Efficacy of Robot-Assisted Ureteroureterostomy in Patients with Complex Ureteral Stricture after Ureteroscopic Lithotripsy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Data Collection
2.3. Surgical Technique
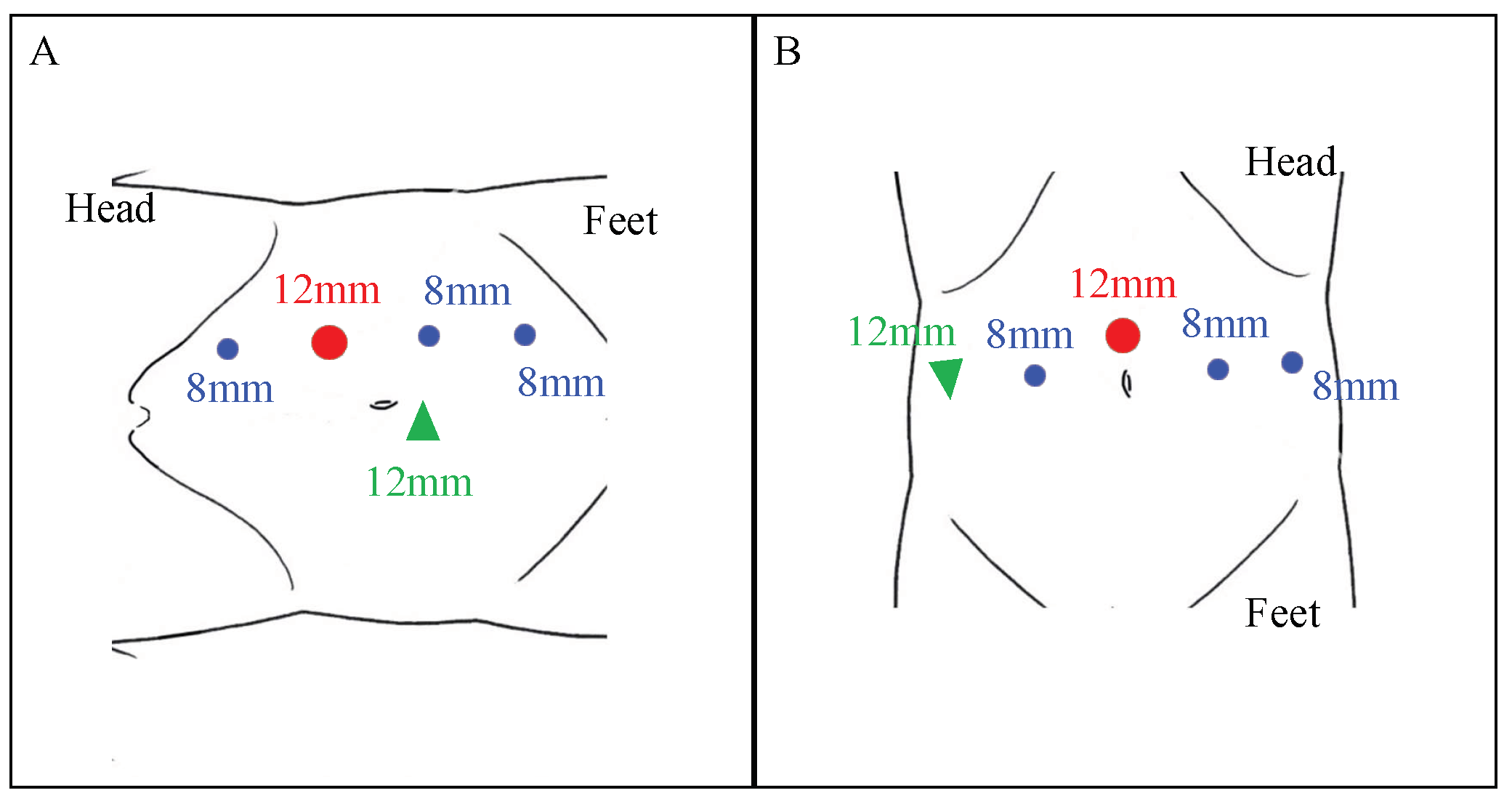
Patient and Robot Setup
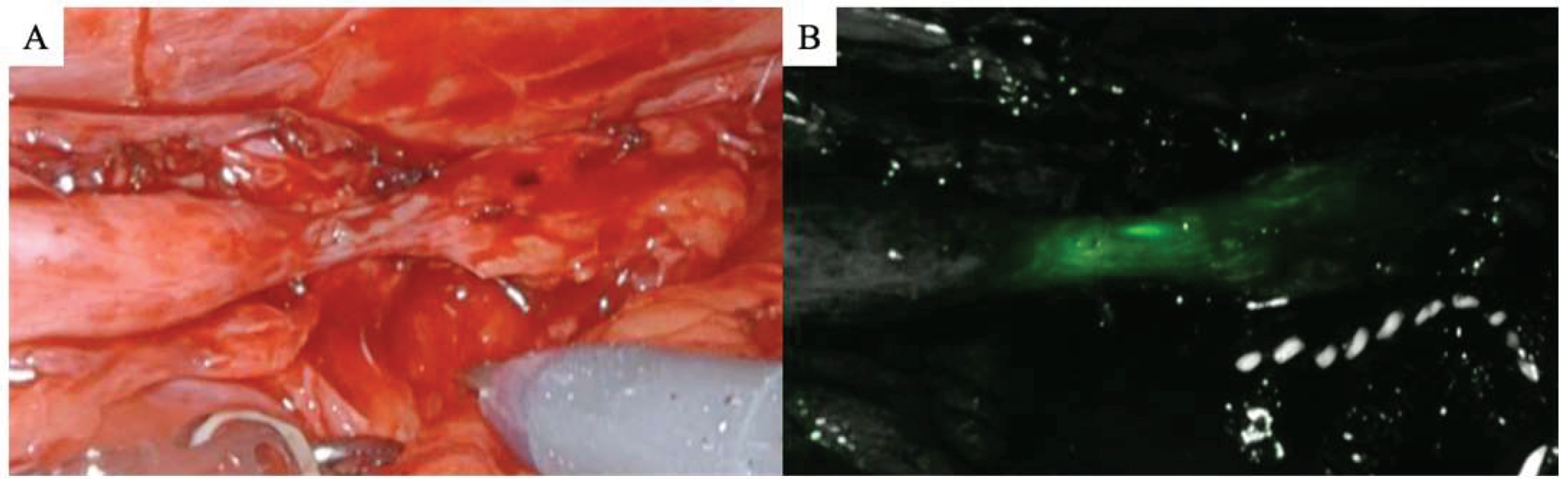
2.4. Stricture Identification
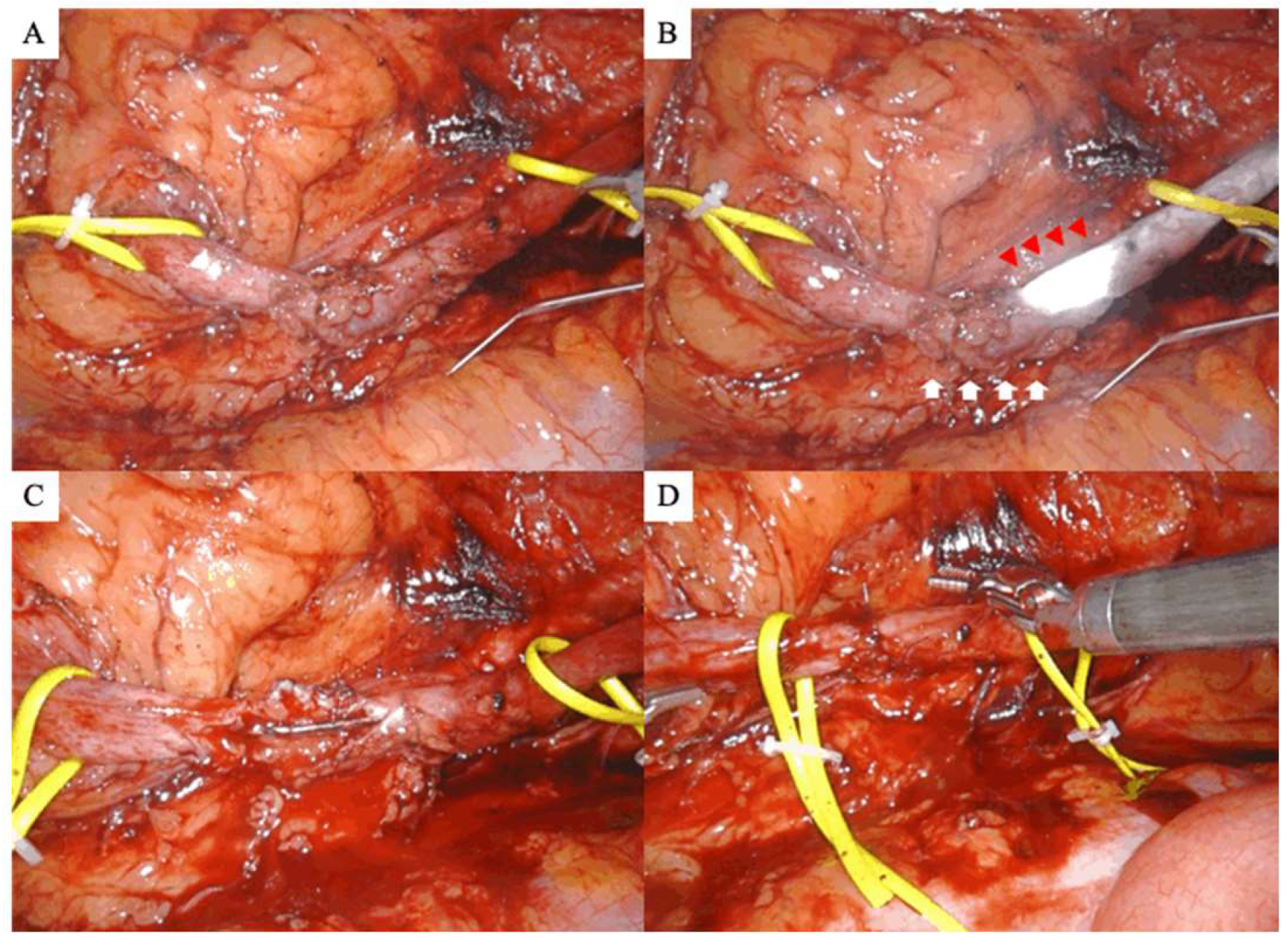
2.5. Dissection of the Ureteral Stricture, Anastomosis, and Stenting
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Surgical Outcomes
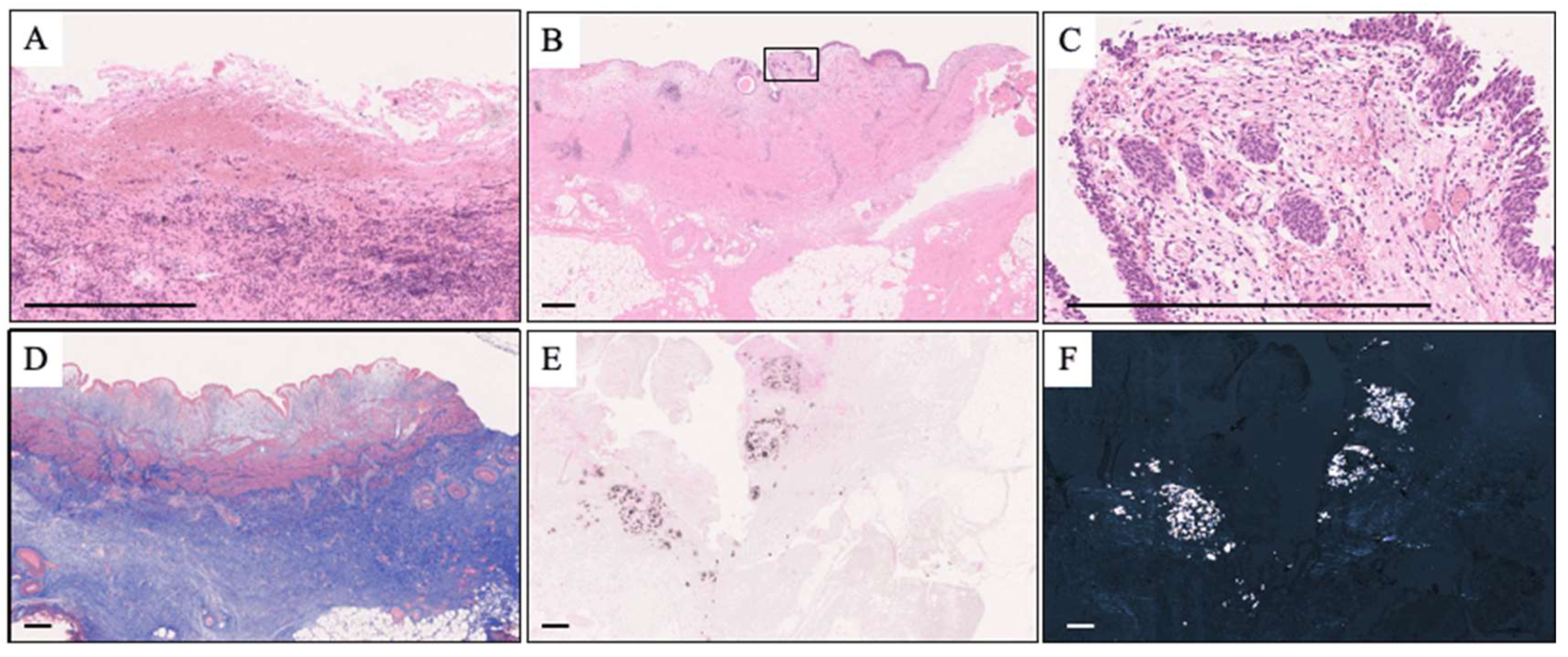
3.3. Pathological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, K.J.; Kim, J.H.; Min, G.E.; Park, H.K.; Li, S.; Del Giudice, F.; Han, D.H.; Chung, B.I. Changing trends in the treatment of nephrolithiasis in the real world. J. Endourol. 2019, 33, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Miyazawa, K.; Yasui, T.; Iguchi, T.; Fujita, M.; Nishimatsu, H.; Masaki, T.; Hasegawa, T.; Hibi, H.; Arakawa, T.; et al. Chronological changes in epidemiological characteristics of lower urinary tract urolithiasis in Japan. Int. J. Urol. 2019, 26, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Sunaryo, P.L.; May, P.C.; Holt, S.K.; Sorensen, M.D.; Sweet, R.M.; Harper, J.D. Ureteral strictures following ureteroscopy for kidney stone disease: A population-based assessment. J. Urol. 2022, 208, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Ulvik, Ø.; Harneshaug, J.R.; Gjengstø, P. Ureteral strictures following ureteroscopic stone treatment. J. Endourol. 2021, 35, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Razdan, S.; Silberstein, I.K.; Bagley, D.H. Ureteroscopic endoureterotomy. BJU Int. 2005, 95 (Suppl. 2), 94–101. [Google Scholar] [CrossRef] [PubMed]
