Intestinal Microbiome Changes and Clinical Outcomes of Patients with Ulcerative Colitis after Fecal Microbiota Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Desing
2.2. Healthy Volunteers and Donors Patients
2.3. Patients
2.4. FMT Enema Preparation and Application
2.5. Clinical Outcomes
2.6. Sequencing
2.7. Data Analysis and Statistics
3. Results
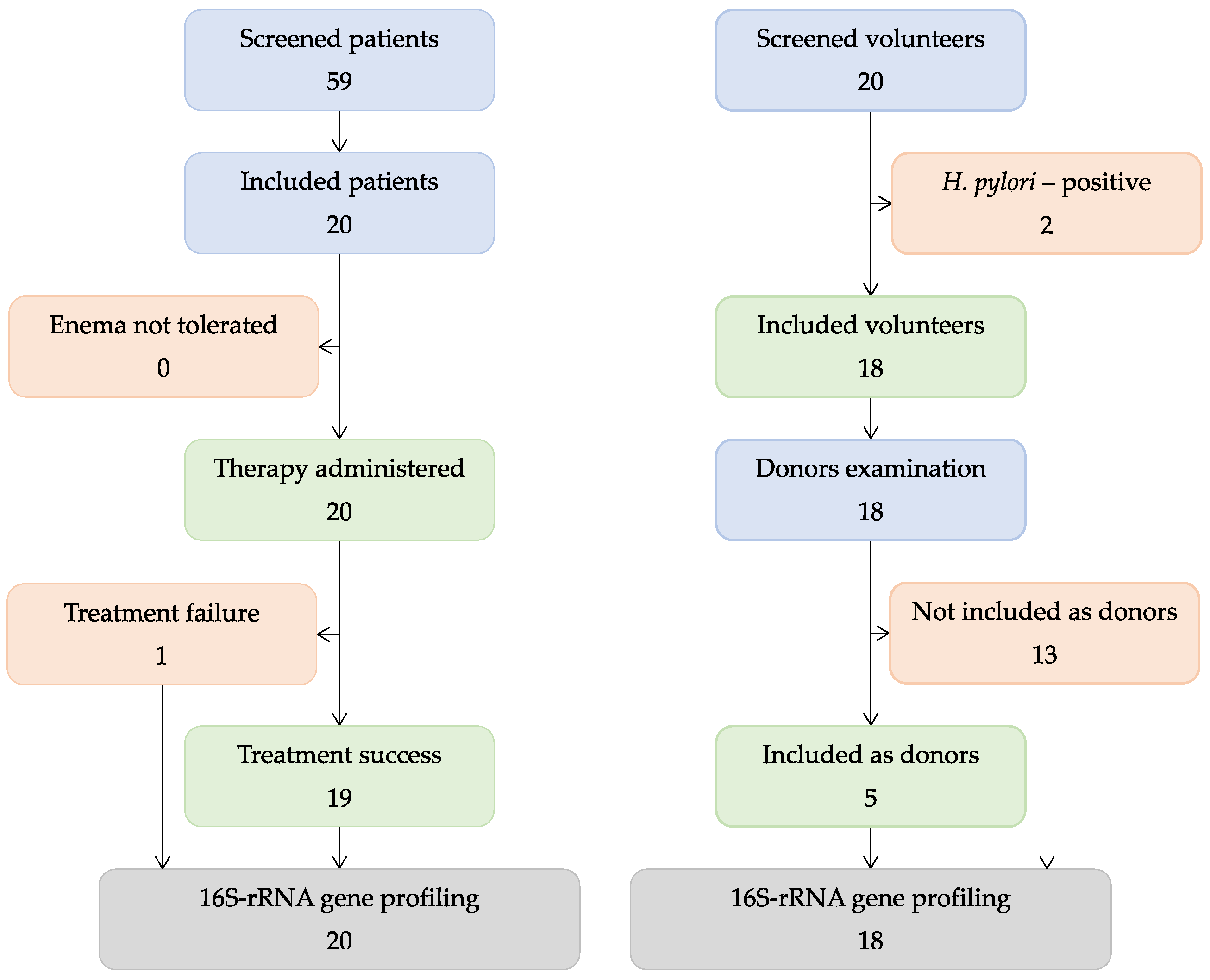
3.1. Patients and Volunteers
3.2. Clinical Outcomes
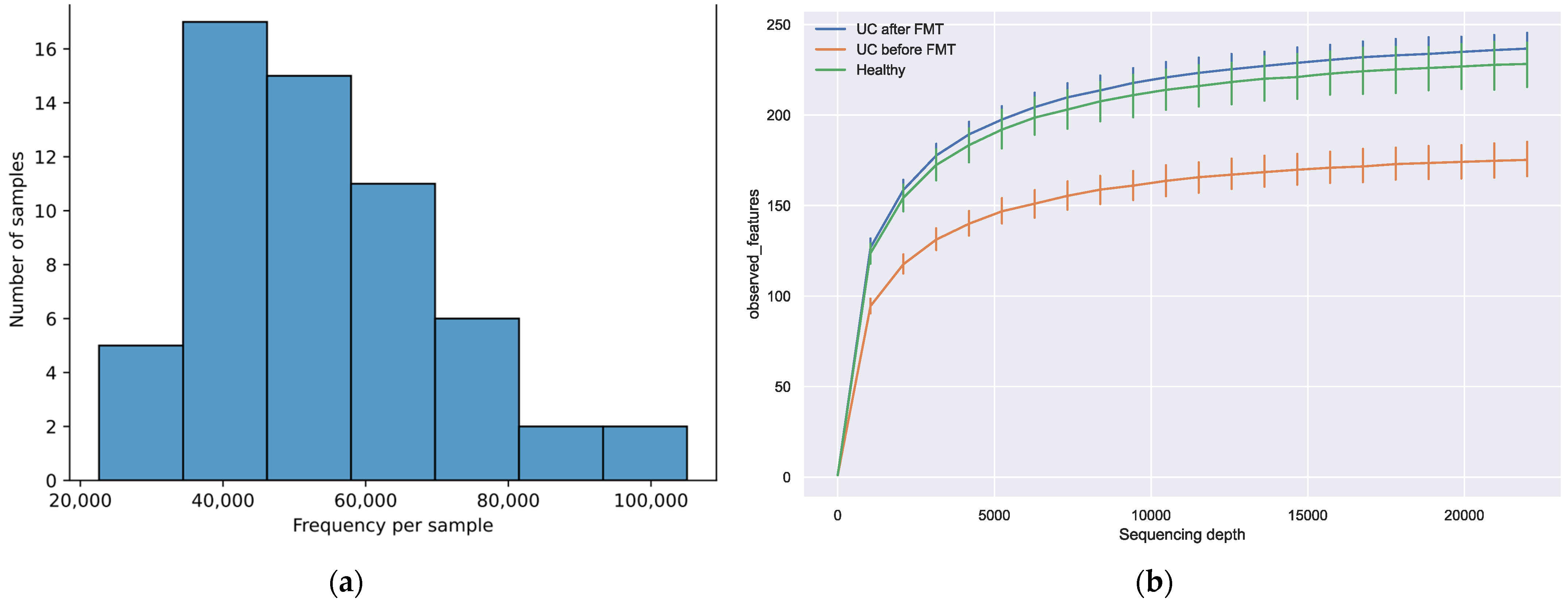
3.3. Sequencing Results
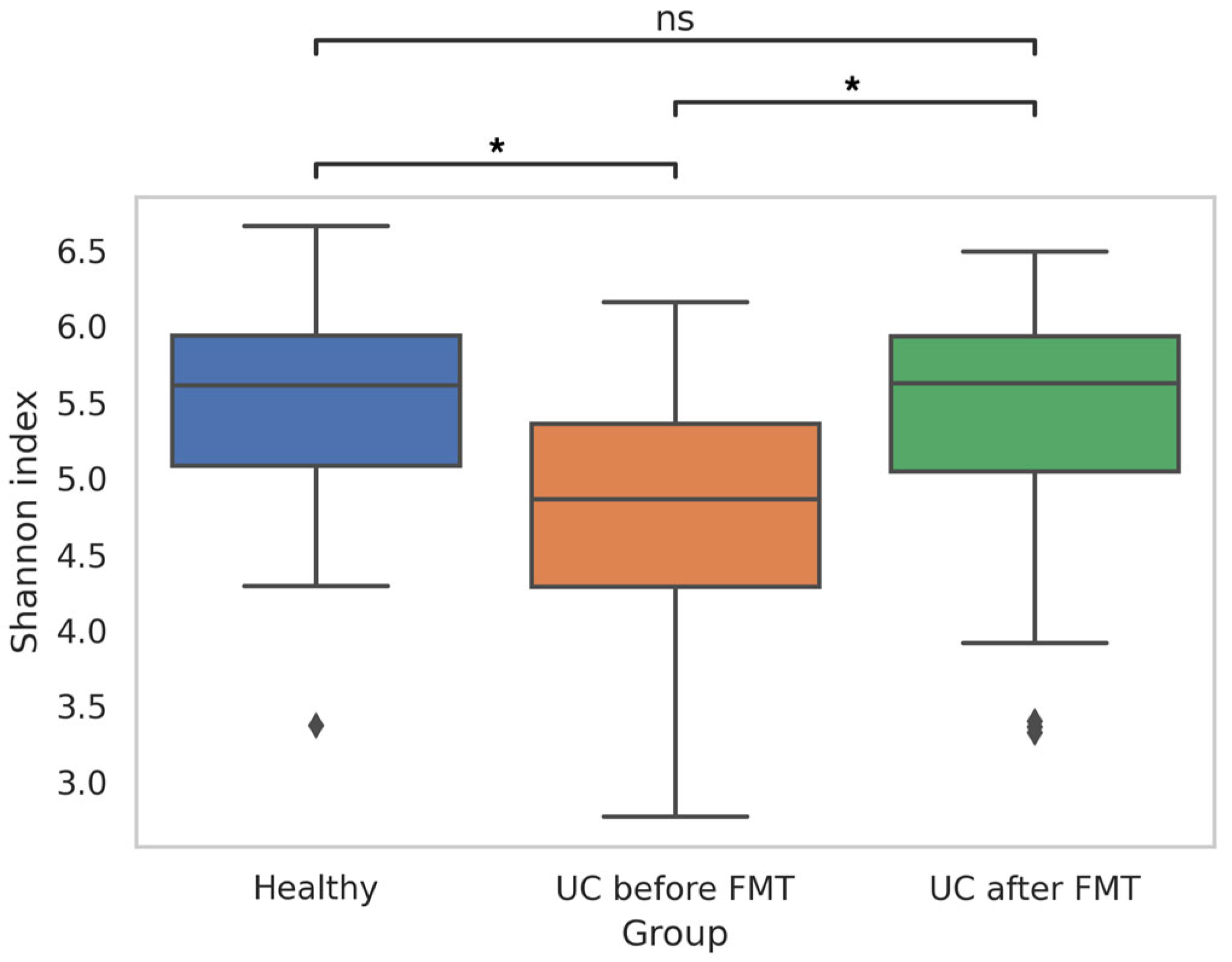
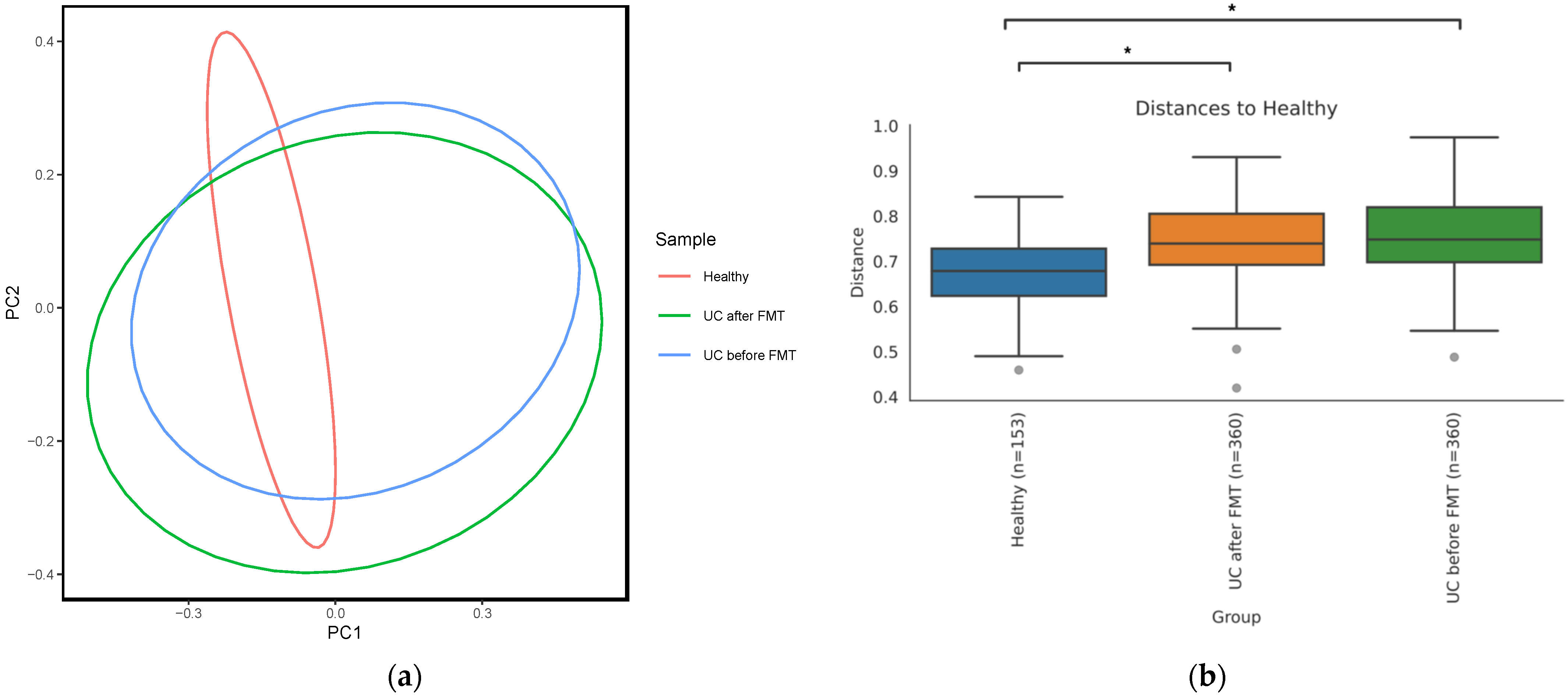
3.4. Comparative Analysis of Microbiota Biodiversity in Healthy Volunteers and Patients with UC before and after FMT
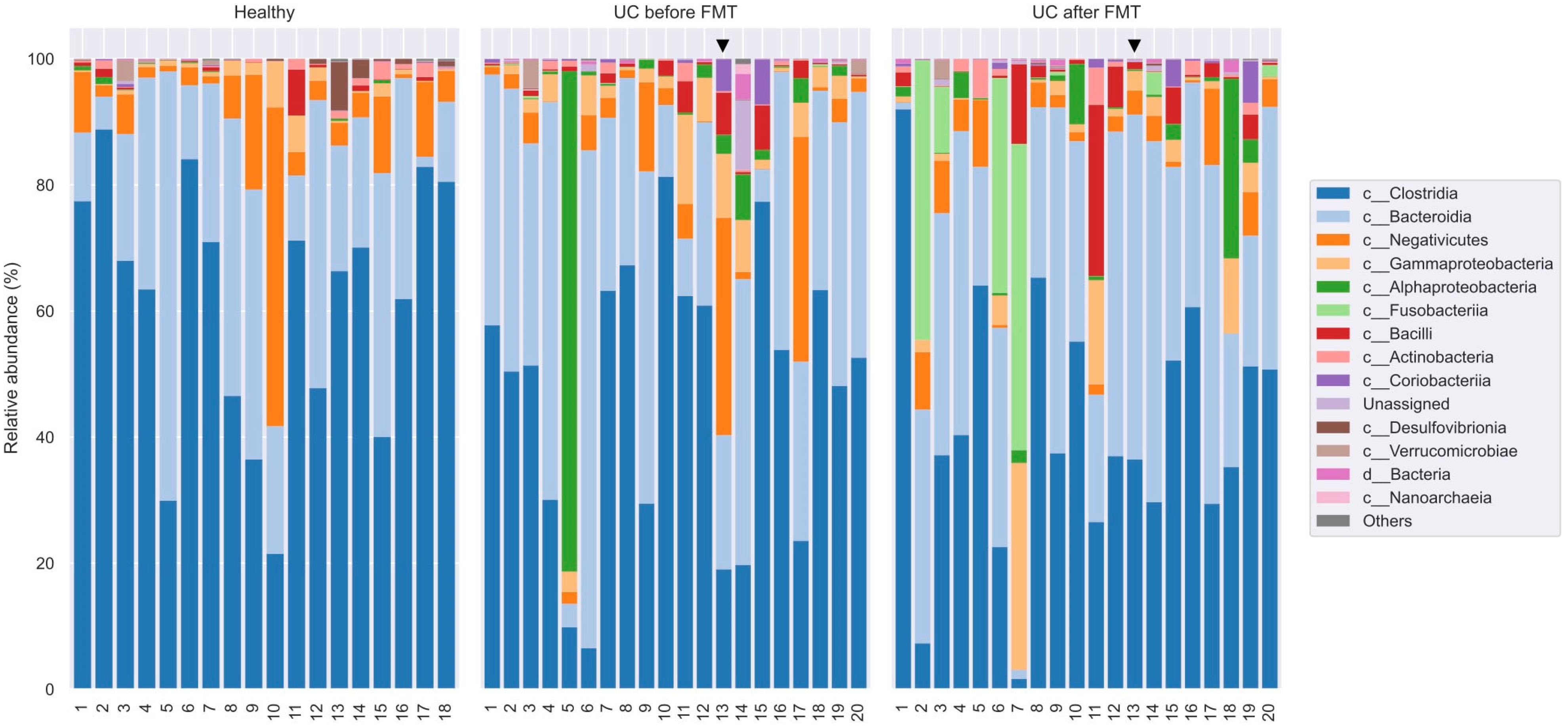
3.5. Comparative Analysis of the Microbiota Taxonomic Composition in Healthy Volunteers and Patients with UC before and after FMT
3.6. Clinical Outcomes and Comparative Microbiome Compositions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmidt, T.S.B.; Raes, J.; Bork, P. The Human Gut Microbiome: From Association to Modulation. Cell 2018, 172, 1198–1215. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- King, C.H.; Desai, H.; Sylvetsky, A.C.; LoTempio, J.; Ayanyan, S.; Carrie, J.; Crandall, K.A.; Fochtman, B.C.; Gasparyan, L.; Gulzar, N.; et al. Baseline Human Gut Microbiota Profile in Healthy People and Standard Reporting Template. PLoS ONE 2019, 14, e0206484. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human Gut Microbiota in Health and Disease: Unveiling the Relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef] [PubMed]
- Eiseman, B.; Silen, W.; Bascom, G.S.; Kauvar, A.J. Fecal Enema as an Adjunct in the Treatment of Pseudomembranous Enterocolitis. Surgery 1958, 44, 854–859. [Google Scholar] [PubMed]
- Levy, A.N.; Allegretti, J.R. Insights into the Role of Fecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease. Ther. Adv. Gastroenterol. 2019, 12, 1756284819836893. [Google Scholar] [CrossRef] [PubMed]
- de Fátima Caldeira, L.; Borba, H.H.; Tonin, F.S.; Wiens, A.; Fernandez-Llimos, F.; Pontarolo, R. Fecal Microbiota Transplantation in Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0238910. [Google Scholar] [CrossRef]
- Stojek, M.; Jabłońska, A.; Adrych, K. The Role of Fecal Microbiota Transplantation in the Treatment of Inflammatory Bowel Disease. J. Clin. Med. 2021, 10, 4055. [Google Scholar] [CrossRef]
