The Immediate Effects of Immersive Virtual Reality on Autonomic Nervous System Function in Patients with Disorders of Consciousness after Severe Acquired Brain Injury: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Assessment
2.2. Participants
2.3. Virtual Reality Intervention
2.4. Data Analysis
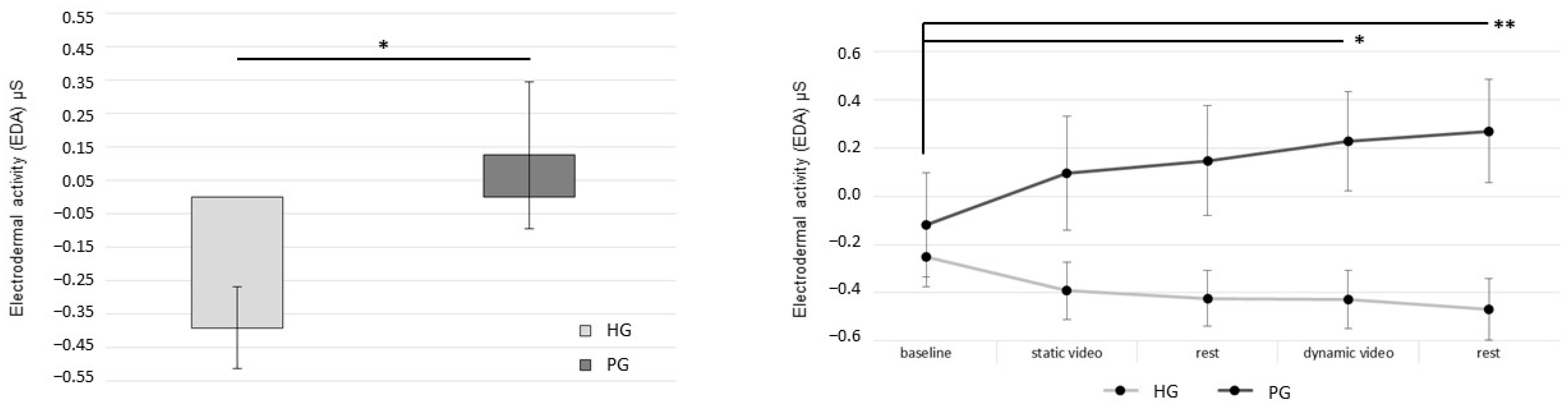
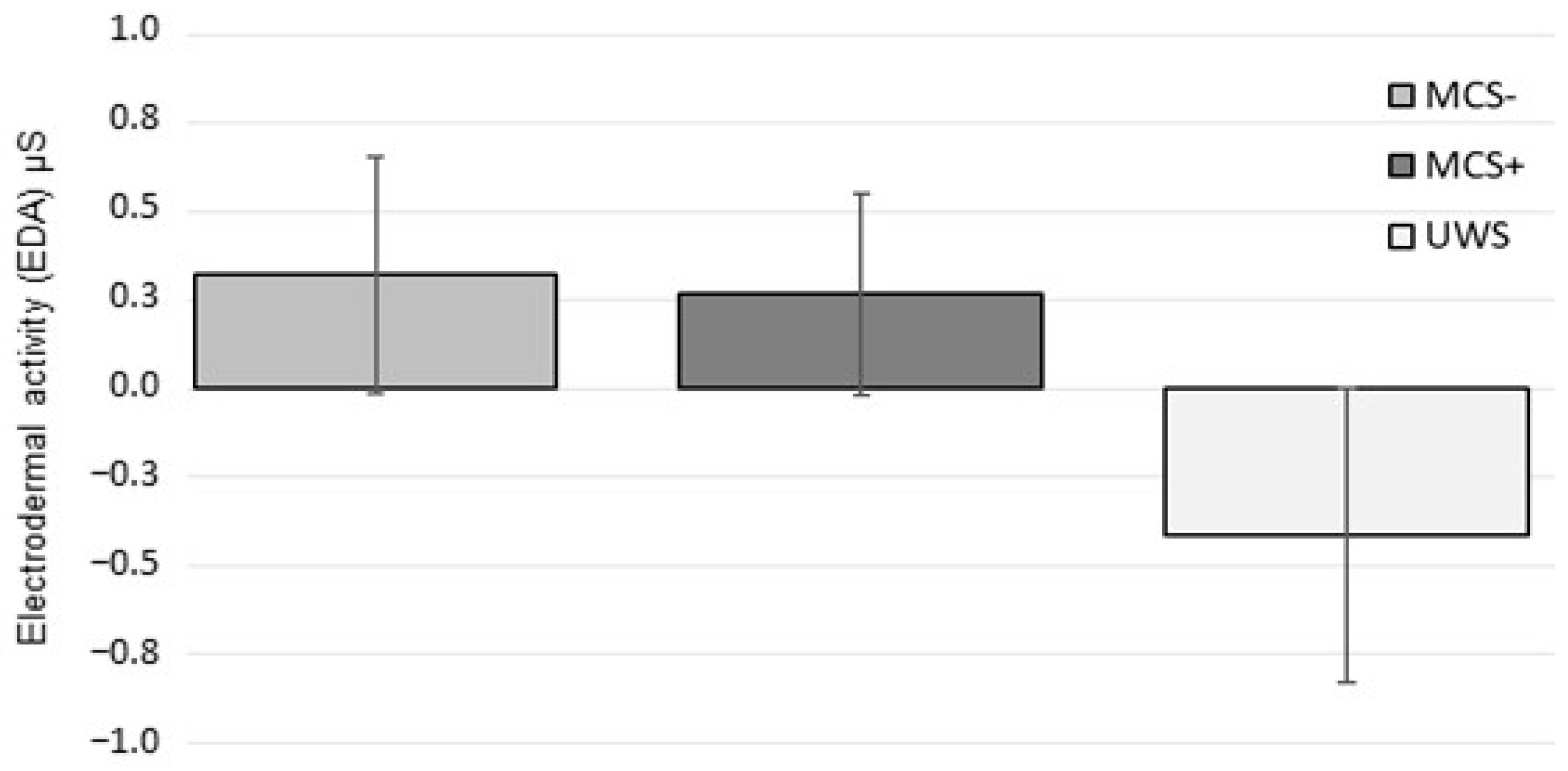
