Association between Subretinal Drusenoid Deposits and Age-Related Macular Degeneration in Multimodal Retinal Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Initial Management
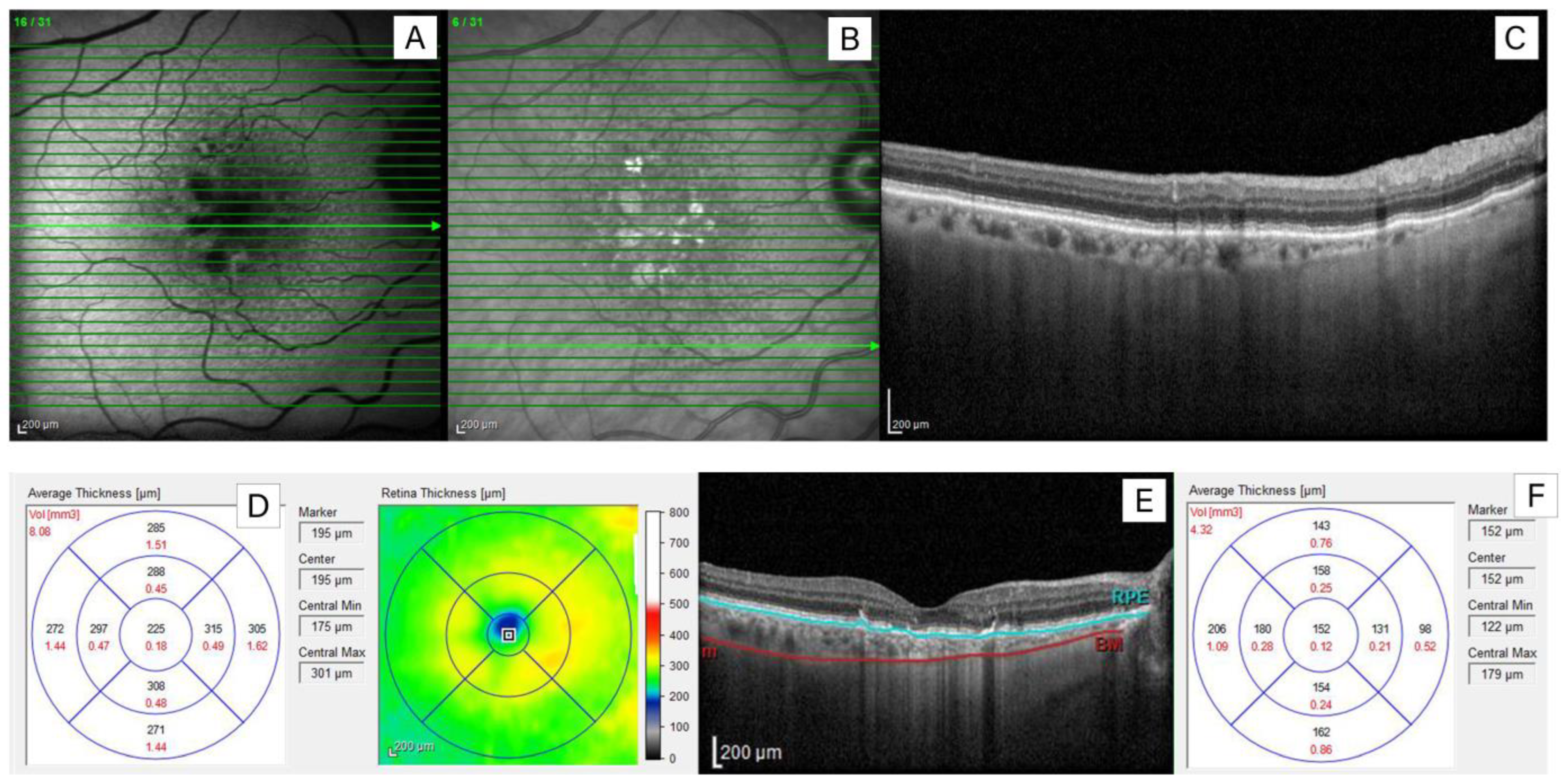
2.2. Imaging Analysis
2.3. Retinal Vessel Analysis
2.4. Serum Lipid Analysis
2.5. Genotyping
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Group
3.2. Relationship between AMD Severity and SDD Presence
3.3. Choroidal Characteristics and SDD Presence
3.4. Relationship between SDD Presence and Retinal Vessel Characteristics
3.5. The Impact of SDD on AMD Progression
3.6. Associations between CFH Y402H and ARMS2 A69S Polymorphisms and SDD Prevalence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mimoun, G.; Soubrane, G.; Coscas, G. Les drusen maculaires [Macular drusen]. J. Fr. Ophtalmol. 1990, 13, 511–513. [Google Scholar] [PubMed]
- Zweifel, S.A.; Spaide, R.F.; Curcio, C.A.; Malek, G.; Imamura, Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology 2010, 117, 303–312.e1. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.Y.; Dubois, L.; Tadayoni, R.; Delahaye-Mazza, C.; Debibie, C.; Quentel, G. Prevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularisation. Br. J. Ophthalmol. 2007, 91, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Zarubina, A.V.; Neely, D.C.; Clark, M.E.; Huisingh, C.E.; Samuels, B.C.; Zhang, Y.; McGwin, G., Jr.; Owsley, C.; Curcio, C.A. Prevalence of Subretinal Drusenoid Deposits in Older Persons with and without Age-Related Macular Degeneration, by Multimodal Imaging. Ophthalmology 2016, 123, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Rabiolo, A.; Sacconi, R.; Cicinelli, M.V.; Querques, L.; Bandello, F.; Querques, G. Spotlight on reticular pseudodrusen. Clin. Ophthalmol. 2017, 11, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, J.S.; Göbel, A.P.; Fleckenstein, M.; Holz, F.G.; Schmitz-Valckenberg, S. Reticular drusen in eyes with high-risk characteris-tics for progression to late-stage age-related macular degeneration. Br. J. Ophthalmol. 2015, 99, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Çeper, S.B.; Afrashi, F.; Değirmenci, C.; Menteş, J.; Nalçacı, S.; Akkın, C. Multimodal Imaging of Reticular Pseudodrusen in Turkish Patients. Turk. J. Ophthalmol. 2023, 53, 275–280. [Google Scholar] [CrossRef]
- Ueda-Arakawa, N.; Ooto, S.; Tsujikawa, A.; Yamashiro, K.; Oishi, A.; Yoshimura, N. Sensitivity and specificity of detecting reticular pseudodrusen in multimodal imaging in Japanese patients. Retina 2013, 33, 490–497. [Google Scholar] [CrossRef]
- Dutheil, C.; Le Goff, M.; Cougnard-Grégoire, A.; Gattoussi, S.; Korobelnik, J.F.; Rougier, M.B.; Schweitzer, C.; Delcourt, C.; Delyfer, M.N. Incidence and Risk Factors of Reticular Pseudodrusen Using Multimodal Imaging. JAMA Ophthalmol. 2020, 138, 467–477. [Google Scholar] [CrossRef]
- Rudolf, M.; Malek, G.; Messinger, J.D.; Clark, M.E.; Wang, L.; Curcio, C.A. Sub-retinal drusenoid deposits in human retina: Organization and composition. Exp. Eye Res. 2008, 87, 402–408. [Google Scholar] [CrossRef]
- Curcio, C.A.; Messinger, J.D.; Sloan, K.R.; McGwin, G.; Medeiros, N.E.; Spaide, R.F. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: Morphology, prevalence, topography, and biogenesis model. Retina 2013, 33, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.T.; Sohrab, M.A.; Busuioc, M.; Barile, G. Reticular macular disease. Am. J. Ophthalmol. 2009, 148, 733–743.e2. [Google Scholar] [CrossRef] [PubMed]
- Cymerman, R.M.; Skolnick, A.H.; Cole, W.J.; Nabati, C.; Curcio, C.A.; Smith, R.T. Coronary Artery Disease and Reticular Macular Disease, a Subphenotype of Early Age-Related Macular Degeneration. Curr. Eye Res. 2016, 41, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, N.; Smith, R.T. Association of age-related macular degeneration and reticular macular disease with cardiovascular disease. Surv. Ophthalmol. 2016, 61, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Meuer, S.M.; Knudtson, M.D.; Iyengar, S.K.; Klein, B.E. The epidemiology of retinal reticular drusen. Am. J. Ophthalmol. 2008, 145, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Querques, G.; Querques, L.; Forte, R.; Massamba, N.; Coscas, F.; Souied, E.H. Choroidal changes associated with reticular pseudodrusen. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1258–1263. [Google Scholar] [CrossRef]
