1. Introduction
Baroreceptors and vestibulosympathetic reflexes (VSRs) play important roles in regulating blood pressure in the autonomic nervous system. Studies have been conducted on the role of vestibular organs connected to the VSR in controlling blood pressure [
1]. In a study that observed changes in blood pressure and pulse rate through horizontal linear acceleration that activated the otolith organ, systolic blood pressure increased by 7–9 mmHg and diastolic blood pressure increased by 3–5 mmHg. In addition, patients with bilateral reduction in vestibular input show much smaller cardiovascular alterations during acceleration than those with normal vestibular function [
2]. Cardiovascular control is associated with breathing. In a study of cold vestibular caloric simulation, heart rate and blood pressure variabilities increased during spontaneous breathing; however, during paced breathing, no consistent effect on heart rate variability or blood pressure variability was evident. This change was not observed in the labyrinthine-defective patient [
3]. Changes in posture require rapid cardiovascular adjustments to maintain blood pressure and volume distribution. In healthy volunteers, heart rate is accelerated by head drops that occur within 500–600 ms of a beat; however, no rapid effects are observed in patients with vestibular defects [
4,
5].
These data suggest that vestibular stimulation can produce cardiovascular responses in humans and support the hypothesis that the vestibular system contributes to maintaining stable blood pressure during movement and changes in posture. Recent studies have indicated that otolithic organ dysfunction affects the autonomic nervous system. Studies have indicated that male patients in the absent vestibular-evoked myogenic potential (VEMP) group have a significant decrease in diastolic blood pressure 1 min after active standing up [
6], indicating that utricular dysfunction is associated with orthostatic hypotension (OH). OH is associated with high baseline systolic blood pressure, heart failure, and unilateral VEMP abnormalities [
7]. Studies have also indicated that the ratio of arrhythmia or heart rate variability markers is higher in patients with benign paroxysmal positional vertigo (BPPV) than in control groups [
8,
9]. OH, which accompanies BPPV, affects the recurrence of BPPV [