- Tan, P.; Li, X.; Shen, J.; Feng, Q. Fecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease: An Update. Front. Pharmacol. 2020, 11, 574533. [Google Scholar] [CrossRef]
- Goldenberg, S.D.; Batra, R.; Beales, I.; Digby-Bell, J.L.; Irving, P.M.; Kellingray, L.; Narbad, A.; Franslem-Elumogo, N. Comparison of Different Strategies for Providing Fecal Microbiota Transplantation to Treat Patients with Recurrent Clostridium Difficile Infection in Two English Hospitals: A Review. Infect. Dis. Ther. 2018, 7, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Sipe, B.; Cheng, Y.W.; Phelps, E.; Rogers, N.; Sagi, S.; Bohm, M.; Xu, H.; Kassam, Z. Fecal Microbiota Transplant in Severe and Severe-Complicated Clostridium Difficile: A Promising Treatment Approach. Gut Microbes 2017, 8, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Baktash, A.; Terveer, E.M.; Zwittink, R.D.; Hornung, B.V.H.; Corver, J.; Kuijper, E.J.; Smits, W.K. Mechanistic Insights in the Success of Fecal Microbiota Transplants for the Treatment of Clostridium Difficile Infections. Front. Microbiol. 2018, 9, 387843. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.E.; Garborg, K.; Bretthauer, M.; Skudal, H.; Øines, M.N.; Wiig, H.; Rose, Ø.; Seip, B.; Lamont, J.T.; Midtvedt, T.; et al. Fecal Microbiota Transplantation for Primary Clostridium Difficile Infection. N. Engl. J. Med. 2018, 378, 2535–2536. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Yuan, Y.; Baharith, H.; Ford, A.C. Faecal Microbiota Transplantation for Clostridium Difficile-Associated Diarrhoea: A Systematic Review of Randomised Controlled Trials. Med. J. Aust. 2017, 207, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Masucci, L.; Quaranta, G.; Simonelli, C.; Lopetuso, L.R.; Sanguinetti, M.; Gasbarrini, A.; Cammarota, G. Randomised Clinical Trial: Faecal Microbiota Transplantation by Colonoscopy plus Vancomycin for the Treatment of Severe Refractory Clostridium Difficile Infection—Single versus Multiple Infusions. Aliment. Pharmacol. Ther. 2018, 48, 152–159. [Google Scholar] [CrossRef]
- Ashraf, M.F.; Tageldin, O.; Nassar, Y.; Batool, A. Fecal Microbiota Transplantation in Patients With Recurrent Clostridium Difficile Infection: A Four-Year Single-Center Retrospective Review. Gastroenterol. Res. 2021, 14, 237. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G. FMT for Ulcerative Colitis: Closer to the Turning Point. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 266–268. [Google Scholar] [CrossRef]
