Arterial Stiffness, Assessed Using the Cardio–Ankle Vascular Index, before and 2 Years after Total Knee Arthroplasty in Patients with Knee Osteoarthritis
Abstract
1. Introduction
2. Material and Methods
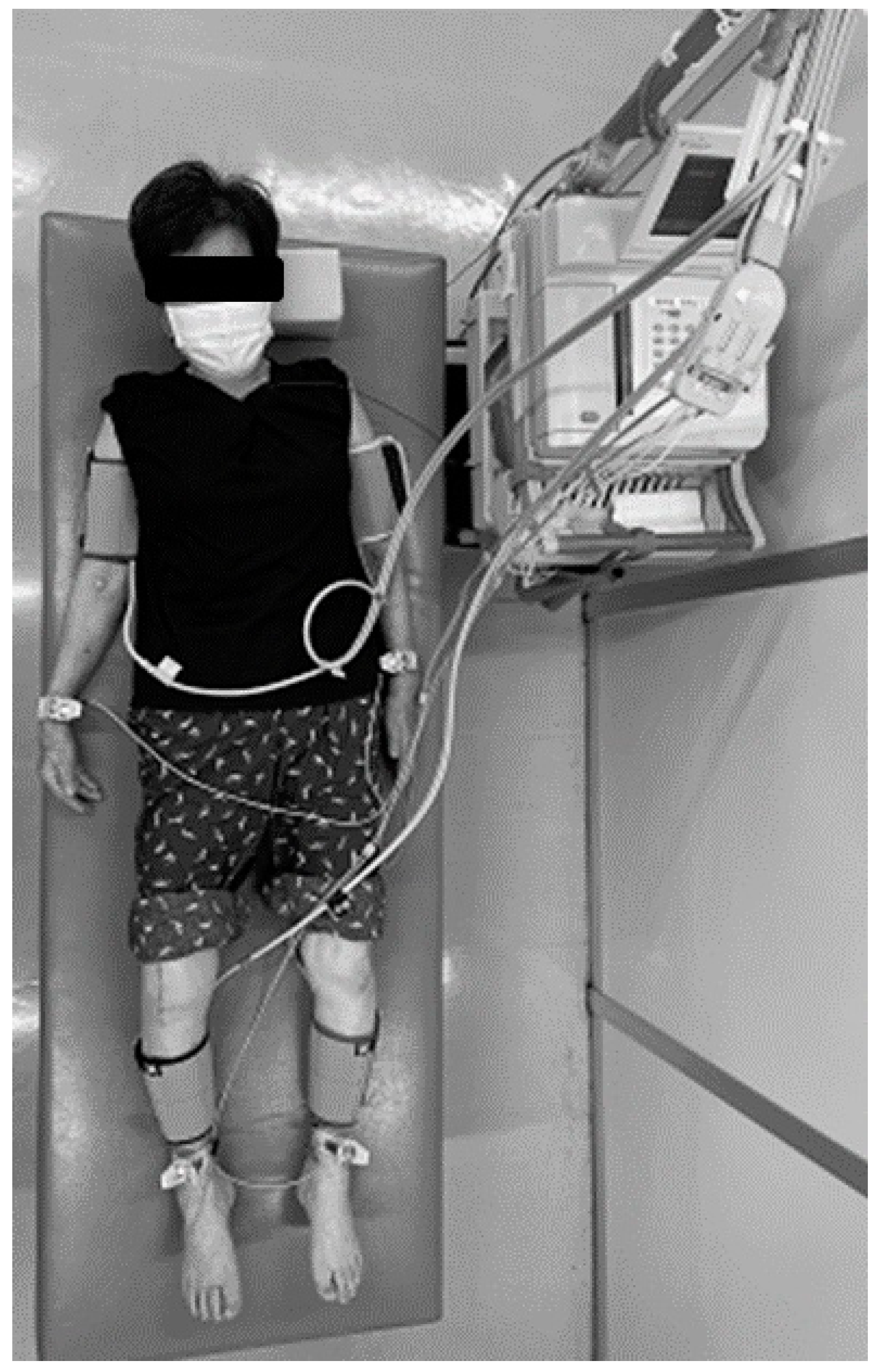
2.1. Measurement of CAVI
2.2. Reproducibility
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, L.K.; Kendzerska, T.; Waugh, E.J.; Hawker, G.A. Impact of osteoarthritis on difficulty walking: A population-based study. Arthritis Care Res. 2018, 70, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kendzerska, T.; Jüni, P.; King, L.K.; Croxford, R.; Stanaitis, I.; Hawker, G.A. The longitudinal relationship between hand, hip and knee osteoarthritis and cardiovascular events: A population-based cohort study. Osteoarthr. Cartil. 2017, 25, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Trevisan, C.; De Rui, M.; Bolzetta, F.; Maggi, S.; Zambon, S.; Musacchio, E.; Sartori, L.; Perissinotto, E.; Crepaldi, G.; et al. Association of osteoarthritis with increased risk of cardiovascular diseases in the elderly: Findings from the Progetto Veneto Anziano study cohort. Arthritis Rheumatol. 2016, 68, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Noguchi, H.; Sato, J.; Sakurai, T.; Toyabe, S.I. Quadriceps strength impairment in the mid- to long-term follow-up period after TKA. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3372–3377. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Noguchi, H.; Takeda, M.; Sato, J.; Kishimoto, Y.; Toyabe, S. Changes of body balance before and after total knee arthroplasty in patients who suffered from bilateral knee osteoarthritis. J. Orthop. Sci. 2013, 18, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Swanik, C.B.; Lephart, S.M.; Rubash, H.E. Proprioception, kinesthesia, and balance after total knee arthroplasty with cruciate-retaining and posterior stabilized prostheses. J. Bone Jt. Surg. Am. 2004, 86, 328–334. [Google Scholar] [CrossRef]