10]. The above findings also show that labyrinthine defective patients have a lower cardiovascular response to changes in the body’s position, and abnormalities in otolith organs affect changes in blood pressure. This study aimed to determine the effects of BPPV on blood pressure control in the autonomic nervous system by observing changes in blood pressure before and after BPPV treatment using the head-up tilt test (HUTT). BPPV increases blood pressure during the initial response to standing in the HUTT.
4. Discussion
In this study, three implications were found: (1) even in the presence of BPPV, blood pressure was normal in the supine position; (2) even in the presence of BPPV, blood pressure showed a pattern similar to a normal response in the HUTT; and (3) BPPV stimulates the otolith organ and activates the VSR, thus increasing blood pressure during the early stages of the HUTT.
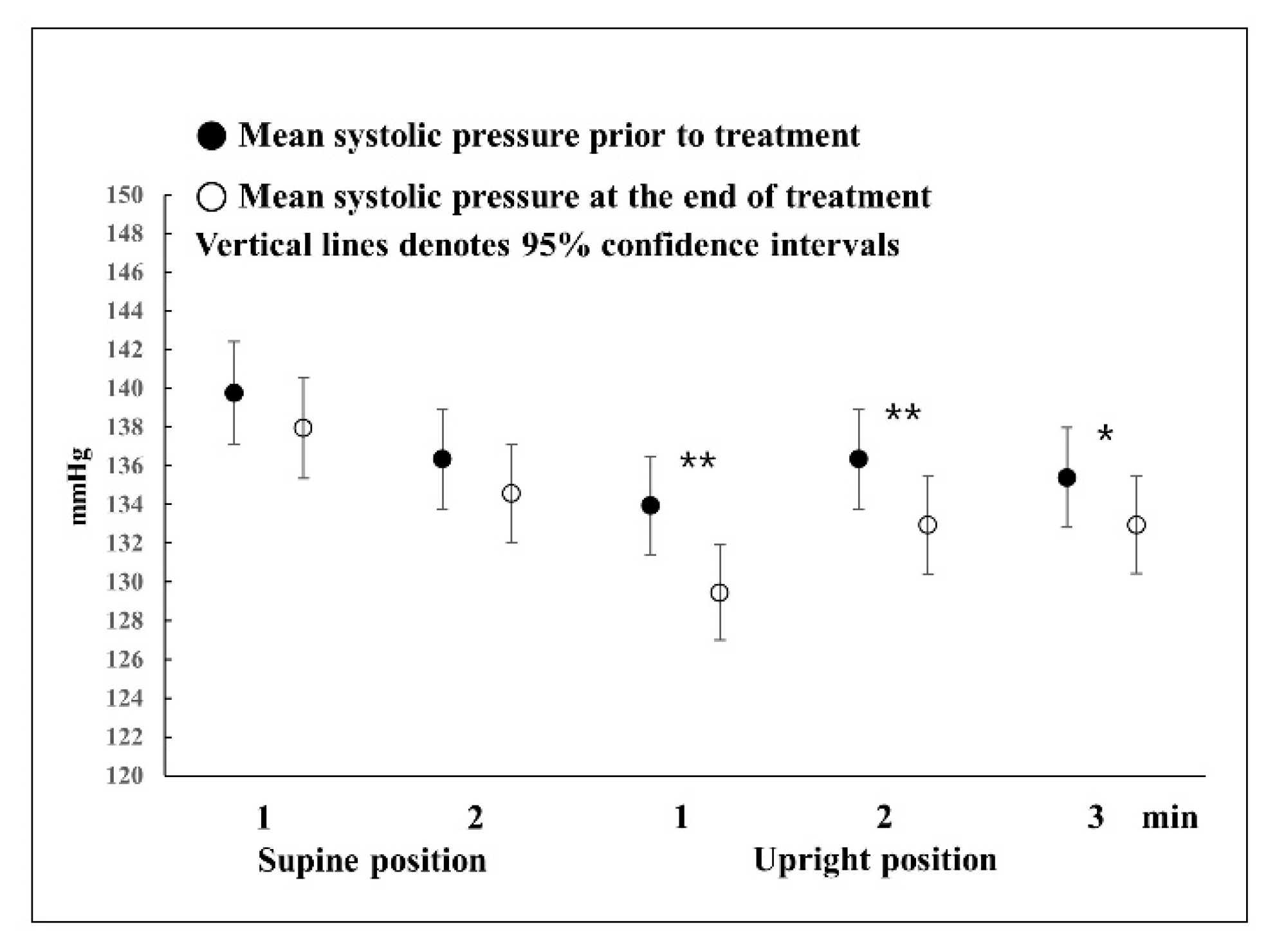
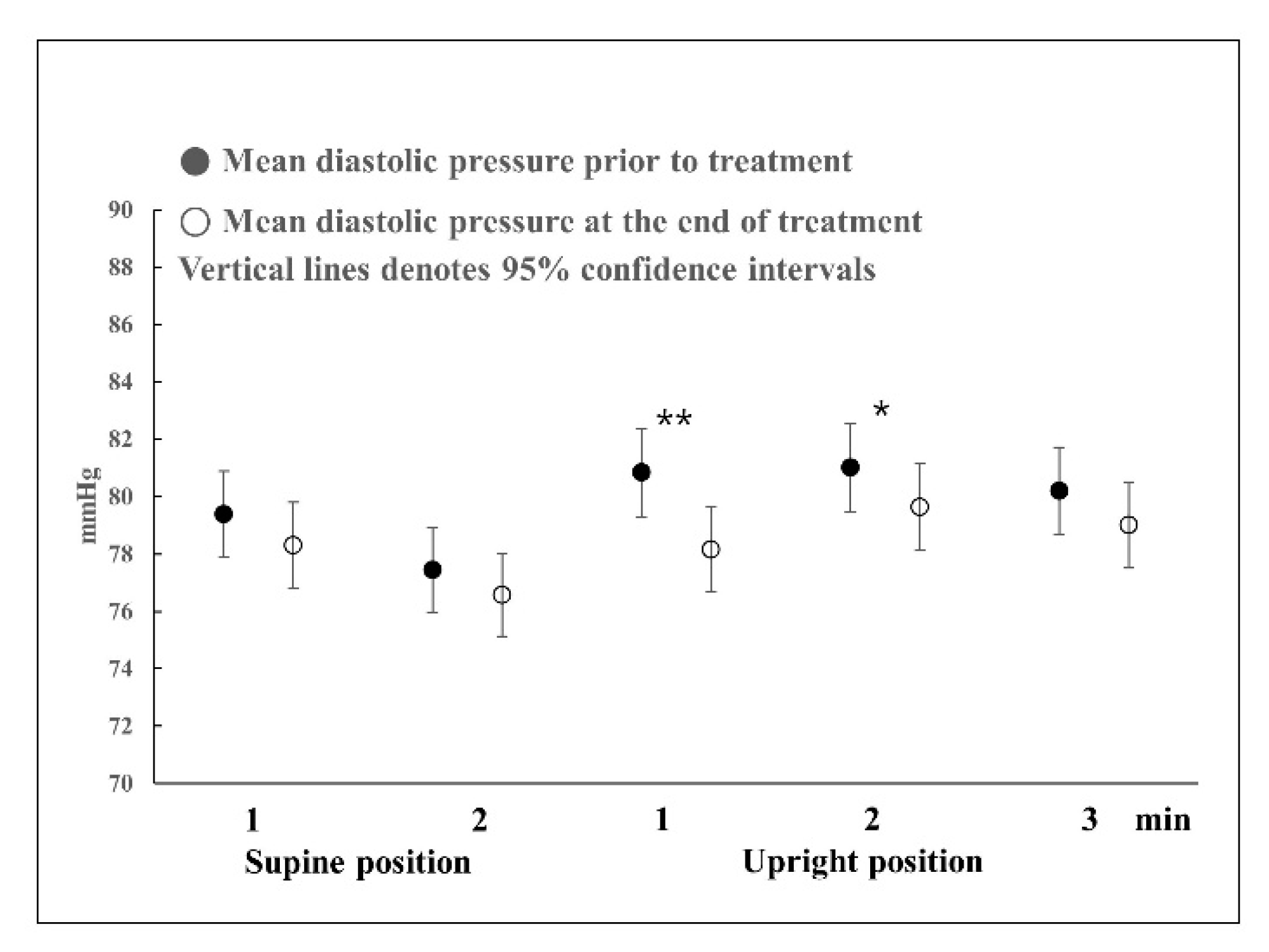
Most studies that use the HUTT have been on syncope, and there are few detailed results on normal responses. Furthermore, most HUTTs are performed until symptoms appear in a tilted state or for up to 45 min for the diagnosis of syncope [
13]. Therefore, only a few studies have mentioned up to 5 min after the onset of the HUTT. According to the findings of several studies on normal responses, in the upright position, the diastolic blood pressure increases slightly and systolic blood pressure decreases slightly before reaching a stable state at 2 min [
14,
15,
16]. In this study, in the HUTT in the supine position, there were also no changes in blood pressure before and after treatment. Systolic blood pressure dropped at 1 min in the upright position and then increased slightly at 2 or 3 min, but the systolic blood pressure pattern was the same before and after treatment. However, the systolic blood pressure before treatment decreased to less than that after complete recovery. Diastolic blood pressure showed slightly different responses before and after treatment. Before treatment, diastolic blood pressure increased at 1 min in the upright position, causing a large change in blood pressure, and then returned to a level similar to that of blood pressure in the supine position at 2 or 3 min. Regarding changes in blood pressure after complete recovery, the increase rate was lower than that of the blood pressure response before treatment; thus, blood pressure slowly increased until 2 min in the upright position and normalized thereafter. That is, in the presence of BPPV, blood pressure showed a rapid response in the upright