- Sugino, T.; Taguchi, K.; Hamamoto, S.; Okada, T.; Isogai, M.; Tanaka, Y.; Unno, R.; Fujii, Y.; Hamakawa, T.; Ando, R.; et al. Risk factors for failure of endoscopic management of stone-related ureteral strictures. Urol. J. 2021, 19, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Carlton, C.E., Jr.; Guthrie, A.G.; Scott, R., Jr. Surgical correction of ureteral injury. J. Trauma 1969, 9, 457–464. [Google Scholar] [CrossRef]
- Simmons, M.N.; Gill, I.S.; Fergany, A.F.; Kaouk, J.H.; Desai, M.M. Laparoscopic ureteral reconstruction for benign stricture disease. Urology 2007, 69, 280–284. [Google Scholar] [CrossRef]
- Kolontarev, K.; Kasyan, G.; Pushkar, D. Robot-assisted laparoscopic ureteral reconstruction: A systematic review of literature. Cent. Eur. J. Urol. 2018, 71, 221–227. [Google Scholar] [CrossRef]
- Elsamra, S.E.; Theckumparampil, N.; Garden, B.; Alom, M.; Waingankar, N.; Leavitt, D.A.; Kreshover, J.; Schwartz, M.; Kavoussi, L.R.; Richstone, L. Open, laparoscopic, and robotic ureteroneocystotomy for benign and malignant ureteral lesions: A comparison of over 100 minimally invasive cases. J. Endourol. 2014, 28, 1455–1459. [Google Scholar] [CrossRef]
- Lee, Z.; Moore, B.; Giusto, L.; Eun, D.D. Use of indocyanine green during robot-assisted ureteral reconstructions. Eur. Urol. 2015, 67, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Elbers, J.R.; Rodríguez Socarrás, M.; Rivas, J.G.; Autran, A.M.; Esperto, F.; Tortolero, L.; Carrion, D.M.; Sancha, F.G. Robotic repair of ureteral strictures: Techniques and review. Curr. Urol. Rep. 2021, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- Ellenbogen, P.H.; Scheible, F.W.; Talner, L.B.; Leopold, G.R. Sensitivity of gray scale ultrasound in detecting urinary tract obstruction. AJR Am. J. Roentgenol. 1978, 130, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Tiselius, H.G.; Andersson, A. Stone burden in an average Swedish population of stone formers requiring active stone removal: How can the stone size be estimated in the clinical routine? Eur. Urol. 2003, 43, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Pizzolato, P. Histochemical recognition of calcium oxalate. J. Histochem. Cytochem. 