- Bonnefoy-Mazure, A.; Lübbeke, A.; Miozzari, H.H.; Armand, S.; Sagawa, Y., Jr.; Turcot, K.; Poncet, A. Walking speed and maximal knee flexion during gait after total knee arthroplasty: Minimal clinically important improvement is not determinable; Patient acceptable symptom state is potentially useful. J. Arthroplast. 2020, 35, 2865–2871. [Google Scholar] [CrossRef]
- Liebensteiner, M.C.; Herten, A.; Gstoettner, M.; Thaler, M.; Krismer, M.; Bach, C.M. Correlation between objective gait parameters and subjective score measurements before and after total knee arthroplasty. Knee 2008, 15, 461–466. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, J.W.; Jang, Y.S. Long-term (Up to 27 Years) prospective, randomized study of mobile-bearing and fixed-bearing total knee arthroplasties in patients <60 years of age with osteoarthritis. J. Arthroplast. 2021, 36, 1330–1335. [Google Scholar]
- Kremers, H.M.; Sierra, R.J.; Schleck, C.D.; Berry, D.J.; Cabanela, M.E.; Hanssen, A.D.; Pagnano, M.W.; Trousdale, R.T.; Lewallen, D.G. Comparative survivorship of different tibial designs in primary total knee arthroplasty. J. Bone Jt. Surg. Am. 2014, 96, e121. [Google Scholar] [CrossRef]
- Nüesch, E.; Dieppe, P.; Reichenbach, S.; Williams, S.; Iff, S.; Jüni, P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: Population based cohort study. BMJ 2011, 342, d1165. [Google Scholar] [CrossRef] [PubMed]
- Shirai, K.; Utino, J.; Otsuka, K.; Takata, M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J. Atheroscler. Thromb. 2006, 13, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, T.; Ito, H. Arterial stiffness in health and disease: The role of cardio-ankle vascular index. J. Cardiol. 2021, 78, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Namba, T.; Masaki, N.; Takase, B.; Adachi, T. Arterial stiffness assessed by cardio-ankle vascular index. Int. J. Mol. Sci. 2019, 20, 3664. [Google Scholar] [CrossRef] [PubMed]