- Ueda-Arakawa, N.; Ooto, S.; Ellabban, A.A.; Takahashi, A.; Oishi, A.; Tamura, H.; Yamashiro, K.; Tsujikawa, A.; Yoshimura, N. Macular choroidal thickness and volume of eyes with reticular pseudodrusen using swept-source optical coherence tomography. Am. J. Ophthalmol. 2014, 157, 994–1004. [Google Scholar] [CrossRef]
- Keenan, T.D.; Klein, B.; Agrón, E.; Chew, E.Y.; Cukras, C.A.; Wong, W.T. Choroidal thickness and vascularity vary with disease severity and subretinal drusenoid deposit presence in nonadvanced age-related macular degeneration. Retina 2020, 40, 632–642. [Google Scholar] [CrossRef]
- Ho, C.Y.; Lek, J.J.; Aung, K.Z.; McGuinness, M.B.; Luu, C.D.; Guymer, R.H. Relationship between reticular pseudodrusen and choroidal thickness in intermediate age-related macular degeneration. Clin. Exp. Ophthalmol. 2018, 46, 485–494. [Google Scholar] [CrossRef]
- Gliem, M.; Müller, P.L.; Mangold, E.; Bolz, H.J.; Stöhr, H.; Weber, B.H.; Holz, F.G.; Charbel Issa, P. Reticular Pseudodrusen in Sorsby Fundus Dystrophy. Ophthalmology 2015, 122, 1555–1562. [Google Scholar] [CrossRef]
- Querques, G. Reticular Pseudodrusen: A Common Pathogenic Mechanism Affecting the Choroid-Bruch’s Membrane Complex and Retinal Pigment Epithelium for Different Retinal and Macular Diseases. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5914–5915. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rabiolo, A.; Benatti, L.; Tomasso, L.; Zucchiatti, I.; Gelormini, F.; Casaluci, M.; Querques, L.; Sacconi, R.; Bandello, F.; Querques, G. Retinal arterial dilation is impaired in eyes with drusen and reticular pseudodrusen. Retina 2019, 39, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Hogg, R.E.; Silva, R.; Staurenghi, G.; Murphy, G.; Santos, A.R.; Rosina, C.; Chakravarthy, U. Clinical characteristics of reticular pseudodrusen in the fellow eye of patients with unilateral neovascular age-related macular degeneration. Ophthalmology 2014, 121, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Mauschitz, M.M.; Hochbein, B.J.; Klinkhammer, H.; Saßmannshausen, M.; Terheyden, J.H.; Krawitz, P.; Finger, R.P. Prevalence and determinants of subretinal drusenoid deposits in patients’ first-degree relatives. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 117, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, S.A.; Imamura, Y.; Spaide, T.C.; Fujiwara, T.; Spaide, R.F. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology 2010, 117, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ayton, L.N.; Luu, C.D.; Baird, P.N.; Guymer, R.H. Reticular Pseudodrusen in Intermediate Age-Related Macular Degeneration: Prevalence, Detection, Clinical, Environmental, and Genetic Associations. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Krytkowska, E.; Grabowicz, A.; Mozolewska-Piotrowska, K.; Ulańczyk, Z.; Safranow, K.; Machalińska, A. The impact of vascular risk factors on the thickness and volume of the choroid in AMD patients. Sci. Rep. 2021, 11, 15106. [Google Scholar] [CrossRef]
- Holladay, J.T. Visual acuity measurements. J. Cataract. Refract. Surg. 2004, 30, 287–290. [Google Scholar] [CrossRef]
- Stevens, S.; Gilbert, C.; Astbury, N. How to measure intraocular pressure: Applanation tonometry. Community Eye Health 2007, 20, 74–75, Erratum in Community Eye Health 2008, 21, 34. [Google Scholar]
- Lam, A.K.; Chan, R.; Pang, P.C. The repeatability and accuracy of axial length and anterior chamber depth measurements from the IOLMaster. Ophthalmic Physiol. Opt. 2001, 21, 477–483. [Google Scholar] [CrossRef]
