google Scholar] [CrossRef]
- Panitch, H.S. Influence of infection on exacerbations of multiple sclerosis. Ann. Neurol. 1994, 36, S25–S28. [Google Scholar] [CrossRef] [PubMed]
- Sibley, W.; Bamford, C.; Clark, K. Clinical viral infections and multiple sclerosis. Lancet 1985, 325, 1313–1315. [Google Scholar] [CrossRef]
- Salvi, F.; Bartolomei, I.; Smolensky, M.H.; Lorusso, A.; Barbarossa, E.; Malagoni, A.M.; Zamboni, P.; Manfredini, R. A seasonal periodicity in relapses of multiple sclerosis? A single-center, population-based, preliminary study conducted in Bologna, Italy. BMC Neurol. 2010, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Spelman, T.; Gray, O.; Trojano, M.; Petersen, T.; Izquierdo, G.; Lugaresi, A.; Hupperts, R.; Bergamaschi, R.; Duquette, P.; Grammond, P.; et al. Seasonal variation of relapse rate in multiple sclerosis is latitude dependent. Ann. Neurol. 2014, 76, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Esper, A.M.; Moss, M.; Lewis, C.A.; Nisbet, R.; Mannino, D.; Martin, G. The role of infection and comorbidity: Factors that influence disparities in sepsis. Crit. Care Med. 2006, 34, 2576–2582. [Google Scholar] [CrossRef]
- Elshout, G.; Monteny, M.; Van Der Wouden, J.C.; Koes, B.W.; Berger, M.Y. Duration of fever and serious bacterial infections in children: A systematic review. BMC Fam. Pract. 2011, 12, 33. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- McDonald, W.I.; Compston, A.; Edan, G.; Goodkin, D.; Hartung, H.-P.; Lublin, F.D.; McFarland, H.F.; Paty, D.W.; Polman, C.H.; Reingold, S.C.; et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the international panel on the diagnosis of multiple sclerosis. Ann. Neurol. 2001, 50, 121–127. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Lublin, F.D.; Häring, D.A.; Ganjgahi, H.; Ocampo, A.; Hatami, F.; Čuklina, J.; Aarden, P.; Dahlke, F.; Arnold, D.L.; Wiendl, H.; et al. How patients with multiple sclerosis acquire disability. Brain 2022, 145, 3147–3161. [Google Scholar] [CrossRef]
- Liu, Z.; Liao, Q.; Wen, H.; Zhang, Y. Disease modifying therapies in relapsing-remitting multiple sclerosis: A systematic review and network meta-analysis. Autoimmun. Rev. 2021, 20, 102826. [Google Scholar] [CrossRef]


| Whole Population RMS (N = 155) | ||
|---|---|---|
| Age (years) | Mean (IQR) | 35.406 (37.5–49.3) |
| Female | N (percentage) | 113 (72.9%) |
| EDSS 1 | Median (IQR) | 3 (2–4.5) |
| Disease duration (years) | Mean (IQR) | 13.25 (6.36–20.4) |
| Patient days reported in the dataset | N | 126,381 |
| Total number of relapses reported in the dataset | N | 78 |
| Total number of infections reported in the dataset | N | 1202 |
| Number of Patients on DMTs 1 at the Enrollment (2018) N (Percentage) | Number of Patients on DMTs 1 after One Year from the Enrollment N (Percentage) | ||||||
|---|---|---|---|---|---|---|---|
| No DMTs | 18/155 (11.61%) | No DMTs | 18/155 (11.61%) | No DMTs | 11/155 (7.10%) | No DMTs | 11/155 (7.10%) |
| Interferon | 28/155 (18.06%) | I Line Therapies | 65/155 (41.93%) | Interferon | 24 (15.48%) | I Line Therapies | 59/155 (38.06%) |
| Glatiramer Acetate | 19/155 (12.26%) | Glatiramer Acetate | 17 (10.97%) | ||||
| Teriflunimide | 12/155 (7.74%) | Teriflunimide | 12 (7.74%) | ||||
| Dimethyl-fumarate | 6/155 (3.87%) | Dimethyl-fumarate | 6 (3.87%) | ||||
| Fingolimod | 48/155 (30.97%) | II Line Therapies | 72/155 (46.45%) | Fingolimod | 52 (33.55%) | II Line Therapies | 85/155 (54.83%) |
| Natalizumab | 11/155 (7.10%) | Natalizumab | 10 (6.45%) | ||||
| Ocrelizumab | 4/155 (2.58%) | Ocrelizumab | 22 (14.2%) | ||||
| Alemtuzumab | 2/155 (1.29%) | Alemtuzumab | 1/155 (0.65%) | ||||
| Others (Rituximab) | 7/155 (4.52%) | Others (Rituximab) | 0/155 (0) | ||||
| Type of Infections | ||
|---|---|---|
| Infections with upper respiratory tract symptoms | N (percentage) | 741/921 (80.4%) |
| Infections with urinary tract symptoms | N (percentage) | 402/921 (43.6%) |
| Infections with gastrointestinal symptoms | N (percentage) | 360/921 (39.1%) |
| Characteristics of infections | ||
| Duration longer than 4 days | N (percentage) | 482/921 (52.33%) |
| Body temperature >37° Celsius | N (percentage) | 82/921 (8.9%) |
| The use of antibiotics and/or antivirals | N (percentage) | 157/921 (17%) |
| Severe infections 1 | N (percentage) | 134/921 (14.54%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miele, G.; Cepparulo, S.; Abbadessa, G.; Lavorgna, L.; Sparaco, M.; Simeon, V.; Guizzaro, L.; Bonavita, S. Clinically Manifest Infections Do Not Increase the Relapse Risk in People with Multiple Sclerosis Treated with Disease-Modifying Therapies: A Prospective Study. J. Clin. Med. 2023, 12, 1023. https://doi.org/10.3390/jcm12031023
Miele G, Cepparulo S, Abbadessa G, Lavorgna L, Sparaco M, Simeon V, Guizzaro L, Bonavita S. Clinically Manifest Infections Do Not Increase the Relapse Risk in People with Multiple Sclerosis Treated with Disease-Modifying Therapies: A Prospective Study. Journal of Clinical Medicine. 2023; 12(3):1023. https://doi.org/10.3390/jcm12031023
Chicago/Turabian StyleMiele, Giuseppina, Simone Cepparulo, Gianmarco Abbadessa, Luigi Lavorgna, Maddalena Sparaco, Vittorio Simeon, Lorenzo Guizzaro, and Simona Bonavita. 2023. "Clinically Manifest Infections Do Not Increase the Relapse Risk in People with Multiple Sclerosis Treated with Disease-Modifying Therapies: A Prospective Study" Journal of Clinical Medicine 12, no. 3: 1023. https://doi.org/10.3390/jcm12031023
APA StyleMiele, G., Cepparulo, S., Abbadessa, G., Lavorgna, L., Sparaco, M., Simeon, V., Guizzaro, L., & Bonavita, S. (2023). Clinically Manifest Infections Do Not Increase the Relapse Risk in People with Multiple Sclerosis Treated with Disease-Modifying Therapies: A Prospective Study. Journal of Clinical Medicine, 12(3), 1023. https://doi.org/10.3390/jcm12031023