- Noike, H.; Nakamura, K.; Sugiyama, Y.; Iizuka, T.; Shimizu, K.; Takahashi, M.; Hirano, K.; Suzuki, M.; Mikamo, H.; Nakagami, T.; et al. Changes in cardio-ankle vascular index in smoking cessation. J. Atheroscler. Thromb. 2010, 17, 517–525. [Google Scholar] [CrossRef][Green Version]
- Nagayama, D.; Endo, K.; Ohira, M.; Yamaguchi, T.; Ban, N.; Kawana, H.; Nagumo, A.; Saiki, A.; Oyama, T.; Miyashita, Y.; et al. Effects of body weight reduction on cardio-ankle vascular index (CAVI). Obes. Res. Clin. Pract. 2013, 7, e139–e145. [Google Scholar] [CrossRef]
- Nagayama, D.; Saiki, A.; Endo, K.; Yamaguchi, T.; Ban, N.; Kawana, H.; Ohira, M.; Oyama, T.; Miyashita, Y.; Shirai, K. Improvement of cardio-ankle vascular index by glimepiride in type 2 diabetic patients. Int. J. Clin. Pract. 2010, 64, 1796–1801. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Shirai, K.; Nagayama, D.; Nakamura, S.; Oka, R.; Tanaka, S.; Watanabe, S.; Imamura, H.; Sato, S.; Kawana, H.; et al. Bezafibrate ameliorates arterial stiffness assessed by cardio-ankle vascular index in hypertriglyceridemic patients with type 2 diabetes mellitus. J. Atheroscler. Thromb. 2019, 26, 659–669. [Google Scholar] [CrossRef]
- American Society of Anaesthesiologists. Physical Status Classification System. Available online: https://www.asahq.org/standards-and-practice-parameters/statement-on-asa-physical-status-classification-system (accessed on 24 March 2023).
- Alicea, J. Scoring systems and their validation for the arthritic knee. In Surgery of the Knee, 3rd ed.; Insall, J.N., Scott, W.N., Eds.; Churchill Livingstone: New York, NY, USA, 2001; Volume 2, pp. 1507–1515. [Google Scholar]
