Clinical Utility and Validation of the Acoustic Voice Quality and Acoustic Breathiness Indexes for Voice Disorder Assessment in English Speakers
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Voice Samples
2.3. Validation Process
2.3.1. Phase One
Standardized Syllable Number for the Continuous Speech (CS) Part
2.3.2. Phase Two
Auditory-Perceptual Assessment
Acoustic Measures
2.4. Statistical Analysis
3. Results
3.1. Standardized Syllable Number (SSN) for the Continuous Speech (CS) Part
3.2. Auditory-Perceptual Assessment: Reliability
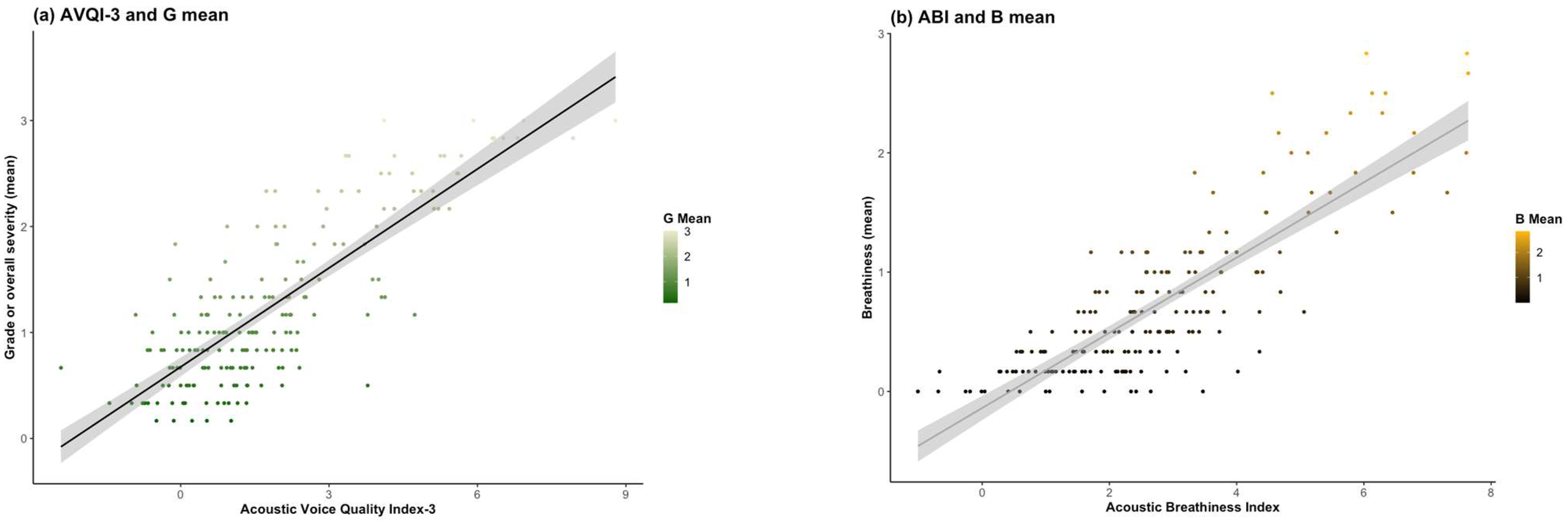
3.3. Concurrent Validity
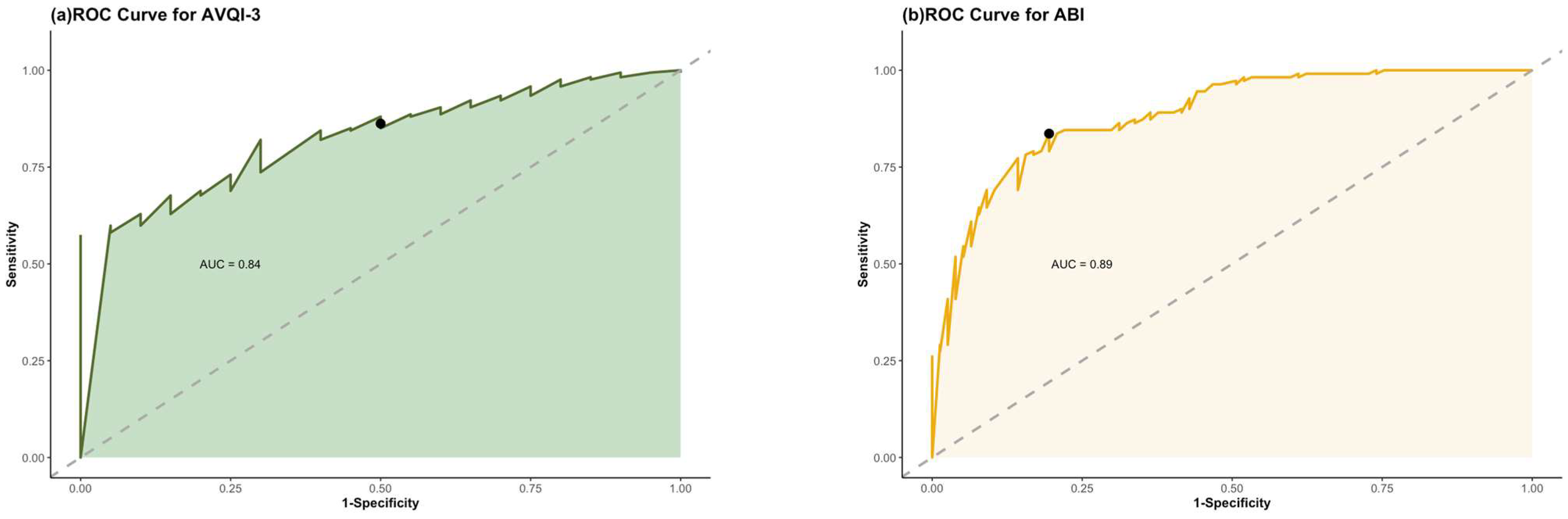
3.4. Discriminatory Accuracy
4. Discussion
4.1. Considerations and Future Directions
4.2. Clinical Utility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dejonckere, P.H.; Bradley, P.; Clemente, P.; Cornut, G.; Crevier-Buchman, L.; Friedrich, G.; Van De Heyning, P.; Remacle, M.; Woisard, V. A Basic Protocol for Functional Assessment of Voice Pathology, Especially for Investigating the Efficacy of (Phonosurgical) Treatments and Evaluating New Assessment Techniques. Eur. Arch. Oto-Rhino-Laryngol. 2001, 258, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; Awan, S.N.; Barkmeier-Kraemer, J.; Courey, M.; Deliyski, D.; Eadie, T.; Paul, D.; Švec, J.G.; Hillman, R. Recommended Protocols for Instrumental Assessment of Voice: American Speech-Language-Hearing Association Expert Panel to Develop a Protocol for Instrumental Assessment of Vocal Function. Am. J. Speech Lang. Pathol. 2018, 27, 887–905. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.M.; Johnson, A.M. Practical Considerations for Instrumental Acoustic and Aerodynamic Assessment of Voice: Discussion Points From an Open Forum of Clinicians. Perspect. ASHA Spec. Interest Groups. 2023, 8, 1354–1362. [Google Scholar] [CrossRef]
- Oates, J. Auditory-Perceptual Evaluation of Disordered Voice Quality: Pros, Cons and Future Directions. Folia Phoniatr. Logop. 2009, 61, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Zraick, R.I.; Kempster, G.B.; Connor, N.P.; Thibeault, S.; Klaben, B.K.; Bursac, Z.; Thrush, C.R.; Glaze, L.E. Establishing Validity of the Consensus Auditory-Perceptual Evaluation of Voice (CAPE-V). Am. J. Speech-Lang. Pathol. 