position, such that systolic and diastolic blood pressure showed the largest change at 1 min; however, after complete recovery, such responses were not observed. When blood pressure was measured after sufficient rest, there was no difference in blood pressure in the supine position before and after treatment; therefore, it can be said that blood pressure at rest was normal even in the presence of BPPV. Meanwhile, the symptoms of vertigo or dizziness could trigger acute stress that increases blood pressure, which is not linked to vestibular stimulation. Although the difference in blood pressure in the supine position before and after treatment was not statistically significant, it can be seen that the blood pressure before treatment was slightly higher than after treatment. The possibility cannot be excluded that the increase in blood pressure was caused by acute stress without stimulation of the vestibular organ in the supine position. Excitement or depression could affect blood pressure. A previous study indicated a significant relationship between low blood pressure and increased depressive symptomatology scored using the Center for Epidemiologic Studies Depression Scale [
17]. Systolic hypotensive subjects scored a Center for Epidemiologic Studies Depression Scale mean of 12.07 ± 0.67 compared to 8.99 ± 0.95 for normotensives (
p < 0.01). Regression analyses supported these findings when controlling for confounders such as gender, age, and the use of antihypertensive medications. However, Menant et al. [
18] reported that participants were classified as having dizziness based on the Dizziness Handicap Inventory. Participants completed health questionnaires and underwent assessments of psychological well-being, lying and standing blood pressure, and vestibular function. Lying and standing blood pressure were not significantly associated with dizziness handicap severity.
However, during standing, when the VSR is overworking, the sympathetic nerve works toward excitation to increase the overall blood pressure; for this reason, it is believed that there is a statistically significant difference in blood pressure before and after treatment.
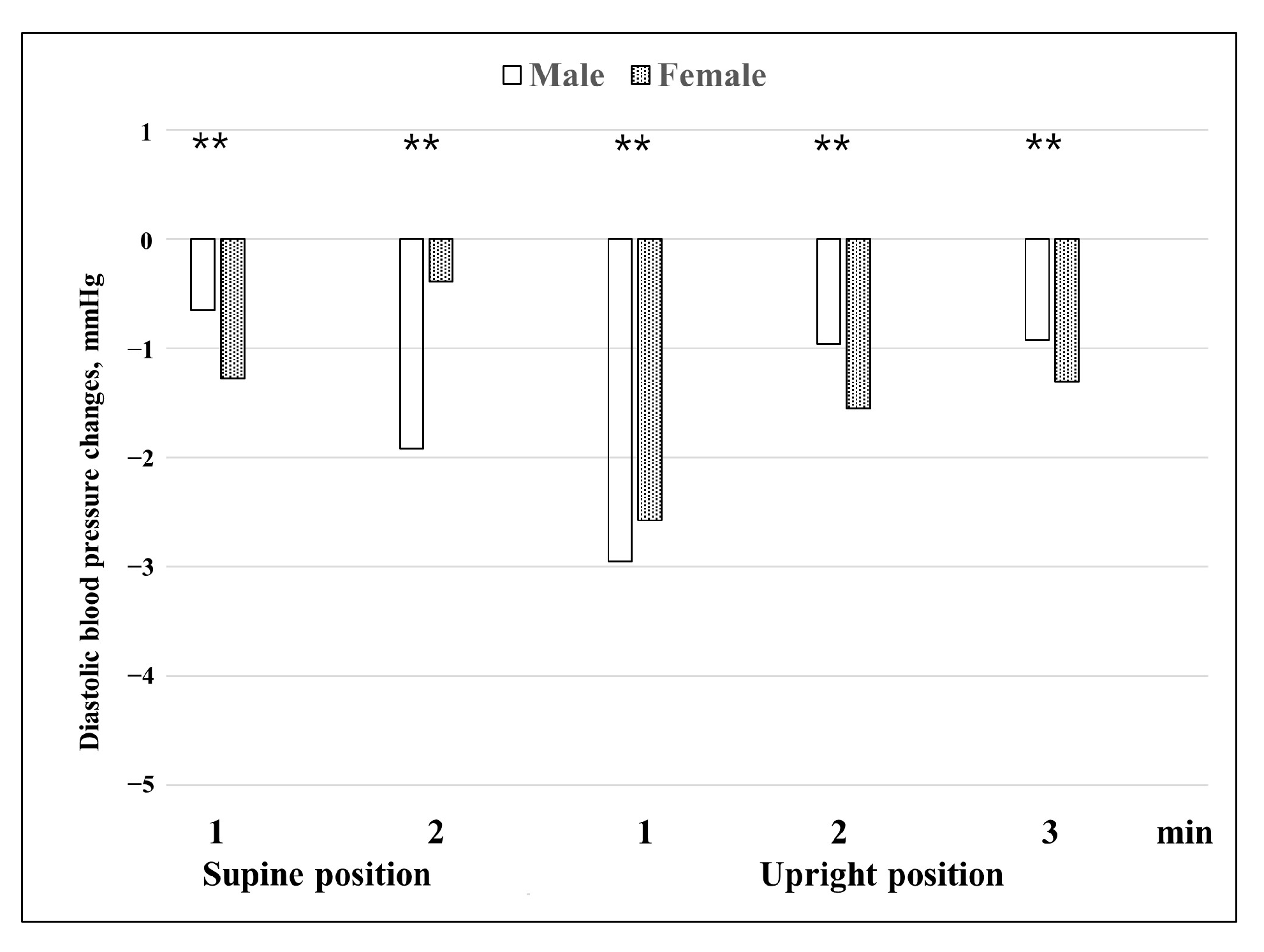
When differences in blood pressure changes before and after treatment between males and females were examined (
Figure 4 and