- Shen, Z.H.; Zhu, C.X.; Quan, Y.S.; Yang, Z.Y.; Wu, S.; Luo, W.W.; Tan, B.; Wang, X.Y. Relationship between Intestinal Microbiota and Ulcerative Colitis: Mechanisms and Clinical Application of Probiotics and Fecal Microbiota Transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef]
- El Hage Chehade, N.; Ghoneim, S.; Shah, S.; Chahine, A.; Mourad, F.H.; Francis, F.F.; Binion, D.G.; Farraye, F.A.; Hashash, J.G. Efficacy of Fecal Microbiota Transplantation in the Treatment of Active Ulcerative Colitis: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Inflamm. Bowel Dis. 2023, 29, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Březina, J.; Bajer, L.; Wohl, P.; Ďuricová, D.; Hrabák, P.; Novotný, A.; Koželuhová, J.; Lukáš, M.; Mrázek, J.; Fliegerová, K.O.; et al. Fecal Microbial Transplantation versus Mesalamine Enema for Treatment of Active Left-Sided Ulcerative Colitis—Results of a Randomized Controlled Trial. J. Clin. Med. 2021, 10, 2753. [Google Scholar] [CrossRef] [PubMed]
- Akbari, H.; Akbari, A.; Ghiasvand, R.; Tamizifar, B.; Saneei, P.; Feizi, A.; Pourmasoumi, M. The Association between Dietary Patterns and the Risk of Developing Ulcerative Colitis. Clin. Nutr. ESPEN 2022, 51, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Keshteli, A.H.; Madsen, K.L.; Dieleman, L.A. Diet in the Pathogenesis and Management of Ulcerative Colitis: A Review of Randomized Controlled Dietary Interventions. Nutrients 2019, 11, 1498. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Meng, X.; Liu, S.; Zhou, P.; Zeng, C.; Fu, C.; Dou, Q.; Wu, A.; Li, C. Gut Microbiota Composition Associated With Clostridium Difficile-Positive Diarrhea and C. Difficile Type in ICU Patients. Front. Cell. Infect. Microbiol. 2020, 10, 523430. [Google Scholar] [CrossRef] [PubMed]
- Shoaei, P.; Shojaei, H.; Jalali, M.; Khorvash, F.; Hosseini, S.M.; Ataei, B.; Vakili, B.; Ebrahimi, F.; Tavakoli, H.; Esfandiari, Z.; et al. Clostridium Difficile Isolated from Faecal Samples in Patients with Ulcerative Colitis. BMC Infect. Dis. 2019, 19, 361. [Google Scholar] [CrossRef] [PubMed]
- Mansour, L.; El-Kalla, F.; Kobtan, A.; Abd-Elsalam, S.; Yousef, M.; Soliman, S.; Ali, L.A.; Elkhalawany, W.; Amer, I.; Harras, H.; et al. Helicobacter Pylori May Be an Initiating Factor in Newly Diagnosed Ulcerative Colitis Patients: A Pilot Study. World J. Clin. Cases 2018, 6, 641. [Google Scholar] [CrossRef]
- Schultz, B.M.; Paduro, C.A.; Salazar, G.A.; Salazar-Echegarai, F.J.; Sebastián, V.P.; Riedel, C.A.; Kalergis, A.M.; Alvarez-Lobos, M.; Bueno, S.M. A Potential Role of Salmonella Infection in the Onset of Inflammatory Bowel Diseases. Front. Immunol. 2017, 8, 236225. [Google Scholar] [CrossRef]