2011, 20, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, S.S.; Gautam, R. Acoustic Analysis Before and After Voice Therapy for Laryngeal Pathology. Kathmandu Univ. Med. J. (KUMJ) 2015, 13, 323–327. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maryn, Y.; Roy, N.; De Bodt, M.; Van Cauwenberge, P.; Corthals, P. Acoustic Measurement of Overall Voice Quality: A Meta-Analysisa). J. Acoust. Soc. Am. 2009, 126, 2619–2634. [Google Scholar] [CrossRef] [PubMed]
- Awan, S.N.; Roy, N.; Zhang, D.; Cohen, S.M. Validation of the Cepstral Spectral Index of Dysphonia (CSID) as a Screening Tool for Voice Disorders: Development of Clinical Cutoff Scores. J. Voice 2016, 30, 130–144. [Google Scholar] [CrossRef]
- Wuyts, F.L.; Bodt, M.S.D.; Molenberghs, G.; Remacle, M.; Heylen, L.; Millet, B.; Lierde, K.V.; Raes, J.; de Heyning, P.H.V. The Dysphonia Severity Index. J. Speech Lang. Hear. Res. 2000, 43, 796–809. [Google Scholar] [CrossRef]
- Batthyany, C.; Latoszek, B.B.V.; Maryn, Y. Meta-Analysis on the Validity of the Acoustic Voice Quality Index. J. Voice 2022, in press. [CrossRef]
- Englert, M.; Lopes, L.; Vieira, V.; Behlau, M. Accuracy of Acoustic Voice Quality Index and Its Isolated Acoustic Measures to Discriminate the Severity of Voice Disorders. J. Voice 2022, 36, 582.e1–582.e10. [Google Scholar] [CrossRef]
- Barsties, V.; Latoszek, B.; Kim, G.-H.; Delgado Hernández, J.; Hosokawa, K.; Englert, M.; Neumann, K.; Hetjens, S. The Validity of the Acoustic Breathiness Index in the Evaluation of Breathy Voice Quality: A Meta-Analysis. Clin. Otolaryngol. 2021, 46, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Branski, K.V.; Rosen Clark, A.; Ryan, C. Structural Pathologies of the Larynx. In Classification Manual for Voice Disorders-I; Psychology Press: London, UK, 2005; ISBN 978-1-4106-1729-3. [Google Scholar]
- Ramos, P.H.; Lagos, A.E.; Napolitano, C.A.; Badía, P.I. Postintubation Phonatory Insufficiency: A Challenging Diagnosis. J. Voice 2022, 36, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, V.; Buckland, A.; Bailey, J.; Lipscombe, J.; Nathan, E.; Vijayasekaran, S.; Kelly, R.; Maryn, Y.; French, N. Objective Assessment of Pediatric Voice Disorders with the Acoustic Voice Quality Index. J. Voice 2012, 26, 672.e1–672.e7. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Benoy, J.J. Acoustic Voice Quality Index (AVQI) in the Measurement of Voice Quality: A Systematic Review and Meta-Analysis. J. Voice 2022, in press. [CrossRef]
