Relationship between Pharmacological Treatment Strategy and Cognitive Function in Geriatric Patients with Atrial Fibrillation
Abstract
:1. Introduction
2. Patients and Methodology
2.1. Study Design
2.2. Data Collection
2.3. Analysis
3. Results
3.1. Patient Characteristics
3.2. Factors Influencing Mini Mental Status
4. Discussion
5. Conclusions
Strengths and Weaknesses of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schnabel, R.B.; Wilde, S.; Wild, P.S.; Munzel, T.; Blankenberg, S. Atrial fibrillation: Its prevalence and risk factor profile in the German general population. Dtsch. Arztebl. Int. 2012, 109, 293–299. [Google Scholar] [CrossRef]
- Ataklte, F.; Huang, Q.; Kornej, J.; Mondesir, F.; Benjamin, E.J.; Trinquart, L. The association of education and household income with the lifetime risk of incident atrial fibrillation: The Framingham Heart study. Am. J. Prev. Cardiol. 2022, 9, 100314. [Google Scholar] [CrossRef]
- Moser, J.; Kuck, K.H.; Andresen, D.; Spitzer, S.G.; Hoffmann, E.; Schumacher, B.; Eckardt, L.; Brachmann, J.; Lewalter, T.; Hochadel, M.; et al. Anticoagulation in high thromboembolic risk after catheter ablation for atrial fibrillation: Results from the German Ablation Registry. Dtsch. Med. Wochenschr. 2014, 139, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Sartipy, U.; Dahlstrom, U.; Fu, M.; Lund, L.H. Atrial Fibrillation in Heart Failure With Preserved, Mid-Range, and Reduced Ejection Fraction. JACC Heart Fail. 2017, 5, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yang, P.S.; Yu, H.T.; Kim, T.H.; Jang, E.; Sung, J.H.; Pak, H.N.; Lee, M.Y.; Lee, M.H.; Lip, G.Y.H.; et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: Data from a population-based cohort. Eur. Heart J. 2019, 40, 2313–2323. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.A.; Dawber, T.R.; Thomas, H.E., Jr.; Kannel, W.B. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: The Framingham study. Neurology 1978, 28, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Vinter, N.; Huang, Q.; Fenger-Gron, M.; Frost, L.; Benjamin, E.J.; Trinquart, L. Trends in excess mortality associated with atrial fibrillation over 45 years (Framingham Heart Study): Community based cohort study. BMJ 2020, 370, m2724. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.K.; Chan, J.Y.; Hirai, H.W.; Wong, S.Y.; Kwok, T.C. Cognitive Tests to Detect Dementia: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2015, 175, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Hylek, E.M.; Go, A.S.; Chang, Y.; Jensvold, N.G.; Henault, L.E.; Selby, J.V.; Singer, D.E. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N. Engl. J. Med. 2003, 349, 1019–1026. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Groenveld, H.F.; Crijns, H.J.; Tuininga, Y.S.; Tijssen, J.G.; Alings, A.M.; Hillege, H.L.; Bergsma-Kadijk, J.A.; Cornel, J.H.; Kamp, O.; et al. Lenient versus strict rate control in patients with atrial fibrillation. N. Engl. J. Med. 2010, 362, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Mulder, B.A.; Van Veldhuisen, D.J.; Crijns, H.J.; Tijssen, J.G.; Hillege, H.L.; Alings, M.; Rienstra, M.; Groenveld, H.F.; Van den Berg, M.P.; Van Gelder, I.C.; et al. Lenient vs. strict rate control in patients with atrial fibrillation and heart failure: A post-hoc analysis of the RACE II study. Eur. J. Heart Fail. 2013, 15, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Wyse, D.G.; Waldo, A.L.; DiMarco, J.P.; Domanski, M.J.; Rosenberg, Y.; Schron, E.B.; Kellen, J.C.; Greene, H.L.; Mickel, M.C.; Dalquist, J.E.; et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Hagens, V.E.; Bosker, H.A.; Kingma, J.H.; Kamp, O.; Kingma, T.; Said, S.A.; Darmanata, J.I.; Timmermans, A.J.; Tijssen, J.G.; et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N. Engl. J. Med. 2002, 347, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Dickow, J.; Kirchhof, P.; Van Houten, H.K.; Sangaralingham, L.R.; Dinshaw, L.H.W.; Friedman, P.A.; Packer, D.L.; Noseworthy, P.A.; Yao, X. Generalizability of the EAST-AFNET 4 Trial: Assessing Outcomes of Early Rhythm-Control Therapy in Patients With Atrial Fibrillation. J. Am. Heart Assoc. 2022, 11, e024214. [Google Scholar] [CrossRef] [PubMed]
- Volgman, A.S.; Nair, G.; Lyubarova, R.; Merchant, F.M.; Mason, P.; Curtis, A.B.; Wenger, N.K.; Aggarwal, N.T.; Kirkpatrick, J.N.; Benjamin, E.J. Management of Atrial Fibrillation in Patients 75 Years and Older: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 166–179. [Google Scholar] [CrossRef]
- Depoorter, L.; Sels, L.; Deschodt, M.; Van Grootven, B.; Van der Linden, L.; Tournoy, J. Clinical Outcomes of Rate vs Rhythm Control for Atrial Fibrillation in Older People: A Systematic Review and Meta-Analysis. Drugs Aging 2020, 37, 19–26. [Google Scholar] [CrossRef]
- Gotze, M.; Ebelt, H. Prevalence, incidence and predictive factors of atrial fibrillation in geriatric patients-A prospective observational study. Z. Gerontol. Geriatr. 2023. [Google Scholar] [CrossRef]
- Doshi, S.K.; Kar, S.; Sadhu, A.; Horton, R.; Osorio, J.; Ellis, C.; Stone, J., Jr.; Shah, M.; Dukkipati, S.R.; Adler, S.; et al. Two-Year Outcomes With a Next-Generation Left Atrial Appendage Device: Final Results of the PINNACLE FLX Trial. J. Am. Heart Assoc. 2023, 12, e026295. [Google Scholar] [CrossRef]
- Sakriss, C.; Roehl, P.; Schwenzky, A.; Hoyme, M.; Ebelt, H. Transition from WATCHMAN V.2.5 to WATCHMAN FLX for closure of the left atrial appendage: Echocardiographic and clinical findings. Open Heart 2023, 10, e002246. [Google Scholar] [CrossRef] [PubMed]
- Offhaus, A.; Linss, L.; Roehl, P.; Sakriss, C.; Pertschy, U.; Schwenzky, A.; Ebelt, H. CT-Based Preplanning Allows Abstaining from Intraprocedural TEE during Interventional Closure of the LAA in Patients with Atrial Fibrillation. J. Clin. Med. 2023, 12, 4019. [Google Scholar] [CrossRef] [PubMed]