- Shirai, K.; Hiruta, N.; Song, M.; Kurosu, T.; Suzuki, J.; Tomaru, T.; Miyashita, Y.; Saiki, A.; Takahashi, M.; Suzuki, K.; et al. Cardioankle vascular index (CAVI) as a novel indicator of arterial stiffness: Theory, evidence and perspectives. J. Atheroscler. Thromb. 2011, 18, 924–938. [Google Scholar] [CrossRef]
- Blum, C.L.; Lepkowsky, E.; Hussein, A.; Wakelin, E.A.; Plaskos, C.; Koenig, J.A. Patient expectations and satisfaction in robotic-assisted total knee arthroplasty: A prospective two-year outcome study. Arch. Orthop. Trauma Surg. 2021, 141, 2155–2164. [Google Scholar] [CrossRef]
- Lakra, A.; Murtaugh, T.; Shah, R.P.; Cooper, H.J.; Geller, J.A. Early Postoperative Pain Predicts 2-Year Functional Outcomes following Knee Arthroplasty. J. Knee Surg. 2020, 33, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Tomiyama, H.; Maruhashi, T.; Matsuzawa, Y.; Miyoshi, T.; Kabutoya, T.; Kario, K.; Sugiyama, S.; Munakata, M.; Ito, H.; et al. Physiological diagnostic criteria for vascular failure. Hypertension 2018, 72, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Tabara, Y.; Setoh, K.; Kawaguchi, T.; Takahashi, Y.; Kosugi, S.; Nakayama, T.; Matsuda, F.; The Nagahama Study Group. Factors affecting longitudinal changes in cardio-ankle vascular index in a large general population: The Nagahama study. J. Hypertens. 2018, 36, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Maiorana, A.; O’Driscoll, G.; Taylor, R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J. Physiol. 2004, 561 Pt 1, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, Y.A.; Al-Shahrani, F.S.; Alanazi, S.S.; Alshammari, F.A.; Alkhudair, A.M.; Jatoi, N.A. The Association of blood glucose levels and arterial stiffness (Cardio-ankle vascular index) in patients with type 2 diabetes mellitus. Cureus 2021, 13, e20408. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, D.; Imamura, H.; Sato, Y.; Yamaguchi, T.; Ban, N.; Kawana, H.; Nagumo, A.; Shirai, K.; Tatsuno, I. Inverse relationship of cardioankle vascular index with BMI in healthy Japanese subjects: A cross-sectional study. Vasc. Health Risk Manag. 