- Aydemir, G.A.; Bayat, A.H.; Aydemir, E.; Cinar, S.S.; Karadag, A.S. Comparison of the Retinal and Choroidal Structures in 3 Refractive Groups. Beyoglu Eye J. 2021, 6, 90–95. [Google Scholar] [PubMed]
- Salehi, M.A.; Nowroozi, A.; Gouravani, M.; Mohammadi, S.; Arevalo, J.F. Associations of refractive errors and retinal changes measured by optical coherence tomography: A systematic review and meta-analysis. Surv. Ophthalmol. 2022, 67, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Nilforushan, N.; Moghimi, S.; Bitrian, E.; Riddle, J.; Lee, G.Y.; Caprioli, J.; Nouri-Mahdavi, K. Peripapillary and macular choroidal thickness in glaucoma. J. Ophthalmic Vis. Res. 2014, 9, 154–161. [Google Scholar] [PubMed]
- Jonas, J.B.; Steinmetz, P.; Forster, T.M.; Schlichtenbrede, F.C.; Harder, B.C. Choroidal Thickness in Open-angle Glaucoma. J. Glaucoma 2015, 24, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Tham, Y.C.; Cheung, N.; Yasuda, M.; Tan, N.Y.Q.; Cheung, C.Y.; Wang, J.J.; Mitchell, P.; Sabanayagam, C.; Wong, T.Y.; et al. Macular thickness profile and diabetic retinopathy: The Singapore Epidemiology of Eye Diseases Study. Br. J. Ophthalmol. 2018, 102, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Narain, S.; Gupta, C.; Gupta, P.; Shroff, D.; Shroff, C.M. Assessment of choroid thickness by swept-source optical coherence tomography (SS-OCT) in patients with posterior uveitis. Ophthalmol. J. 2022, 7, 86–93. [Google Scholar] [CrossRef]
- Fang, I.M.; Chen, L.L. Association of macular choroidal thickness with optical coherent tomography morphology in patients with idiopathic epiretinal membrane. PLoS ONE 2020, 15, e0239992. [Google Scholar] [CrossRef] [PubMed]
- Giacuzzo, C.; Bergin, C.; Potic, J.; Forestier, E.; Daruich, A.; Pournaras, J.A.; Konstantinidis, L.; Wolfensberger, T.J. Evolution and patterns of choroidal thickness changes in rhegmatogenous retinal detachment. Retina 2020, 40, 47–55. [Google Scholar] [CrossRef]
- Burke, M.; Lieu, P.; Boss, J.; Abrams, G.W. Macular Choroidal Thickness in Unilateral Commotio Retinae. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3679. [Google Scholar] [CrossRef]
- Mackenbrock, L.H.B.; Weindler, J.N.; Labuz, G.; Baur, I.D.; Auffarth, G.U.; Khoramnia, R. Change in Subfoveal Choroidal Thickness following Cataract Surgery Imaged with Enhanced Depth Imaging Optical Coherence Tomography. Klin. Monbl Augenheilkd. 2023, 240, 989–996. [Google Scholar] [CrossRef]
- Krytkowska, E.; Grabowicz, A.; Safranow, K.; Machalińska, A. Does the Presence of the Cilioretinal Artery Affect the Incidence, Clinical Picture and Progression of Age-Related Macular Degeneration? Diagnostics 2023, 13, 1593. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, S.; Sakamoto, T.; Yamashita, T.; Shirasawa, M.; Uchino, E.; Terasaki, H.; Tomita, M. Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3893–3899. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Gupta, P.; Tan, K.A.; Cheung, C.M.; Wong, T.Y.; Cheng, C.Y. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci. Rep. 2016, 6, 21090. [Google Scholar] [CrossRef] [PubMed]
- Yung, M.; Klufas, M.A.; Sarraf, D. Clinical applications of fundus autofluorescence in retinal disease. Int. J. Retin. Vitr. 2016, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.L., 3rd; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R.; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef]
- Spaide, R.F.; Curcio, C.A. Drusen characterization with multimodal imaging. Retina 2010, 30, 1441–1454. [Google Scholar] [CrossRef]
- Wightman, A.J.; Guymer, R.H. Reticular pseudodrusen: Current understanding. Clin. Exp. Optom. 2019, 102, 455–462. [Google Scholar] [CrossRef]
- Spaide, R.F. Disease expression in nonexudative age-related macular degeneration varies with choroidal thickness. Retina 2018, 38, 708–716. [Google Scholar] [CrossRef]