- Baumgartner, M.; Zirnbauer, R.; Schlager, S.; Mertens, D.; Gasche, N.; Sladek, B.; Herbold, C.; Bochkareva, O.; Emelianenko, V.; Vogelsang, H.; et al. Atypical Enteropathogenic E. coli Are Associated with Disease Activity in Ulcerative Colitis. Gut Microbes 2022, 14, 2143218. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Fischer, M. The Present Status of Fecal Microbiota Transplantation and Its Value in the Elderly. Curr. Treat. Options Gastroenterol. 2017, 15, 349–362. [Google Scholar] [CrossRef]
- Segal, J.P.; Mullish, B.H.; Quraishi, M.N.; Iqbal, T.; Marchesi, J.R.; Sokol, H. Mechanisms Underpinning the Efficacy of Faecal Microbiota Transplantation in Treating Gastrointestinal Disease. Ther. Adv. Gastroenterol. 2020, 13, 1756284820946904. [Google Scholar] [CrossRef] [PubMed]
- Soveral, L.F.; Korczaguin, G.G.; Schmidt, P.S.; Nunes, I.S.; Fernandes, C.; Zárate-Bladés, C.R. Immunological Mechanisms of Fecal Microbiota Transplantation in Recurrent Clostridioides Difficile Infection. World J. Gastroenterol. 2022, 28, 4762. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.F.; Reinisch, W.; et al. Subcutaneous Golimumab Induces Clinical Response and Remission in Patients with Moderate-to-Severe Ulcerative Colitis. Gastroenterology 2014, 146, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). Wiley StatsRef Stat. Ref. Online 2017, 1–15. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Pedregosa, F.; Michel, V.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Vanderplas, J.; Cournapeau, D.; Pedregosa, F.; Varoquaux, G.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2′s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Van Treuren, W.; White, R.A.; Eggesbø, M.; Knight, R.; Peddada, S.D. Analysis of Composition of Microbiomes: A Novel Method for Studying Microbial Composition. Microb. Ecol. Health Dis. 2015, 26, 27663. [Google Scholar] [CrossRef] [PubMed]
- GitHub. Sbslee/Dokdo: A Python Package for Microbiome Sequencing Analysis with QIIME 2. Available online: https://github.com/sbslee/dokdo (accessed on 16 May 2023).
- Chakravorty, S.; Helb, D.; Burday, M.; Connell, N.; Alland, D. A Detailed Analysis of 16S Ribosomal RNA Gene Segments for the Diagnosis of Pathogenic Bacteria. J. Microbiol. Methods 2007, 69, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, P.Y. Conservative Fragments in Bacterial 16S RRNA Genes and Primer Design for 16S Ribosomal DNA Amplicons in Metagenomic Studies. PLoS ONE 2009, 4, e7401. [Google Scholar] [CrossRef]