2016, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pavlovska, I.; Kunzova, S.; Jakubik, J.; Hruskova, J.; Skladana, M.; Rivas-Serna, I.M.; Medina-Inojosa, J.R.; Lopez-Jimenez, F.; Vysoky, R.; Geda, Y.E.; et al. Associations between high triglycerides and arterial stiffness in a population-based sample: Kardiovize Brno 2030 study. Lipids Health Dis. 2020, 19, 170. [Google Scholar] [CrossRef]
- Satoh, N.; Shimatsu, A.; Kato, Y.; Araki, R.; Koyama, K.; Okajima, T.; Tanabe, M.; Ooishi, M.; Kotani, K.; Ogawa, Y. Evaluation of the cardio-ankle vascular index, a new indicator of arterial stiffness independent of blood pressure, in obesity and metabolic syndrome. Hypertens. Res. 2008, 31, 1921–1930. [Google Scholar] [CrossRef]
- Dobsak, P.; Soska, V.; Sochor, O.; Jarkovsky, J.; Novakova, M.; Homolka, M.; Soucek, M.; Palanova, P.; Lopez-Jimenez, F.; Shirai, K. Increased cardio-ankle vascular index in hyper-lipidemic patients without diabetes or hypertension. J. Atheroscler. Thromb. 2015, 22, 272–283. [Google Scholar] [CrossRef]
- Kubozono, T.; Miyata, M.; Ueyama, K.; Hamasaki, S.; Kusano, K.; Kubozono, O.; Tei, C. Acute and chronic effects of smoking on arterial stiffness. Circ. J. 2011, 75, 698–702. [Google Scholar] [CrossRef]
- Uurtuya, S.; Taniguchi, N.; Kotani, K.; Yamada, T.; Kawano, M.; Khurelbaatar, N.; Itoh, K.; Lkhagvasuren, T. Comparative study of the cardio-ankle vascular index and ankle–brachial index between young Japanese and Mongolian subjects. Hypertens. Res. 2009, 32, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shirai, K.; Liu, J.; Lu, N.; Wang, M.; Zhao, H.; Xie, J.; Yu, X.; Fu, X.; Shi, H.; et al. Comparative study of cardio-ankle vascular index between Chinese and Japanese healthy subjects. Clin. Exp. Hypertens. 2014, 36, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Yingchoncharoen, T.; Sritara, P. Cardio-ankle vascular index in a Thai population. Pulse 2017, 4 (Suppl. S1), 8–10. [Google Scholar] [CrossRef] [PubMed]