- Fukuda, Y.; Sakurada, Y.; Yoneyama, S.; Kikushima, W.; Sugiyama, A.; Matsubara, M.; Tanabe, N.; Iijima, H. Clinical and genetic characteristics of pachydrusen in patients with exudative age-related macular degeneration. Sci. Rep. 2019, 9, 11906. [Google Scholar] [CrossRef]
- Castro-Navarro, V.; Behar-Cohen, F.; Chang, W.; Joussen, A.M.; Lai, T.Y.Y.; Navarro, R.; Pearce, I.; Yanagi, Y.; Okada, A.A. Pachychoroid: Current concepts on clinical features and pathogenesis. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 1385–1400. [Google Scholar] [CrossRef]
- Baek, J.; Lee, J.H.; Chung, B.J.; Lee, K.; Lee, W.K. Choroidal morphology under pachydrusen. Clin. Exp. Ophthalmol. 2019, 47, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Mitchell, P.; Freund, K.B.; Sadda, S.; Holz, F.G.; Brittain, C.; Henry, E.C.; Ferrara, D. The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology 2018, 125, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Garhofer, G.; Bek, T.; Boehm, A.G.; Gherghel, D.; Grunwald, J.; Jeppesen, P.; Kergoat, H.; Kotliar, K.; Lanzl, I.; Lovasik, J.V.; et al. Ocular Blood Flow Research Association. Use of the retinal vessel analyzer in ocular blood flow research. Acta Ophthalmol. 2010, 88, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Ulańczyk, Z.; Grabowicz, A.; Mozolewska-Piotrowska, K.; Safranow, K.; Kawa, M.P.; Pałucha, A.; Krawczyk, M.; Sikora, P.; Matczyńska, E.; Machaliński, B.; et al. Genetic factors associated with age-related macular degeneration: Identification of a novel PRPH2 single nucleotide polymorphism associated with increased risk of the disease. Acta Ophthalmol. 2021, 99, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Ooto, S.; Curcio, C.A. Subretinal drusenoid deposits AKA pseudodrusen. Surv. Ophthalmol. 2018, 63, 782–815. [Google Scholar] [CrossRef] [PubMed]
- Monge, M.; Araya, A.; Wu, L. Subretinal drusenoid deposits: An update. Taiwan. J. Ophthalmol. 2022, 12, 138–146. [Google Scholar] [PubMed]
- De Bats, F.; Mathis, T.; Mauget-Faÿsse, M.; Joubert, F.; Denis, P.; Kodjikian, L. Prevalence of reticular pseudodrusen in age-related macular degeneration using multimodal imaging. Retina 2016, 36, 46–52. [Google Scholar] [CrossRef]
- Chan, H.; Cougnard-Grégoire, A.; Delyfer, M.N.; Combillet, F.; Rougier, M.B.; Schweitzer, C.; Dartigues, J.F.; Korobelnik, J.F.; Delcourt, C. Multimodal Imaging of Reticular Pseudodrusen in a Population-Based Setting: The Alienor Study. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3058–3065. [Google Scholar] [CrossRef]
- Boddu, S.; Lee, M.D.; Marsiglia, M.; Marmor, M.; Freund, K.B.; Smith, R.T. Risk factors associated with reticular pseudodrusen versus large soft drusen. Am. J. Ophthalmol. 2014, 157, 985–993.e2. [Google Scholar] [CrossRef]
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Kovach, J.L.; Schwartz, S.G.; Agarwal, A.; Brantley, M.A.; Pan, S.S.; Haines, J.L.; Scott, W.K.; Pericak-Vance, M.A. The Relationship Between Reticular Pseudodrusen and Severity of AMD. Ophthalmology 2016, 123, 921–923. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Domalpally, A.; Agrón, E.; Pak, J.W.; Keenan, T.D.; Ferris, F.L., 3rd; Clemons, T.E.; Chew, E.Y. Prevalence, Risk, and Genetic Association of Reticular Pseudodrusen in Age-related Macular Degeneration: Age-Related Eye Disease Study 2 Report 21. Ophthalmology 2019, 126, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Ueda-Arakawa, N.; Ooto, S.; Nakata, I.; Yamashiro, K.; Tsujikawa, A.; Oishi, A.; Yoshimura, N. Prevalence and genomic association of reticular pseudodrusen in age-related macular degeneration. Am. J. Ophthalmol. 2013, 155, 260–269.e2. [Google Scholar] [CrossRef] [PubMed]