- Dietsch, A.M.; Mocarski, R.; Hope, D.A.; Woodruff, N.; McKelvey, M. Revisiting the Rainbow: Culturally Responsive Updates to a Standard Clinical Resource. Am. J. Speech-Lang. Pathol. 2023, 32, 377–380. [Google Scholar] [CrossRef]
- Deliyski, D.D.; Shaw, H.S.; Evans, M.K. Influence of Sampling Rate on Accuracy and Reliability of Acoustic Voice Analysis. Logop. Phoniatr. Vocol. 2005, 30, 55–62. [Google Scholar] [CrossRef]
- Boersma, P.; Weenink, D. PRAAT, a System for Doing Phonetics by Computer. Glot Int. 2001, 5, 341–345. [Google Scholar]
- Barsties, B.; Maryn, Y. The Improvement of Internal Consistency of the Acoustic Voice Quality Index. Am. J. Otolaryngol. 2015, 36, 647–656. [Google Scholar] [CrossRef]
- Karnell, M.P.; Melton, S.D.; Childes, J.M.; Coleman, T.C.; Dailey, S.A.; Hoffman, H.T. Reliability of Clinician-Based (GRBAS and CAPE-V) and Patient-Based (V-RQOL and IPVI) Documentation of Voice Disorders. J. Voice 2007, 21, 576–590. [Google Scholar] [CrossRef]
- Maryn, Y.; Roy, N. Sustained Vowels and Continuous Speech in the Auditory-Perceptual Evaluation of Dysphonia Severity. J. Soc. Bras. Fonoaudiol. 2012, 24, 107–112. [Google Scholar] [CrossRef]
- McDonald, N.; Schoenebeck, S.; Forte, A. Reliability and Inter-Rater Reliability in Qualitative Research: Norms and Guidelines for CSCW and HCI Practice. Proc. ACM Hum.-Comput. Interact. 2019, 3, 72:1–72:23. [Google Scholar] [CrossRef]
- Dejonckere, P.H.; Lebacq, J. Harmonic Emergence in Formant Zone of a Sustained [a] as a Parameter for Evaluating Hoarseness. Acta Oto-Rhino-Laryngol. Belg. 1987, 41, 988–996. [Google Scholar]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Vanbelle, S.; Albert, A. Agreement between an Isolated Rater and a Group of Raters. Stat. Neerl. 2009, 63, 82–100. [Google Scholar] [CrossRef]
- Swets, J.A.; Dawes, R.M.; Monahan, J. Psychological Science Can Improve Diagnostic Decisions. Psychol. Sci. Public Interest 2000, 1, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and Its Associated Cutoff Point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, R.; Guyatt, G.H.; Sackett, D.L.; Bass, E.; Edwards, P.B.; Browman, G.; Cook, D.; Farkouh, M.; Gerstein, H.; Haynes, B.; et al. Users’ Guides to the Medical Literature: III. How to Use an Article About a Diagnostic Test B. What Are the Results and Will They Help Me in Caring for My Patients? JAMA J. Am. Med. Assoc. 1994, 271, 703–707. [Google Scholar] [CrossRef]