Figure 5), both systolic and diastolic blood pressures showed changes in a V shape around 1 min in the upright position. This means that in the presence of BPPV, blood pressure is most affected at 1 min in the upright position, and it can be seen that the VSR is affected by BPPV. In general, the effects of vestibular organs on the cardiovascular system occur within one or two heartbeats after rapid head movement, and the VSR has a latency of approximately 400–500 ms [
5,
19]. From the name of VSR, it can be seen that vestibular autonomic nerve reflection mainly affects the sympathetic nervous system. VSR suggests that otolith signals are important and operate more rapidly than baroreceptors in response to changes in blood pressure caused by body movement [
1,
2,
3]. The otolith is a linear accelerometer that measures gravitational and inertial acceleration [
20]. Raphan et al. [
21] reported that VSR modulates blood pressure and heart rate in an oscillating system by manipulating the parameters of the baroreflex feedback and the signals that maintain the oscillations.
Furthermore, it appears that in the case of BPPV, the autonomic nervous system is more vulnerable in females; therefore, the amount of change in blood pressure is generally higher in females than in males. Our findings that the difference in blood pressure changes before and after treatment between males and females (
Figure 4 and
Figure 5) are consistent with previously reported data obtained using shorter HUTT protocols. Braune et al. [
22] reported that sex had a significant influence on changes in blood pressure during passive HUTTs. Females showed more changes in blood pressure than males during the HUTT. In the orthostatic maneuvers, males showed lower blood pressure values compared with females. With increasing age, this difference became even more evident. The data suggest that the blood pressure responses to orthostatic stressors are attenuated with increasing age in contrast to the preserved blood pressure activation during non-orthostatic challenges. The reasons for the different impacts of age on blood pressure regulation remain to be identified. Some studies have found that there are sex effects on cardiovascular autonomic function during passive HUTTs [
23,