- Finger, R.P.; Wu, Z.; Luu, C.D.; Kearney, F.; Ayton, L.N.; Lucci, L.M.; Hubbard, W.C.; Hageman, J.L.; Hageman, G.S.; Guymer, R.H. Reticular pseudodrusen: A risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularization. Ophthalmology 2014, 121, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Agrón, E.; Domalpally, A.; Cukras, C.A.; Clemons, T.E.; Chen, Q.; Lu, Z.; Chew, E.Y.; Keenan, T.D.L.; AREDS and AREDS2 Research Groups. Reticular Pseudodrusen: The Third Macular Risk Feature for Progression to Late Age-Related Macular Degeneration: Age-Related Eye Disease Study 2 Report 30. Ophthalmology 2022, 129, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Finger, R.P.; Chong, E.; McGuinness, M.B.; Robman, L.D.; Aung, K.Z.; Giles, G.; Baird, P.N.; Guymer, R.H. Reticular Pseudodrusen and Their Association with Age-Related Macular Degeneration: The Melbourne Collaborative Cohort Study. Ophthalmology 2016, 123, 599–608. [Google Scholar] [CrossRef]
- Agrón, E.; Domalpally, A.; Cukras, C.A.; Clemons, T.E.; Chen, Q.; Swaroop, A.; Lu, Z.; Chew, E.Y.; Keenan, T.D.L.; AREDS and AREDS2 Research Groups. Reticular Pseudodrusen Status, ARMS2/HTRA1 Genotype, and Geographic Atrophy Enlargement: Age-Related Eye Disease Study 2 Report 32. Ophthalmology 2023, 130, 488–500. [Google Scholar] [CrossRef]
- Marsiglia, M.; Boddu, S.; Bearelly, S.; Xu, L.; Breaux BEJr Freund, K.B.; Breaux, B.E., Jr.; Yannuzzi, L.A.; Smith, R.T. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7362–7369. [Google Scholar] [CrossRef]
- Thiele, S.; Nadal, J.; Pfau, M.; Saßmannshausen, M.; Fleckenstein, M.; Holz, F.G.; Schmid, M.; Schmitz-Valckenberg, S. Prognostic value of intermediate age-related macular degeneration phenotypes for geographic atrophy progression. Br. J. Ophthalmol. 2021, 105, 239–245. [Google Scholar] [CrossRef]
- Sadda, S.R.; Abdelfattah, N.S.; Lei, J.; Shi, Y.; Marion, K.M.; Morgenthien, E.; Gune, S.; Balasubramanian, S. Spectral-Domain OCT Analysis of Risk Factors for Macular Atrophy Development in the HARBOR Study for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Guymer, R.H.; Wu, Z.; Hodgson, L.A.B.; Caruso, E.; Brassington, K.H.; Tindill, N.; Aung, K.Z.; McGuinness, M.B.; Fletcher, E.L.; Chen, F.K.; et al. Subthreshold Nanosecond Laser Intervention in Age-Related Macular Degeneration: The LEAD Randomized Controlled Clinical Trial. Ophthalmology 2019, 126, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina 2013, 33, 1800–1808. [Google Scholar] [CrossRef]
- Sassmannshausen, M.; Pfau, M.; Thiele, S.; Fimmers, R.; Steinberg, J.S.; Fleckenstein, M.; Holz, F.G.; Schmitz-Valckenberg, S. Longitudinal Analysis of Structural and Functional Changes in Presence of Reticular Pseudodrusen Associated with Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 19. [Google Scholar] [CrossRef] [PubMed]
- Abdolrahimzadeh, S.; Di Pippo, M.; Sordi, E.; Cusato, M.; Lotery, A.J. Subretinal drusenoid deposits as a biomarker of age-related macular degeneration progression via reduction of the choroidal vascularity index. Eye 2023, 37, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Oll, M.; Yzer, S.; Chang, S.; Barile, G.R.; Merriam, J.C.; Tsang, S.H.; Bearelly, S. Reticular pseudodrusen in early age-related macular degeneration are associated with choroidal thinning. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7075–7081. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.; Oh, J.; Ahn, S.E.; Hwang, S.Y.; Kim, S.W.; Huh, K. Peripapillary choroidal thickness in patients with early age-related macular degeneration and reticular pseudodrusen. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 427–435. [Google Scholar] [CrossRef]
- Viggiano, P.; Toto, L.; Ferro, G.; Evangelista, F.; Porreca, A.; Mastropasqua, R. Choroidal structural changes in different intermediate AMD patterns. Eur. J. Ophthalmol. 2022, 32, 460–467. [Google Scholar] [CrossRef]
- Thorell, M.R.; Goldhardt, R.; Nunes, R.P.; de Amorim Garcia Filho, C.A.; Abbey, A.M.; Kuriyan, A.E.; Modi, Y.S.; Gregori, G.; Yehoshua, Z.; Feuer, W.; et al. Association Between Subfoveal Choroidal Thickness, Reticular Pseudodrusen, and Geographic Atrophy in Age-Related Macular Degeneration. Ophthalmic Surg. Lasers Imaging Retina 2015, 46, 513–521. [Google Scholar] [CrossRef]