- Sataloff, R.T. Professional Voice Users: The Evaluation of Voice Disorders. Occup. Med. 2001, 16, 633–647. [Google Scholar] [PubMed]
- Chiolero, A.; Paccaud, F.; Aujesky, D.; Santschi, V.; Rodondi, N. How to Prevent Overdiagnosis. Swiss Med. Wkly. 2015, 145, w14060. [Google Scholar] [CrossRef]
- Kale, M.S.; Korenstein, D. Overdiagnosis in Primary Care: Framing the Problem and Finding Solutions. BMJ 2018, 362, k2820. [Google Scholar] [CrossRef]
- Behlau, M.; Madazio, G.; Oliveira, G. Functional Dysphonia: Strategies to Improve Patient Outcomes. Patient Relat. Outcome Meas. 2015, 6, 243–253. [Google Scholar] [CrossRef]
- Rubin, A.D.; Jackson-Menaldi, C.; Kopf, L.M.; Marks, K.; Skeffington, J.; Skowronski, M.D.; Shrivastav, R.; Hunter, E.J. Comparison of Pitch Strength with Perceptual and Other Acoustic Metric Outcome Measures Following Medialization Laryngoplasty. J. Voice 2019, 33, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Roy, N.; Peterson, E.; Merrill, R.M. Comparison of Two Multiparameter Acoustic Indices of Dysphonia Severity: The Acoustic Voice Quality Index and Cepstral Spectral Index of Dysphonia. J. Voice 2018, 32, 515.e1–515.e13. [Google Scholar] [CrossRef] [PubMed]
- Wolk, L.; Abdelli-Beruh, N.B.; Slavin, D. Habitual Use of Vocal Fry in Young Adult Female Speakers. J. Voice 2012, 26, e111–e116. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Klofstad, C.A.; Mayew, W.J.; Venkatachalam, M. Vocal Fry May Undermine the Success of Young Women in the Labor Market. PLoS ONE 2014, 9, e97506. [Google Scholar] [CrossRef] [PubMed]
- Gibson, T.A. The Role of Lexical Stress on the Use of Vocal Fry in Young Adult Female Speakers. J. Voice 2017, 31, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, V.; Roesner, I.; Wendt, F.; Schoentgen, J.; Aichinger, P. Auditory Perception of Impulsiveness and Tonality in Vocal Fry. Appl. Sci. 2023, 13, 4186. [Google Scholar] [CrossRef]
- Pineda-Pérez, E.; Calvache, C.; Cantor-Cutiva, L.C. Bibliometric Analysis and Review of Literature on the Relationship Between Voice Production and Bilingualism. J. Voice 2021, in press. [CrossRef]