- Svedbom, A.; Hernlund, E.; Ivergard, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A.; EU review panel of the IOF. Osteoporosis in the European Union: A compendium of country-specific reports. Arch. Osteoporos. 2013, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, A.; Oristrell, J.; Pla, X.; Ruggiero, C.; Ferretti, R.; Diestre, G.; Clarfield, A.M.; Crome, P.; Hertogh, C.; Lesauskaite, V.; et al. The persistent exclusion of older patients from ongoing clinical trials regarding heart failure. Arch. Intern. Med. 2011, 171, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Fratiglioni, L.; Johnell, K.; Santoni, G.; Fastbom, J.; Ljungman, P.; Marengoni, A.; Qiu, C. Atrial fibrillation, antithrombotic treatment, and cognitive aging: A population-based study. Neurology 2018, 91, e1732–e1740. [Google Scholar] [CrossRef] [PubMed]
- Wilke, T.; Groth, A.; Mueller, S.; Pfannkuche, M.; Verheyen, F.; Linder, R.; Maywald, U.; Bauersachs, R.; Breithardt, G. Incidence and prevalence of atrial fibrillation: An analysis based on 8.3 million patients. Europace 2013, 15, 486–493. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Igras, E.; Ramesh, A.; Power, B.; O’Connor, K.; Liston, R. Assessing the Appropriateness of Oral Anticoagulation for Atrial Fibrillation in Advanced Frailty: Use of Stroke and Bleeding Risk-Prediction Models. J. Frailty Aging 2017, 6, 46–52. [Google Scholar] [CrossRef]
- Liczko, J.; Schulein, S.; Tumena, T.; Gassmann, K.G. Prevalence and treatment of atrial fibrillation in older adults. Z. Gerontol. Geriatr. 2023, 56, 146–152. [Google Scholar] [CrossRef]
- Ekerstad, N.; Karlsson, T.; Soderqvist, S.; Karlson, B.W. Hospitalized frail elderly patients—Atrial fibrillation, anticoagulation and 12 months’ outcomes. Clin. Interv. Aging 2018, 13, 749–756. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
| Variable | Patients with Rhythm Control | Patients with Frequency Control | p-Value |
|---|---|---|---|
| number | 43 (6%) | 672 (94%) | - |
| age (years) | 79.7 ± 5.8 | 84 ± 5.8 | <0.001 |
| female gender | 28 (65.1%) | 444 (66.1%) | 0.898 |
| height (cm) | 167 ± 9 | 166 ± 9 | 0.581 |
| weight (kg) | 84.8 ± 16.3 | 76.8 ± 25.9 | 0.046 |
| Barthel index | 36 ± 12 | 34 ± 13 | 0.374 |
| MMS | 22 ± 7 | 21 ± 7 | 0.398 |
| CHA2DS2Vasc | 3.86 ± 1.06 | 4.18 ± 1.16 | 0.082 |
| HASBLED | 3.42 ± 0.91 | 3.56 ± 0.90 | 0.334 |
| heart rate (bpm) | 73 ± 19 | 75 ± 15 | 0.527 |
| creatinine (µmol/L) | 126 ± 48 | 105 ± 51 | 0.008 |
| ALAT (µmol/L) | 0.44 ± 0.28 | 0.45 ± 0.70 | 0.904 |
| ASAT (µmol/L) | 0.54 ± 0.36 | 0.54 ± 0.57 | 0.994 |
| bilirubin (µmol/L) | 13 ± 13 | 12 ± 7 | 0.704 |
| hypertension | 34 (79.1%) | 579 (86.2%) | 0.197 |
| diabetes | 5 (11.6%) | 79 (11.8%) | 0.98 |
| heart failure | 32 (74.4%) | 421 (62.6%) | 0.12 |