- Gabrielle, P.H.; Seydou, A.; Arnould, L.; Acar, N.; Devilliers, H.; Baudin, F.; Ben Ghezala, I.; Binquet, C.; Bron, A.M.; Creuzot-Garcher, C. Subretinal Drusenoid Deposits in the Elderly in a Population-Based Study (the Montrachet Study). Investig. Ophthalmol. Vis. Sci. 2019, 60, 4838–4848. [Google Scholar] [CrossRef]
- Arnold, J.J.; Sarks, S.H.; Killingsworth, M.C.; Sarks, J.P. Reticular pseudodrusen. A risk factor in age-related maculopathy. Retina 1995, 15, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Chatziralli, I.; Theodossiadis, G.; Panagiotidis, D.; Pousoulidi, P.; Theodossiadis, P. Choriocapillaris’ alterations in the presence of reticular pseudodrusen compared to drusen: Study based on OCTA findings. Int. Ophthalmol. 2018, 38, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.T.; Chung, H.W.; Jang, S.; Kim, S.W.; Oh, J.; Yun, C. Features of the macular and peripapillary choroid and choriocapillaris in eyes with nonexudative age-related macular degeneration. Retina 2020, 40, 2270–2276. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhou, X.; Chu, Z.; Gregori, G.; Wang, R.K.; Rosenfeld, P.J.; Guymer, R.H. Impact of Reticular Pseudodrusen on Choriocapillaris Flow Deficits and Choroidal Structure on Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2022, 63, 1. [Google Scholar] [CrossRef] [PubMed]
- Nesper, P.L.; Soetikno, B.T.; Fawzi, A.A. Choriocapillaris Nonperfusion is Associated with Poor Visual Acuity in Eyes With Reticular Pseudodrusen. Am. J. Ophthalmol. 2017, 54, 3250–3257. [Google Scholar] [CrossRef] [PubMed]
- Alten, F.; Clemens, C.R.; Heiduschka, P.; Eter, N. Localized reticular pseudodrusen and their topographic relation to choroidal watershed zones and changes in choroidal volumes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3250–3257. [Google Scholar] [CrossRef] [PubMed]
- Nickla, D.L.; Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef]
- Yoon, J.M.; Choi, Y.J.; Ham, D.I. Longitudinal change of reticular pseudodrusen area in ultrawide-field imaging. Sci. Rep. 2022, 12, 22383. [Google Scholar] [CrossRef]
- Xu, W.; Grunwald, J.E.; Metelitsina, T.I.; DuPont, J.C.; Ying, G.S.; Martin, E.R.; Dunaief, J.L.; Brucker, A.J. Association of risk factors for choroidal neovascularization in age-related macular degeneration with decreased foveolar choroidal circulation. Am. J. Ophthalmol. 2010, 150, 40–47.e2. [Google Scholar] [CrossRef]
- Zhang, X.; Sivaprasad, S. Drusen and pachydrusen: The definition, pathogenesis, and clinical significance. Eye 2021, 35, 121–133. [Google Scholar] [CrossRef]
- Kim, Y.H.; Chung, Y.R.; Kim, C.; Lee, K.; Lee, W.K. The Association of Pachydrusen Characteristics with Choroidal Thickness and Patient’s Age in Polypoidal Choroidal Vasculopathy versus Central Serous Chorioretinopathy. Int. J. Mol. Sci. 2022, 23, 8353. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Byeon, S.H. Prevalence and clinical characteristics of pachydrusen in polypoidal choroidal vasculopathy: Multimodal Image Study. Retina 2019, 39, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Sato-Akushichi, M.; Kinouchi, R.; Ishiko, S.; Hanada, K.; Hayashi, H.; Mikami, D.; Ono, S.; Yanagi, Y. Population-Based Prevalence and 5-Year Change of Soft Drusen, Pseudodrusen, and Pachydrusen in a Japanese Population. Ophthalmol. Sci. 2021, 1, 100081. [Google Scholar] [CrossRef] [PubMed]
- McCarter, R.V.; McKay, G.J.; Quinn, N.B.; Chakravarthy, U.; MacGillivray, T.J.; Robertson, G.; Pellegrini, E.; Trucco, E.; Williams, M.C.; Peto, T.; et al. Evaluation of coronary artery disease as a risk factor for reticular pseudodrusen. Br. J. Ophthalmol. 2018, 102, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Gil, G.; Otero-Marquez, O.; Alauddin, S.; Tong, Y.; Tai, K.; Lloyd, H.; Koci, M.; Scolaro, M.; Pillai, C.; Ye, C.; et al. Subretinal drusenoid deposits are strongly associated with coexistent high-risk vascular diseases. BMJ Open Ophthalmol. 2022, 7, e001154. [Google Scholar] [CrossRef]