1964, 12, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Roberts, W.W.; Cadeddu, J.A.; Micali, S.; Kavoussi, L.R.; Moore, R.G. Ureteral stricture formation after removal of impacted calculi. J. Urol. 1998, 159, 723–726. [Google Scholar] [CrossRef]
- Netto Júnior, N.R.; Ferreira, U.; Lemos, G.C.; Claro, J.F. Endourological management of ureteral strictures. J. Urol. 1990, 144, 631–634. [Google Scholar] [CrossRef]
- Vannahme, M.; Mathur, S.; Davenport, K.; Timoney, A.G.; Keeley, F.X., Jr. The management of secondary pelvi-ureteric junction obstruction—A comparison of pyeloplasty and endopyelotomy. BJU Int. 2014, 113, 108–112. [Google Scholar] [CrossRef]
- Moscardi, P.R.; Barbosa, J.A.; Andrade, H.S.; Mello, M.F.; Cezarino, B.N.; Oliveira, L.M.; Srougi, M.; Dénes, F.T.; Lopes, R.I. Reoperative laparoscopic ureteropelvic junction obstruction repair in children: Safety and efficacy of the technique. J. Urol. 2017, 197, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, Z.; Houston, N.; Strauss, D.; Lee, R.; Asghar, A.M.; Corse, T.; Zhao, L.C.; Stifelman, M.D.; Eun, D.D.; et al. Robotic ureteral reconstruction for recurrent strictures after prior failed management. BJUI Compass 2023, 4, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Buffi, N.; Cestari, A.; Lughezzani, G.; Bellinzoni, P.; Sangalli, M.; Scapaticci, E.; Zanoni, M.; Annino, F.; Larcher, A.; Lazzeri, M.; et al. Robot-assisted uretero-ureterostomy for iatrogenic lumbar and iliac ureteral stricture: Technical details and preliminary clinical results. Eur. Urol. 2011, 60, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Stifelman, M. Ureteral reimplantation, psoas hitch, and Boari flap. J. Endourol. 2020, 34, S25–S30. [Google Scholar] [CrossRef] [PubMed]