| Parameter (patients/knees) | N = 119/140 |
| Sex (Male (knees)/Female (knees)) | 15 (17)/104 (123) |
| Height (cm) | 150 (145, 155) |
| Body mass (kg) | 58 (52, 66) |
| Body mass index (kg/m2) | 26 (24, 28) |
| Age (years) | 73 (69, 79) |
| Smoking history (yes/no) number of knees | 6/134 |
| Diabetes mellitus (yes/no) number of knees | 19/121 |
| Hypertension (yes/no) | 93/47 |
| Preoperative serum cholesterol concentration (nmol/L) | 5.2 (4.8, 5.9) |
| Preoperative serum triglyceride concentration (nmol/L) | 1.5 (1.1, 2.0) |
| Preoperative range of knee motion (°) | 100 (90, 120) |
| Postoperative range of knee motion (°) | 110 (100, 120) |
| ASA Grade | I 20, II 120 |
| Preoperative HSS | 44 (35, 51) |
| Postoperative HSS | 92 (92, 94) |
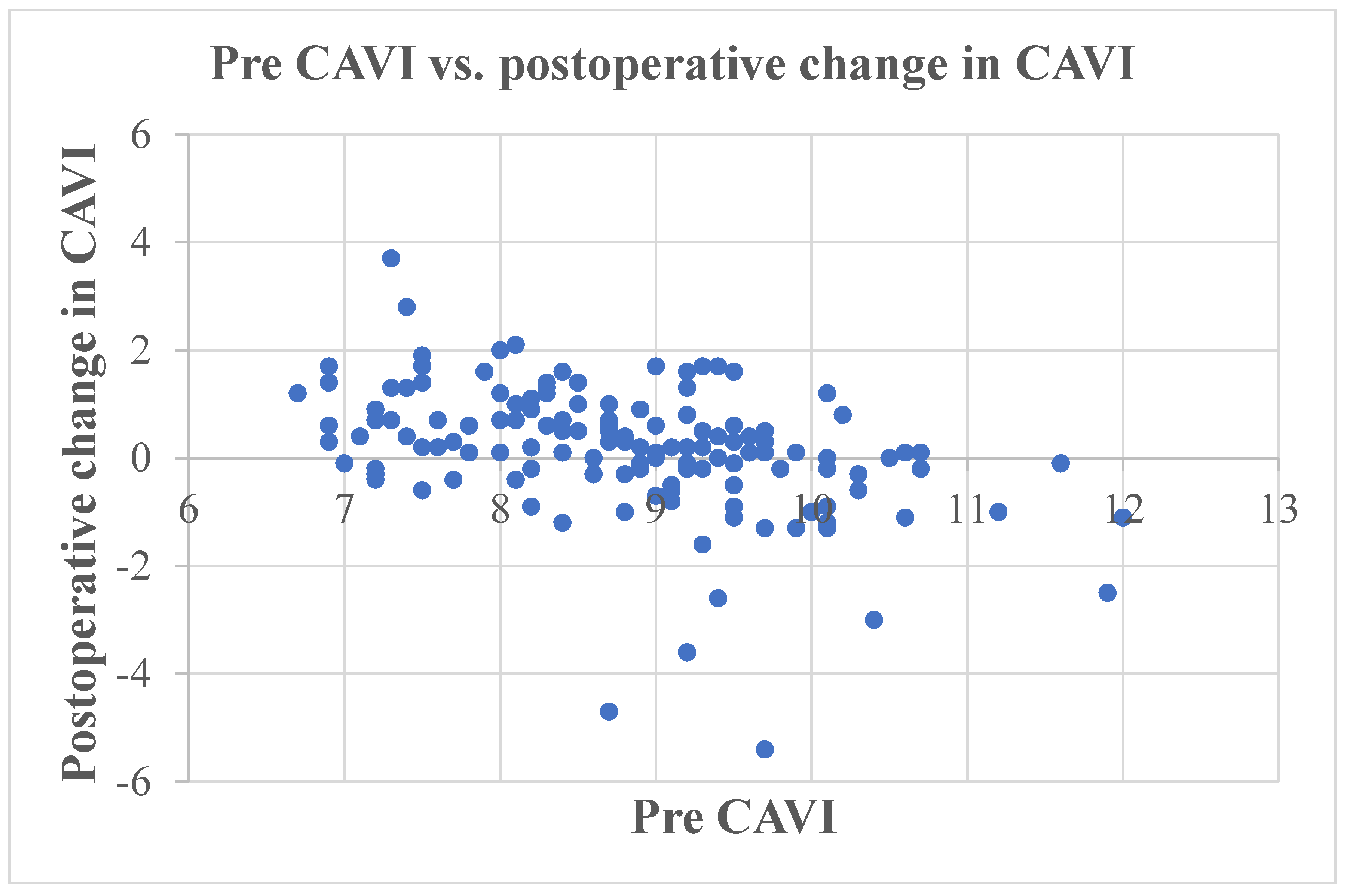
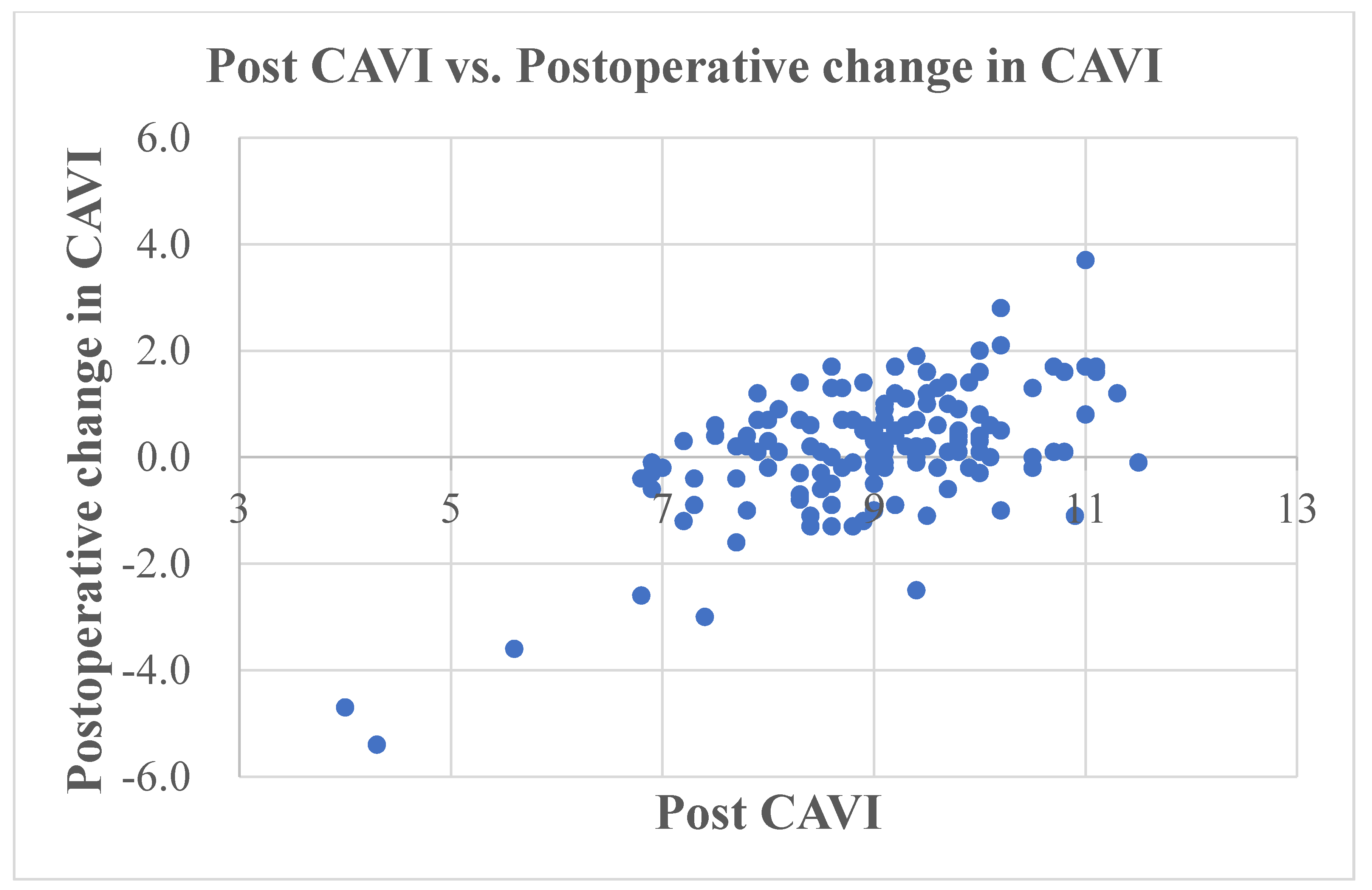
| Preoperative CAVI | 8.8 (8.0, 9.5) |
| Postoperative CAVI | 9.1 (8.3, 9.8) |
| Variable | r | p |
|---|---|---|
| Pre CAVI | −0.469 | <0.001 |
| Age | 0.652 | |
| Height | 0.278 | |
| Body mass | 0.744 | |
| Body mass index | 0.367 | |
| HSS score | 0.246 | |
| ROM | 0.175 | 0.039 |
| Cholesterol | 0.306 | |
| Triglycerides | 0.713 |
| Preoperative Arteriosclerosis | CAVI < 9.0 (No Arteriosclerosis) | CAVI ≥ 9.0 (Arteriosclerosis) | Total |
|---|---|---|---|
| Maintained or improved | 17 (23%) | 37 (57%) | 54 (39%) |
| Deteriorated | 58 (77%) | 28 (43%) | 86 (61%) |
| p < 0.001 | 75 | 65 | 140 |
| Parameter | B | S.E. | Β | Sig. | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Preoperative CAVI | −0.478 | 0.085 | −0.432 | <0.001 | −0.646 | −0.310 |
| Constant | 4.368 | 0.753 | <0.001 | 2.879 | 5.857 | |
| Variable | Change in CAVI Following TKA in Each Subgroup | p Value | |
|---|---|---|---|