- Liang, C.; Wang, N. Subretinal Drusenoid Deposits and Lower Serum High-Density Lipoprotein Cholesterol Levels Possess Latent Relation to Cardiovascular Disease and Can Be a Feasible Predictor. Comput. Math. Methods Med. 2022, 2022, 313510. [Google Scholar] [CrossRef] [PubMed]
- Gelinas, N.; Lynch, A.M.; Mathias, M.T.; Palestine, A.G.; Mandava, N.; Christopher, K.L.; Patnaik, J.L.; University of Colorado Retina Research Group. Gender as an effect modifier in the relationship between hypertension and reticular pseudodrusen in patients with early or intermediate age-related macular degeneration. Int. J. Ophthalmol. 2022, 15, 461–465. [Google Scholar] [CrossRef]
- Thomson, R.J.; Chazaro, J.; Otero-Marquez, O.; Ledesma-Gil, G.; Tong, Y.; Coughlin, A.C.; Teibel, Z.R.; Alauddin, S.; Tai, K.; Lloyd, H.; et al. Subretinal drusenoid deposits and soft drusen: Are they markers for distinct retinal diseases? Retina 2022, 42, 1311–1318. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Jia, C.; Sowers, J.R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1802–1809. [Google Scholar] [CrossRef]
- Machalińska, A.; Pius-Sadowska, E.; Babiak, K.; Sałacka, A.; Safranow, K.; Kawa, M.P.; Machaliński, B. Correlation between Flicker-Induced Retinal Vessel Vasodilatation and Plasma Biomarkers of Endothelial Dysfunction in Hypertensive Patients. Curr. Eye Res. 2018, 43, 128–134. [Google Scholar] [PubMed]
- Jabbarpoor Bonyadi, M.H.; Yaseri, M.; Nikkhah, H.; Bonyadi, M.; Soheilian, M. Association of risk genotypes of ARMS2/LOC387715 A69S and CFH Y402H with age-related macular degeneration with and without reticular pseudodrusen: A meta-analysis. Acta Ophthalmol. 2018, 96, e105–e110. [Google Scholar] [CrossRef] [PubMed]
- Altay, L.; Liakopoulos, S.; Berghold, A.; Rosenberger, K.D.; Ernst, A.; de Breuk, A.; den Hollander, A.I.; Fauser, S.; Schick, T. Genetic and environmental risk factors for reticular pseudodrusen in the EUGENDA study. Mol. Vis. 2021, 27, 757–767. [Google Scholar] [PubMed]
- Joachim, N.; Mitchell, P.; Rochtchina, E.; Tan, A.G.; Wang, J.J. Incidence and progression of reticular drusen in age-related macular degeneration: Findings from an older Australian cohort. Ophthalmology 2014, 121, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.T.; Merriam, J.E.; Sohrab, M.A.; Pumariega, N.M.; Barile, G.; Blonska, A.M.; Haans, R.; Madigan, D.; Allikmets, R. Complement factor H 402H variant and reticular macular disease. Arch. Ophthalmol. 2011, 129, 1061–1066. [Google Scholar] [CrossRef]
- Joachim, N.; Mitchell, P.; Younan, C.; Burlutsky, G.; Cheng, C.Y.; Cheung, C.M.; Zheng, Y.; Moffitt, M.; Wong, T.Y.; Wang, J.J. Ethnic variation in early age-related macular degeneration lesions between white Australians and Singaporean Asians. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4421–4429. [Google Scholar] [CrossRef]
- Puche, N.; Blanco-Garavito, R.; Richard, F.; Leveziel, N.; Zerbib, J.; Tilleul, J.; Mimoun, G.; Querques, G.; Cohen, S.Y.; Souied, E.H. Genetic and environmental factors associated with reticular pseudodrusen in age-related macular degeneration. Retina 2013, 33, 998–1004. [Google Scholar] [CrossRef]
- Spaide, R.F.; Yannuzzi, L.; Freund, K.B.; Mullins, R.; Stone, E. Eyes with subretinal drusenoid deposits and no drusen: Progression of Macular Findings. Retina 2019, 39, 12–26. [Google Scholar] [CrossRef]
| Parameter | SDD Present | SDD Absent | p-Value * |
|---|---|---|---|
| Number of subjects | 121 | 218 | - |
| Sex (male/female) [%] | 40/60 | 33.6/66.4 | 0.28 |
| Age [years] (mean ± SD) | 76.95 (6.36) | 71.56 (8.17) | <0.001 |
| Hypertension [%] | 70.75 | 61.58 | 0.13 |
| Duration of hypertension [years] (mean ± SD) | 9.62 (10.86) | 7.76 (8.94) | 0.16 |
| History of ischemic heart disease [%] | 16.04 | 17.46 | 0.87 |
| Duration of ischemic heart disease [years] (mean ± SD) | 1.36 (4.64) | 1.29 (4.14) | 0.41 |
| History of myocardial infarction [%] | 6.6 | 6.88 | 1.0 |
| Stroke [%] | 3.81 | 1.59 | 0.25 |