- Buffi, N.M.; Lughezzani, G.; Hurle, R.; Lazzeri, M.; Taverna, G.; Bozzini, G.; Bertolo, R.; Checcucci, E.; Porpiglia, F.; Fossati, N.; et al. Robot-assisted surgery for benign ureteral strictures: Experience and outcomes from four tertiary care institutions. Eur. Urol. 2017, 71, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Heijkoop, B.; Kahokehr, A.A. Buccal mucosal ureteroplasty for the management of ureteric strictures: A systematic review of the literature. Int. J. Urol. 2021, 28, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, X.; Xu, C.; Li, Z.; Han, G.; Fan, S.; Chen, S.; Li, X.; Zhu, H.; Zhou, L.; et al. Totally intracorporeal robot-assisted unilateral or bilateral ileal ureter replacement for the treatment of ureteral strictures: Technique and outcomes from a Single Center. Eur. Urol. 2023, 84, 561–570. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, Y.; Hu, H.; Zhang, J.; Qin, B.; Zhu, J.; Dirie, N.I.; Zhang, Z.; Wang, S. Management of recurrent ureteral stricture: A retrospectively comparative study with robot-assisted laparoscopic surgery versus open approach. PeerJ 2019, 7, e8166. [Google Scholar] [CrossRef]
- Hemal, A.K.; Nayyar, R.; Gupta, N.P.; Dorairajan, L.N. Experience with robot assisted laparoscopic surgery for upper and lower benign and malignant ureteral pathologies. Urology 2010, 76, 1387–1393. [Google Scholar] [CrossRef]
- Yang, K.K.; Asghar, A.M.; Lee, R.A.; Strauss, D.; Kuppa, S.; Lee, Z.; Metro, M.; Eun, D.D. Robot-assisted laparoscopic distal ureteroureterostomy for distal benign ureteral strictures with long-term follow-up. J. Endourol. 2022, 36, 203–208. [Google Scholar] [CrossRef]