| previous stroke | 9 (20.9%) | 194 (28.9%) | 0.263 |
| any antiplatelet medication | 7 (16.3%) | 84 (12.5%) | 0.49 |
| aspirin | 4 (9.3%) | 62 (9.2%) | 0.987 |
| clopidogrel | 5 (11.6%) | 34 (5.1%) | 0.066 |
| any OAC | 35 (81.4%) | 553 (82.3%) | 0.881 |
| VKA | 2 (4.7%) | 52 (7.7%) | 0.458 |
| dabigatran | 0 (0%) | 28 (4.2%) | 0.172 |
| rivaroxaban | 5 (11.6%) | 98 (14.6%) | 0.593 |
| apixaban | 18 (41.9%) | 245 (36.5%) | 0.476 |
| edoxaban | 10 (23.3%) | 133 (19.8%) | 0.582 |
| CCB | 10 (23.3%) | 204 (30.4%) | 0.324 |
| beta blockers | 31 (72.1%) | 515 (76.6%) | 0.497 |
| ACE inhibitor | 28 (65.1%) | 402 (59.8%) | 0.492 |
| digitalis glycosides | 4 (9.3%) | 98 (14.6%) | 0.337 |
| amiodarone | 33 (76.7%) | 0 (0%) | <0.001 |
| history of ablation | 10 (23.3%) | 0 (0%) | <0.001 |
| LAAC | 2 (4.7%) | 12 (1.8%) | 0.189 |
| Variable | Regression Coefficient B | p-Value |
|---|---|---|
| age (years) | −0.212 | <0.001 |
| CHA2DS2Vasc | −0.396 | 0.088 |
| HASBLED | −0.881 | 0.003 |
| OAC | 1.832 | 0.008 |
| creatinine (µmol/L) | −0.011 | 0.03 |
| CCB | 0.067 | 0.908 |
| beta blockers | 0.434 | 0.488 |
| digitalis glykosides | −0.573 | 0.462 |
| amiodarone | 1.594 | 0.193 |
| heart rate (bpm) | 0.007 | 0.768 |
| rhythm control | 0.91 | 0.398 |
| Variable | RHY (n = 42) | RATE (n = 42) | p-Value |
|---|---|---|---|
| MMS | 22.0 ± 7.4 | 19.9 ± 7.3 | 0.20 |
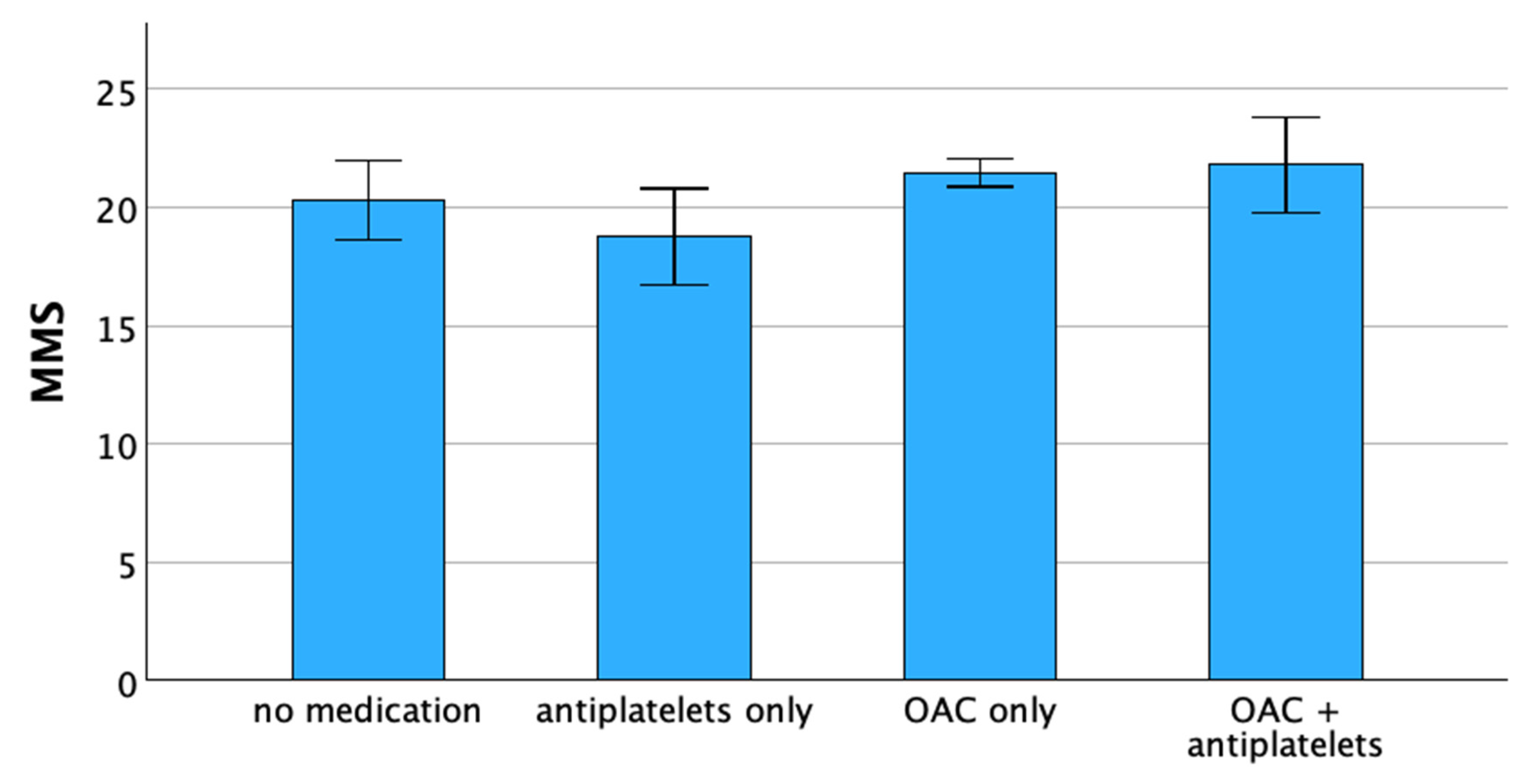
| Total | No Anticoagulation | Antiplatelet Medication Only | OAC Only | Combination of Antiplatelets and OAC |
|---|---|---|---|---|
| 715 (100%) | 76 (10.6%) | 51 (7.1%) | 541 (75.7%) | 47 (6.6%) |
| Variable | Regression Coefficient B | p-Value |
|---|---|---|
| no anticoagulation | −0.974 | 0.258 |
| antiplatelets only | −2.621 | 0.01 |
| OAC only | 1.262 | 0.042 |
| OAC + antiplatelets | 0.669 | 0.544 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goetze, M.; Knauf, T.; Ebelt, H. Relationship between Pharmacological Treatment Strategy and Cognitive Function in Geriatric Patients with Atrial Fibrillation. J. Clin. Med. 2023, 12, 7724. https://doi.org/10.3390/jcm12247724
Goetze M, Knauf T, Ebelt H. Relationship between Pharmacological Treatment Strategy and Cognitive Function in Geriatric Patients with Atrial Fibrillation. Journal of Clinical Medicine. 2023; 12(24):7724. https://doi.org/10.3390/jcm12247724
Chicago/Turabian StyleGoetze, Markus, Tim Knauf, and Henning Ebelt. 2023. "Relationship between Pharmacological Treatment Strategy and Cognitive Function in Geriatric Patients with Atrial Fibrillation" Journal of Clinical Medicine 12, no. 24: 7724. https://doi.org/10.3390/jcm12247724
APA StyleGoetze, M., Knauf, T., & Ebelt, H. (2023). Relationship between Pharmacological Treatment Strategy and Cognitive Function in Geriatric Patients with Atrial Fibrillation. Journal of Clinical Medicine, 12(24), 7724. https://doi.org/10.3390/jcm12247724