- Zhu, S.; Chong, S.; Chen, Y.; Wang, T.; Ng, M.L. Effect of Language on Voice Quality: An Acoustic Study of Bilingual Speakers of Mandarin Chinese and English. Folia Phoniatr. Logop. 2022, 74, 421–430. [Google Scholar] [CrossRef]
- Fox, R.A.; Jacewicz, E. Cross-Dialectal Variation in Formant Dynamics of American English Vowels. J. Acoust. Soc. Am. 2009, 126, 2603–2618. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.L.A.; Majid, A.; van Hout, R. The Geographical Configuration of a Language Area Influences Linguistic Diversity. PLoS ONE 2019, 14, e0217363. [Google Scholar] [CrossRef] [PubMed]
- Narayana, S.; Franklin, C.; Peterson, E.; Hunter, E.J.; Robin, D.A.; Halpern, A.; Spielman, J.; Fox, P.T.; Ramig, L.O. Immediate and Long-Term Effects of Speech Treatment Targets and Intensive Dosage on Parkinson’s Disease Dysphonia and the Speech Motor Network: Randomized Controlled Trial. Hum. Brain Mapp. 2022, 43, 2328–2347. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, P.; Codino, J.; Cantor-Cutiva, L.C.; Marks, K.; Nudelman, C.J.; Skeffington, J.; Shrivastav, R.; Jackson-Menaldi, M.C.; Hunter, E.J.; Rubin, A.D. Reproducibility of Voice Parameters: The Effect of Room Acoustics and Microphones. J. Voice 2020, 34, 320–334. [Google Scholar] [CrossRef]
- de Lima Andrade, E.; da Cunha e Silva, D.C.; de Lima, E.A.; de Oliveira, R.A.; Zannin, P.H.T.; Martins, A.C.G. Environmental Noise in Hospitals: A Systematic Review. Environ. Sci. Pollut. Res. Int. 2021, 28, 19629–19642. [Google Scholar] [CrossRef]
- Erman Mahmut, E.; Stoicu-Tivadar, V. Current Challenges in the Computer-Based Assessment of Speech Sound Disorders. In Proceedings of the 2018 IEEE 12th International Symposium on Applied Computational Intelligence and Informatics (SACI), Timisoara, Romania, 17–19 May 2018; pp. 000431–000436. [Google Scholar]

| Diagnosis | Female | Male | ||
|---|---|---|---|---|
| Frequency | Mean Age (SD) in Years | Frequency | Mean Age (SD) in Years | |
| Normal Voice | 29 | 25.55 (5.58) | 20 | 34.30 (12.77) |
| Atrophy | 18 | 71.06 (11.92) | 7 | 76.57 (6.60) |
| Bacterial Laryngitis | 1 | 49 | 0 | 0 |
| Carcinoma in Situ | 0 | 0 | 2 | 64.00 (15.56) |
| Cyst | 1 | 47 | 0 | 0 |
| Erythema | 1 | 50 | 1 | 57 |
| Granuloma | 2 | 58.50 (3.54) | 1 | 35 |
| Hemorrhage | 1 | 49 | 0 | 0 |
| Laryngeal Dystonia | 7 | 56.00 (16.56) | 3 | 74.00 (14.73) |
| Laryngeal Trauma | 0 | 0 | 1 | 58 |
| Laryngocele | 1 | 80 | 0 | 0 |