- Lee, Z.; Llukani, E.; Reilly, C.E.; Mydlo, J.H.; Lee, D.I.; Eun, D.D. Single surgeon experience with robot-assisted ureteroureterostomy for pathologies at the proximal, middle, and distal ureter in adults. J. Endourol. 2013, 27, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Breda, A.; Diana, P.; Territo, A.; Gallioli, A.; Piana, A.; Gaya, J.M.; Gavrilov, P.; Desender, L.; Van Parys, B.; Van Praet, C.; et al. Intracorporeal versus extracorporeal robot-assisted kidney autotransplantation: Experience of the ERUS RAKT Working Group. Eur. Urol. 2022, 81, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.; Waldorf, B.T.; Cho, E.Y.; Liu, J.C.; Metro, M.J.; Eun, D.D. Robotic ureteroplasty with buccal mucosa graft for the management of complex ureteral strictures. J. Urol. 2017, 198, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, E.; Fujimori, M.; Kodama, H.; Felsen, D.; Chen, J.; Durack, J.C.; Solomon, S.B.; Coleman, J.A.; Srimathveeravalli, G. Macrophage-secreted TGF-β1 contributes to fibroblast activation and ureteral stricture after ablation injury. Am. J. Physiol. Ren. Physiol. 2019, 317, F52–F64. [Google Scholar] [CrossRef] [PubMed]
- Dretler, S.P.; Young, R.H. Stone granuloma: A cause of ureteral stricture. J. Urol. 1993, 150, 1800–1802. [Google Scholar] [CrossRef] [PubMed]
- Traxer, O.; Thomas, A. Prospective evaluation and classification of ureteral wall injuries resulting from insertion of a ureteral access sheath during retrograde intrarenal surgery. J. Urol. 2013, 189, 580–584. [Google Scholar] [CrossRef]
- Wollin, D.A.; Carlos, E.C.; Tom, W.R.; Simmons, W.N.; Preminger, G.M.; Lipkin, M.E. Effect of laser settings and irrigation rates on ureteral temperature during holmium laser lithotripsy, an in vitro model. J. Endourol. 2018, 32, 59–63. [Google Scholar] [CrossRef]
- Pietropaolo, A.; Massella, V.; Ripa, F.; Sinha, M.M.; Somani, B.K. Ureteroscopy and lasertripsy with pop dusting using high power holmium laser for large urinary stones > 15 mm: 6.5-year prospective outcomes from a high-volume stone center. World J. Urol. 2023, 41, 1935–1941. [Google Scholar] [CrossRef]