24]. In young women, variability in resting sympathetic nerve activity (SNA) is similar to that seen in men, but the ‘balancing’ mechanisms are strikingly different. Women exhibit greater β-adrenergic vasodilatation compared with men, which minimizes the pressor effects of a given level of SNA. In healthy men, the variability in SNA is balanced by variability in cardiac output and vascular adrenergic responses, such that blood pressure remains similar. Loss of estrogen with menopause in women appears to be linked mechanistically with a decrease in β-adrenergic vasodilatation and that suggest that β-adrenergic receptor sensitivity to noradrenaline is reduced in postmenopausal women. Another study observed that in men, mean blood pressure at rest (
p = 0.001 systolic and
p = 0.004 diastolic) and during HUTT (
p = 0.001 systolic and
p = 0.001 diastolic), mean total peripheral resistance at rest (
p = 0.034), and mean stroke volume during HUTT (
p = 0.001) were significantly higher. Particularly in older women, orthostatic changes in heart rate and diastolic blood pressure, the deep breathing ratio, and the Valsalva ratio become attenuated with age [
24]. Regarding the average age of females, 52.5 years in this study, the difference in the change in blood pressure between the sexes was partially explained. A previous study on the otolith organ and the autonomic nervous system by Aoki et al. [
6] classified the otolith organ according to the VEMP responses and reported that in the case of males, when the VEMP response was absent, the diastolic blood pressure dropped significantly compared to other groups at 1 min of active standing, and that in the case of the asymmetry and normal VEMP groups, the systolic blood pressure increased at 1 min of standing. The study reported that, in the case of females, there was no difference between the absent and other groups. Compared to our study, the study by Aoki et al. was an active standing test study, while our study was a passive standing test study; however, the most significant results were shown at 1 min of standing in both studies. Therefore, it can be seen that the time in which VSR has the largest effect is 1 min of standing. Furthermore, the fact that the responses of males and females differed can be considered a common point.
Kim et al. [
7] reported that in a HUTT using a finometer, OH was associated with high baseline systolic blood pressure, heart failure, and unilateral ocular VEMP abnormalities. The n1 latency of VEMP was negatively correlated with maximal changes in systolic blood pressure and utricular dysfunction related to OH. In a similar study, Kim et al. [
10] reported that when BPPV was present, the group without accompanying OH had fewer recurrences. In a study on the autonomic nervous system and BPPV associated with the heart, Günlü et al. [