| Sex | Male (17) | Female (123) | |
| 0.2 (−0.2, 0.8) | 0.2 (−0.3, 0.8) | 0.848 | |
| ASA | I (20) | II (120) | |
| 0.2 (−0.3, 0.7) | 0.2 (−0.2, 0.8) | 0.383 | |
| Smoking | Yes (6) | No (134) | |
| 0.1 (−0.5, 0.7) | 0.2 (−0.3, 0.8) | 0.248 | |
| Hypertension | Yes (93) | No (47) | |
| 0.2 (−0.2, 0.8) | 0.3 (−0.4, 0.8) | 0.788 | |
| Diabetes mellitus | Yes (19) | No (121) | |
| 0.3 (−0.1, 1.0) | 0.2 (−0.4, 0.7) | 0.469 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii, Y.; Noguchi, H.; Sato, J.; Takahashi, I.; Ishii, H.; Ishii, R.; Ishii, K.; Ishii, K.; Toyabe, S.-i. Arterial Stiffness, Assessed Using the Cardio–Ankle Vascular Index, before and 2 Years after Total Knee Arthroplasty in Patients with Knee Osteoarthritis. J. Clin. Med. 2023, 12, 7734. https://doi.org/10.3390/jcm12247734
Ishii Y, Noguchi H, Sato J, Takahashi I, Ishii H, Ishii R, Ishii K, Ishii K, Toyabe S-i. Arterial Stiffness, Assessed Using the Cardio–Ankle Vascular Index, before and 2 Years after Total Knee Arthroplasty in Patients with Knee Osteoarthritis. Journal of Clinical Medicine. 2023; 12(24):7734. https://doi.org/10.3390/jcm12247734
Chicago/Turabian StyleIshii, Yoshinori, Hideo Noguchi, Junko Sato, Ikuko Takahashi, Hana Ishii, Ryo Ishii, Kei Ishii, Kai Ishii, and Shin-ichi Toyabe. 2023. "Arterial Stiffness, Assessed Using the Cardio–Ankle Vascular Index, before and 2 Years after Total Knee Arthroplasty in Patients with Knee Osteoarthritis" Journal of Clinical Medicine 12, no. 24: 7734. https://doi.org/10.3390/jcm12247734
APA StyleIshii, Y., Noguchi, H., Sato, J., Takahashi, I., Ishii, H., Ishii, R., Ishii, K., Ishii, K., & Toyabe, S.-i. (2023). Arterial Stiffness, Assessed Using the Cardio–Ankle Vascular Index, before and 2 Years after Total Knee Arthroplasty in Patients with Knee Osteoarthritis. Journal of Clinical Medicine, 12(24), 7734. https://doi.org/10.3390/jcm12247734