- Bajer, L.; Kverka, M.; Kostovcik, M.; Macinga, P.; Dvorak, J.; Stehlikova, Z.; Brezina, J.; Wohl, P.; Spicak, J.; Drastich, P. Distinct Gut Microbiota Profiles in Patients with Primary Sclerosing Cholangitis and Ulcerative Colitis. World J. Gastroenterol. 2017, 23, 4548–4558. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A Decrease of the Butyrate-Producing Species Roseburia hominis and Faecalibacterium prausnitzii Defines Dysbiosis in Patients with Ulcerative Colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The Gut Microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef]
- Angelberger, S.; Reinisch, W.; Makristathis, A.; Lichtenberger, C.; Dejaco, C.; Papay, P.; Novacek, G.; Trauner, M.; Loy, A.; Berry, D. Temporal Bacterial Community Dynamics Vary among Ulcerative Colitis Patients after Fecal Microbiota Transplantation. Am. J. Gastroenterol. 2013, 108, 1620–1630. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediat. Inflamm. 2018, 2018, 2037838. [Google Scholar] [CrossRef]
- Bashir, A.; Miskeen, A.Y.; Hazari, Y.M.; Asrafuzzaman, S.; Fazili, K.M. Fusobacterium Nucleatum, Inflammation, and Immunity: The Fire within Human Gut. Tumor Biol. 2016, 37, 2805–2810. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Flint, H.J. Cultivable Bacterial Diversity from the Human Colon. Lett. Appl. Microbiol. 2007, 44, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial Degradation of Complex Carbohydrates in the Gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E. Prevotella in the Gut: Choose Carefully. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, M.I.; Neagoe, D.Ș.; Crăciun, A.M.; Moldovan, O.T. The Gram-Negative Bacilli Isolated from Caves—Sphingomonas paucimobilis and Hafnia alvei and a Review of Their Involvement in Human Infections. Int. J. Environ. Res. Public Health 2022, 19, 2324. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. Infections. Nat. Rev. Dis. Primers 2018, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Seck, E.H.; Fournier, P.E.; Raoult, D.; Khelaifia, S. ‘Halomonas massiliensis’ sp. Nov., a New Halotolerant Bacterium Isolated from the Human Gut. New Microbes New Infect. 2016, 14, 19–20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Newsome, R.C.; Gharaibeh, R.Z.; Pierce, C.M.; da Silva, W.V.; Paul, S.; Hogue, S.R.; Yu, Q.; Antonia, S.; Conejo-Garcia, J.R.; Robinson, L.A.; et al. Interaction of Bacterial Genera Associated with Therapeutic Response to Immune Checkpoint PD-1 Blockade in a United States Cohort. Genome Med. 2022, 14, 35. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 522172. [Google Scholar] [CrossRef]