| Patient Characteristics | ||
|---|---|---|
| age (years) | 49.8 | (10.2) |
| sex (%) | ||
| male | 10 | (71.4) |
| female | 4 | (28.6) |
| laterality (%) | ||
| right | 6 | (42.9) |
| left | 8 | (57.1) |
| BMI (kg/m2) | 23.07 | (2.11) |
| Characteristics of previous urinary calculi | ||
| location (%) | ||
| UPJ | 4 | (28.6) |
| proximal ureter | 7 | (50.0) |
| middle ureter | 2 | (14.3) |
| distal ureter | 1 | (7.1) |
| stone size (mm) | 11.85 | (11.00, 14.75) |
| stone volume (mm2) | 78.11 | (72.76, 99.11) |
| number of previous stone treatment | ||
| SWL | 0.00 | (0.00, 0.00) |
| URSL | 0.00 | (0.00, 0.00) |
| PCNL | 2.00 | (1.00, 2.00) |
| stone analysis (%) | ||
| calcium stone | 8 | (57.1) |
| ammonium Acid Urate | 1 | (7.1) |
| cystine | 1 | (7.1) |
| unknown | 4 | (28.6) |
| Stricture Location (%) | ||
|---|---|---|
| UPJ | 3 | (21.4) |
| proximal ureter | 7 | (50.0) |
| middle ureter | 3 | (21.4) |
| distal ureter | 1 | (7.1) |
| deviation from the previous stone site (%) | 4 | (28.6) |
| stricture length on radiography (mm) | 22.66 | (7.38) |
| prior treatment for stricture (%) | ||
| none | 5 | (35.7) |
| dilation | 1 | (7.1) |
| endoscopic incision plus dilation | 8 | (57.1) |
| frequency of prior treatment for stricture times | 1.00 | (0.00, 1.00) |
| preoperative hydronephrosis (%) | ||
| none | 0 | (0) |
| mild | 0 | (0) |
| moderate | 4 | (28.6) |
| severe | 10 | (71.4) |
| preoperative drainage (%) | ||
| none | 1 | (7.1) |
| ureteral stent | 10 | (71.4) |
| percutaneous nephrostomy | 3 | (21.4) |
| split renal function of the affected side (%) | 29.28 | (15.31) |
| Stricture Identification | ||
|---|---|---|
| ICG | 8 | (57.1) |
| IRIS catheter | 6 | (42.9) |
| transected length of the ureter (mm) | 26.36 | (9.60) |
| surgery time (min) | 205.53 | −46.51 |
| console time (min) | 164.36 | (44.18) |
| blood loss (mL) | 10.00 | (0.00, 57.00) |