| Leukoplakia | 2 | 50.00 (2.83) | 4 | 54.50 (15.15) |
| Muscle Tension Dysphonia | 7 | 42.14 (13.51) | 5 | 43.40 (23.16) |
| Nodules | 10 | 37.80 (17.24) | 1 | 34 |
| Papilloma | 0 | 0 | 1 | 80 |
| Paradoxical Vocal Fold Motion | 3 | 67.67 (14.15) | 0 | 0 |
| Paresis | 6 | 67.50 (10.59) | 2 | 57.5 (17.68) |
| Paralysis | 4 | 41.00 (18.78) | 2 | 55.50 (14.95) |
| Posterior Glottic Diastasis | 2 | 47.00 (1.41) | 1 | 51 |
| Reinke’s Edema | 11 | 11.00 (57.91) | 0 | 0 |
| Scar | 8 | 49.50 (45.67) | 3 | 22.21 (17.62) |
| Supraglottic Mass | 1 | 62 | 0 | 0 |
| Tremor | 3 | 65.33 (10.01) | 1 | 74 |
| Selection Method | Time (in Seconds) | AVQI-3 | ABI | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Hand-marked | 2.97272 | 0.26653 | 1.85 | 1.95 | 2.74 | 1.68 |
| SSN (22 syllables) | 2.96955 | 0.51102 | 1.82 | 1.95 | 2.72 | 1.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Allendes, A.; Codino, J.; Cantor-Cutiva, L.C.; Nudelman, C.J.; Rubin, A.D.; Barsties v. Latoszek, B.; Hunter, E.J. Clinical Utility and Validation of the Acoustic Voice Quality and Acoustic Breathiness Indexes for Voice Disorder Assessment in English Speakers. J. Clin. Med. 2023, 12, 7679. https://doi.org/10.3390/jcm12247679
Castillo-Allendes A, Codino J, Cantor-Cutiva LC, Nudelman CJ, Rubin AD, Barsties v. Latoszek B, Hunter EJ. Clinical Utility and Validation of the Acoustic Voice Quality and Acoustic Breathiness Indexes for Voice Disorder Assessment in English Speakers. Journal of Clinical Medicine. 2023; 12(24):7679. https://doi.org/10.3390/jcm12247679
Chicago/Turabian StyleCastillo-Allendes, Adrián, Juliana Codino, Lady Catherine Cantor-Cutiva, Charles J. Nudelman, Adam D. Rubin, Ben Barsties v. Latoszek, and Eric J. Hunter. 2023. "Clinical Utility and Validation of the Acoustic Voice Quality and Acoustic Breathiness Indexes for Voice Disorder Assessment in English Speakers" Journal of Clinical Medicine 12, no. 24: 7679. https://doi.org/10.3390/jcm12247679
APA StyleCastillo-Allendes, A., Codino, J., Cantor-Cutiva, L. C., Nudelman, C. J., Rubin, A. D., Barsties v. Latoszek, B., & Hunter, E. J. (2023). Clinical Utility and Validation of the Acoustic Voice Quality and Acoustic Breathiness Indexes for Voice Disorder Assessment in English Speakers. Journal of Clinical Medicine, 12(24), 7679. https://doi.org/10.3390/jcm12247679