- Chaima, D.; Pickering, H.; Hart, J.D.; Burr, S.E.; Maleta, K.M.; Kalua, K.; Bailey, R.L.; Holland, M.J. Fecal Biomarkers of Environmental Enteric Dysfunction and the Gut Microbiota of Rural Malawian Children: An Observational Study. Heliyon 2021, 7, e08194. [Google Scholar] [CrossRef]
- Lam, Y.Y.; Ha, C.W.Y.; Campbell, C.R.; Mitchell, A.J.; Dinudom, A.; Oscarsson, J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; Holmes, A.J.; et al. Increased Gut Permeability and Microbiota Change Associate with Mesenteric Fat Inflammation and Metabolic Dysfunction in Diet-Induced Obese Mice. PLoS ONE 2012, 7, e34233. [Google Scholar] [CrossRef] [PubMed]
- Gryaznova, M.V.; Solodskikh, S.A.; Panevina, A.V.; Syromyatnikov, M.Y.; Dvoretskaya, Y.D.; Sviridova, T.N.; Popov, E.S.; Popov, V.N. Study of Microbiome Changes in Patients with Ulcerative Colitis in the Central European Part of Russia. Heliyon 2021, 7, e06432. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, C.; Hou, S.; Wu, X.; Liu, J.; Wan, X. Analyses of Potential Driver and Passenger Bacteria in Human Colorectal Cancer. Cancer Manag. Res. 2020, 12, 11553. [Google Scholar] [CrossRef] [PubMed]
- Krych, L.; Hansen, C.H.F.; Hansen, A.K.; van den Berg, F.W.J.; Nielsen, D.S. Quantitatively Different, yet Qualitatively Alike: A Meta-Analysis of the Mouse Core Gut Microbiome with a View towards the Human Gut Microbiome. PLoS ONE 2013, 8, e62578. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, S.L.; Leth, M.L.; Michalak, L.; Hansen, M.E.; Pudlo, N.A.; Glowacki, R.; Pereira, G.; Workman, C.T.; Arntzen, M.; Pope, P.B.; et al. The Human Gut Firmicute Roseburia Intestinalis Is a Primary Degrader of Dietary β-Mannans. Nat. Commun. 2019, 10, 905. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Musmeci, E.; Candeliere, F.; Amaretti, A.; Rossi, M. Identification of Mucin Degraders of the Human Gut Microbiota. Sci. Rep. 2021, 11, 11094. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bogert, B.; Meijerink, M.; Zoetendal, E.G.; Wells, J.M.; Kleerebezem, M. Immunomodulatory Properties of Streptococcus and Veillonella Isolates from the Human Small Intestine Microbiota. PLoS ONE 2014, 9, e114277. [Google Scholar] [CrossRef]