| perioperative complication (Clavien–Dindo grade; %) | ||
| 0 | 12 | (85.7) |
| I | 1 | (7.1) |
| II | 1 | (7.1) |
| ≥III | 0 | (0) |
| postoperative hydronephrosis after 3 months (%) | ||
| none | 2 | (14.3) |
| mild | 11 | (78.6) |
| moderate | 1 | (7.1) |
| severe | 0 | |
| success rate (%) | 13 | (92.9) |
| pathological features (%) | ||
| inflammatory cell infiltration | 13 | (92.9) |
| fibrosis | 13 | (92.9) |
| calcification | 8 | (57.1) |
| loss of urothelium | 4 | (28.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamamoto, S.; Taguchi, K.; Kawase, K.; Unno, R.; Isogai, M.; Torii, K.; Iwatsuki, S.; Etani, T.; Naiki, T.; Okada, A.; et al. Efficacy of Robot-Assisted Ureteroureterostomy in Patients with Complex Ureteral Stricture after Ureteroscopic Lithotripsy. J. Clin. Med. 2023, 12, 7726. https://doi.org/10.3390/jcm12247726
Hamamoto S, Taguchi K, Kawase K, Unno R, Isogai M, Torii K, Iwatsuki S, Etani T, Naiki T, Okada A, et al. Efficacy of Robot-Assisted Ureteroureterostomy in Patients with Complex Ureteral Stricture after Ureteroscopic Lithotripsy. Journal of Clinical Medicine. 2023; 12(24):7726. https://doi.org/10.3390/jcm12247726
Chicago/Turabian StyleHamamoto, Shuzo, Kazumi Taguchi, Kengo Kawase, Rei Unno, Masahiko Isogai, Koei Torii, Shoichiro Iwatsuki, Toshiki Etani, Taku Naiki, Atsushi Okada, and et al. 2023. "Efficacy of Robot-Assisted Ureteroureterostomy in Patients with Complex Ureteral Stricture after Ureteroscopic Lithotripsy" Journal of Clinical Medicine 12, no. 24: 7726. https://doi.org/10.3390/jcm12247726
APA StyleHamamoto, S., Taguchi, K., Kawase, K., Unno, R., Isogai, M., Torii, K., Iwatsuki, S., Etani, T., Naiki, T., Okada, A., & Yasui, T. (2023). Efficacy of Robot-Assisted Ureteroureterostomy in Patients with Complex Ureteral Stricture after Ureteroscopic Lithotripsy. Journal of Clinical Medicine, 12(24), 7726. https://doi.org/10.3390/jcm12247726