| Peripheral artery disease [%] | 6.6 | 4.76 | 0.59 |
| Aortic aneurysm [%] | 1.9 | 1.6 | 1.0 |
| Current smokers [%] | 9.43 | 16.32 | 0.12 |
| Former smokers [%] | 50.1 | 51.58 | 0.81 |
| Period without smoking [years] (mean ± SD) | 7.75 (11.65) | 5.9 (9.98) | 0.21 |
| Smoking pack years (mean ± SD) | 14.93 (19.87) | 13.37 (19.2) | 0.73 |
| BMI [kg/m2] (mean ± SD) | 26.98 (4.44) | 26.88 (4.23) | 0.52 |
| WHR [arbitrary units] (mean ± SD) | 0.9 (0.1) | 0.9 (0.09) | 0.71 |
| MAP [mmHg] (mean ± SD) | 98.22 (11.05) | 97.9 (11.18) | 0.85 |
| Physical activity [MET] (mean ± SD) | 1200.35 (1155.42) | 1723.77 (2444.57) | 0.12 |
| Total cholesterol [mg/dL] (mean ± SD) | 202.4 (43.55) | 204.21(44.62) | 0.85 |
| HDL [mg/dL] (mean ± SD) | 58.6 (12.69) | 61.13 (14.97) | 0.28 |
| LDL [mg/dL] (mean ± SD) | 119.5 (38.93) | 118.97 (38.82) | 0.87 |
| TG [mg/dL] (mean ± SD) | 101.32 (43.31) | 108.04 (55.31) | 0.48 |
| Clinical Feature | SDD Present | SDD Absent | p-Value * | |
|---|---|---|---|---|
| AMD stage | Early (yes [%]) | 8.99 | 25.11 | 0.008 |
| Intermediate (yes [%]) | 46.11 | 33.92 | ||
| Late (yes [%]) | 44.91 | 40.97 | ||
| Geographic atrophy (Yes/No) | 24/53 | 18/181 | 0.00003 | |
| MNV (Yes/No | 39/16 | 112/60 | 0.51 | |
| Pachychoroid (Yes/No) | 2/175 | 34/430 | 0.004 | |
| Pachyvessels (Yes/No) | 33/145 | 125/339 | 0.035 | |
| Pachydrusen (Yes/No) | 0/178 | 38/428 | 0.0002 | |
| Clinical Parameter | SDD Present Median (IQR) | SDD Absent Median (IQR) | p-Value * |
|---|---|---|---|
| Visual acuity (logMAR) | 0.4 (0.5) | 0.4 (0.52) | 0.21 |
| SFCT (μm) | 152 (113) | 203 (138) | <0.001 |
| ATC (μm) | 210.5 (144) | 294 (136) | <0.001 |
| AV (mm3) | 5.42 (2.57) | 7.08 (3.29) | <0.001 |
| AVC (mm3) | 0.17 (0.12) | 0.23 (0.11) | <0.001 |
| CVI | 0.65 (0.04) | 0.65 (0.05) | 0.05 |
| CRT (μm) | 277 (64) | 295 (104) | <0.001 |
| CRV (mm3) | 8.29 (0.78) | 8.54 (0.88) | <0.001 |
| CRAE | 179.3 (20.3) | 183.4 (22.1) | 0.003 |
| CRVE | 209 (26.65) | 216.5 (27.8) | 0.002 |
| AVR | 0.85 (0.11) | 0.85 (0.09) | 0.93 |
| DAA (%) | 2.9 (3.2) | 2.7 (3.1) | 0.84 |
| DAV (%) | 4.25 (2.5) | 3.9 (2.7) | 0.06 |
| Clinical Parameter | SFCT | ATC | AV | AVC | CVI | CRT | CRV | CRAE | CRVE |
|---|---|---|---|---|---|---|---|---|---|
| β | −0.14 | −0.22 | −0.19 | −0.22 | −0.06 | −0.19 | −0.16 | −0.04 | −0.002 |
| p-value | 0.002 | <0.001 | <0.001 | <0.001 | 0.21 | <0.001 | <0.001 | 0.34 | 0.97 |
| Tested SNP | Genotype | % of AMD Patients with SDD | % of AMD Patients without SDD | p-Value * |
|---|---|---|---|---|
| CFH Y402H | TT | 35.71% | 64.29% | 0.92 |
| TC | 35.43% | 64.57% | ||
| CC | 38.04% | 61.96% | ||
| ARMS2 A69S | GG | 37.50% | 62.50% | 0.89 |
| GT | 34.93% | 65.07% | ||
| TT | 38.10% | 61.90% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krytkowska, E.; Olejnik-Wojciechowska, J.; Grabowicz, A.; Safranow, K.; Machalińska, A. Association between Subretinal Drusenoid Deposits and Age-Related Macular Degeneration in Multimodal Retinal Imaging. J. Clin. Med. 2023, 12, 7728. https://doi.org/10.3390/jcm12247728
Krytkowska E, Olejnik-Wojciechowska J, Grabowicz A, Safranow K, Machalińska A. Association between Subretinal Drusenoid Deposits and Age-Related Macular Degeneration in Multimodal Retinal Imaging. Journal of Clinical Medicine. 2023; 12(24):7728. https://doi.org/10.3390/jcm12247728
Chicago/Turabian StyleKrytkowska, Elżbieta, Joanna Olejnik-Wojciechowska, Aleksandra Grabowicz, Krzysztof Safranow, and Anna Machalińska. 2023. "Association between Subretinal Drusenoid Deposits and Age-Related Macular Degeneration in Multimodal Retinal Imaging" Journal of Clinical Medicine 12, no. 24: 7728. https://doi.org/10.3390/jcm12247728
APA StyleKrytkowska, E., Olejnik-Wojciechowska, J., Grabowicz, A., Safranow, K., & Machalińska, A. (2023). Association between Subretinal Drusenoid Deposits and Age-Related Macular Degeneration in Multimodal Retinal Imaging. Journal of Clinical Medicine, 12(24), 7728. https://doi.org/10.3390/jcm12247728