- Higashi, D.L.; Krieger, M.C.; Qin, H.; Zou, Z.; Palmer, E.A.; Kreth, J.; Merritt, J. Who Is in the Driver’s Seat? Parvimonas micra: An Understudied Pathobiont at the Crossroads of Dysbiotic Disease and Cancer. Environ. Microbiol. Rep. 2023, 15, 254–264. [Google Scholar] [CrossRef]
- Moustafa, A.M.; Velusamy, S.K.; Denu, L.; Narechania, A.; Fine, D.H.; Planet, P.J. Adaptation by Ancient Horizontal Acquisition of Butyrate Metabolism Genes in Aggregatibacter Actinomycetemcomitans. mBio 2021, 12, e03581-20. [Google Scholar] [CrossRef]
- De Amorim, A.M.B.; Nascimento, J.D.S. Acinetobacter: An Underrated Foodborne Pathogen? J. Infect. Dev. Ctries. 2017, 11, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M. The Clinical Importance of Emerging Campylobacter Species. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Long, W.; Hao, B.; Ding, D.; Ma, X.; Zhao, L.; Pang, X. A Human Stool-Derived Bilophila Wadsworthia Strain Caused Systemic Inflammation in Specific-Pathogen-Free Mice. Gut Pathog. 2017, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Claus, S.P. The Strange Case of Prevotella Copri: Dr. Jekyll or Mr. Hyde? Cell Host Microbe 2019, 26, 577–578. [Google Scholar] [CrossRef]
- Tikunov, A.Y.; Morozov, V.V.; Shvalov, A.N.; Bardasheva, A.V.; Shrayner, E.V.; Maksimova, O.A.; Voloshina, I.O.; Morozova, V.V.; Vlasov, V.V.; Tikunova, N.V. Fecal Microbiome Change in Patients with Ulcerative Colitis after Fecal Microbiota Transplantation. Vavilovskii Zhurnal Genet. Sel. 2020, 24, 168–175. [Google Scholar] [CrossRef]
- Nakase, H.; Sato, N.; Mizuno, N.; Ikawa, Y. The Influence of Cytokines on the Complex Pathology of Ulcerative Colitis. Autoimmun. Rev. 2022, 21, 103017. [Google Scholar] [CrossRef]


| Male | 11 (55%) | |
| Female | 9 (45%) | |
| Age | 36 (25–65) | |
| Disease duration (years) | 4 (1–12) | |
| Total Mayo score | 3–5 | 19 (95%) |
| 6 | 1 (5%) | |
| Fecal calprotectin (µg/g) | 750.5 (122–2000) | |
| C-reactive protein (mg/L) | 5.1 (0.43–39.4) | |
| Hemoglobin (g/L) | 133 (87–167) | |
| Used therapy | 5-aminosalicylate | 18 (90%) |
| Steroids | 4 (20%) | |
| Immunomodulator | 3 (15%) | |
| Antispasmodic | 1 (5%) |
| Time Period | Clinical Outcome | Number of Patients (%) |
|---|---|---|
| Week 2 | Clinical response | 19 (95%) |
| Week 8 | Clinical remission | 12 (60%) |
| Clinical response | 19 (95%) | |
| Week 12 | Clinical remission | 9 from 13 interviewed patients (69%) |
| Clinical response | 11 from 13 interviewed patients (85%) | |