9] reported that Holter monitoring detected abnormal rhythms in 41 (63%) of the patients in the study group and three (6.2%) of the patients in the control group. Yıldırım et al. [
8] reported that the Tp-e and Tp-e/QTc ratios were significantly higher in patients compared to controls. These findings suggest that the risk of ventricular arrhythmia is higher in patients with BPPV. The findings of the previous studies mentioned above indicate that abnormalities in the otolith organs are related to the autonomic nervous system. Patients with vestibular defects have a lower response to postural changes than those with normal vestibular function [
3,
4,
5]. However, in general, when the otolith organ is stimulated, the responses of the sympathetic nerves are diverse. Ray et al. [
25] reported that yaw head rotation, which stimulates horizontal semicircular canals, elicits a sympathetic nerve response. Yaw head rotation did not significantly change sympathetic nerve activity or mean arterial pressure. In another study, age-related VSR experiments showed that head-down rotation performance during simultaneous orthostatic stress further increased total muscle sympathetic nerve activity in young subjects, but not in older subjects. Older subjects consistently showed significant hypotension during head-down rotations [
19]. VSRs differ from responses triggered by the unloading of cardiovascular receptors, such as baroreceptors and cardiopulmonary receptors, as they can be elicited before a change in blood distribution occurs in the body [
1]. Therefore, it can be seen that in various studies in which VSR works, different results are produced according to various conditions.
This is one of the studies on the various autonomic nervous system responses of the otolith organ and helps verify that BPPV affects the autonomic nervous system through VSR and increases blood pressure during the initial response to the HUTT. Compared to other studies showing that patients with vestibular defects have small changes in blood pressure due to stimuli, such as changes in posture, this study did not show that BPPV reduces vestibular function. Among the participants in this study, patients with ear disease were excluded; however, patients with diabetes, high blood pressure, or heart disease that affected the results of the HUTT could not be excluded. Based on these findings, a follow-up study is needed to identify the potential risk factors for blood pressure response in the presence of BPPV.