| Month 6 | Clinical remission | 9 * from 13 interviewed patients (69%) |
| Month 12 | Clinical remission | 5 * from 13 interviewed patients (38%) |
| Month 18 | Clinical remission | 5 * from 13 interviewed patients (38%) |
| Month 24 | Clinical remission | 4 * ** from 13 interviewed patients (31%) |
| Group | Naverage, (Nmin–Nmax) before Filtration | Naverage, (Nmin–Nmax) after Filtration | Number of OTU |
|---|---|---|---|
| HV | 114,670, (95,892–156,040) | 59,601, (33,893–93,798) | 1794 |
| UC-bef | 139,819, (83,382–175,968) | 51,660, (22,621–81,037) | 1818 |
| UC-aft | 142,060, (107,889–218,576) | 54,773, (32,919–105,051) | 1959 |
| Phylum | Class | Average Proportion, % | ||
|---|---|---|---|---|
| HV | UC-bef | UC-aft | ||
| Firmicutes | Clostridia | 64.7 | 46.7 | 42.4 |
| Negativicutes | 7.7 | 5.7 | 4.0 | |
| Bacilli | 0.8 | 1.3 | 2.8 | |
| Bacteroidota | Bacteroidia | 26.0 | 34.2 | 34.0 |
| Proteobacteria | Alphaproteobacteria | 0.2 | 5.2 | 2.8 |
| Gammaproteobacteria | 1.5 | 3.9 | 4.6 | |
| Fusobacteriota | Fusobacteriia | 0.002 | 0.06 | 7.2 |
| Actinobacteriota | Actinobacteria | 0.7 | 0.46 | 1.1 |
| Coriobacteriia | 0.07 | 0.73 | 0.77 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tikunov, A.Y.; Fedorets, V.A.; Shrainer, E.V.; Morozov, V.V.; Bystrova, V.I.; Tikunova, N.V. Intestinal Microbiome Changes and Clinical Outcomes of Patients with Ulcerative Colitis after Fecal Microbiota Transplantation. J. Clin. Med. 2023, 12, 7702. https://doi.org/10.3390/jcm12247702
Tikunov AY, Fedorets VA, Shrainer EV, Morozov VV, Bystrova VI, Tikunova NV. Intestinal Microbiome Changes and Clinical Outcomes of Patients with Ulcerative Colitis after Fecal Microbiota Transplantation. Journal of Clinical Medicine. 2023; 12(24):7702. https://doi.org/10.3390/jcm12247702
Chicago/Turabian StyleTikunov, Artem Y., Valeria A. Fedorets, Evgenia V. Shrainer, Vitaliy V. Morozov, Valeria I. Bystrova, and Nina V. Tikunova. 2023. "Intestinal Microbiome Changes and Clinical Outcomes of Patients with Ulcerative Colitis after Fecal Microbiota Transplantation" Journal of Clinical Medicine 12, no. 24: 7702. https://doi.org/10.3390/jcm12247702
APA StyleTikunov, A. Y., Fedorets, V. A., Shrainer, E. V., Morozov, V. V., Bystrova, V. I., & Tikunova, N. V. (2023). Intestinal Microbiome Changes and Clinical Outcomes of Patients with Ulcerative Colitis after Fecal Microbiota Transplantation. Journal of Clinical Medicine, 12(24), 7702. https://doi.org/10.3390/jcm12247702