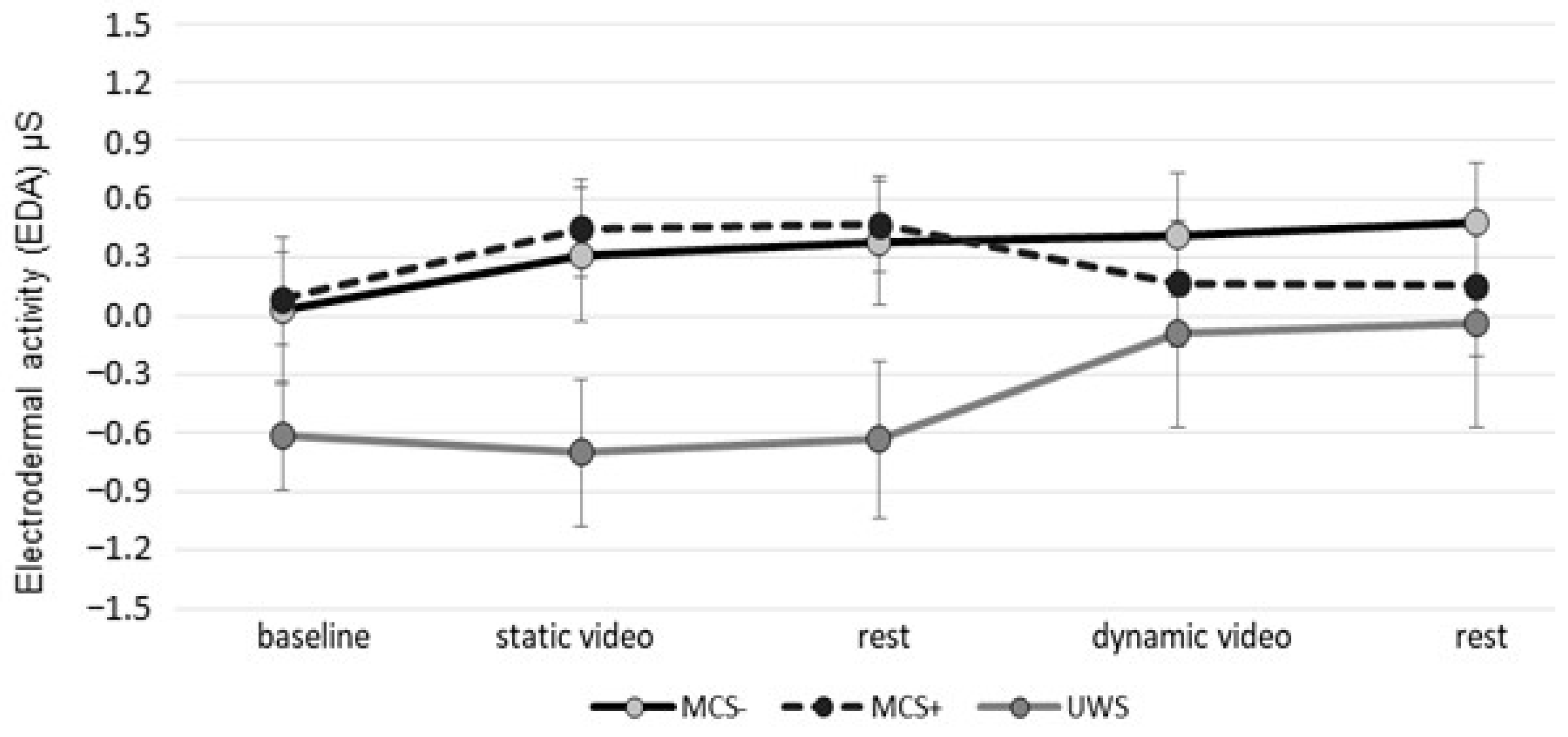
3. Results
4. Discussion
Implications of the Use of Immersive Virtual Reality in Clinical Practice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maegele, M. Traumatic Brain Injury in 2017: Exploring the Secrets of Concussion. Lancet Neurol. 2018, 17, 13–15. [Google Scholar] [CrossRef]
- Kondziella, D.; Bender, A.; Diserens, K.; van Erp, W.; Estraneo, A.; Formisano, R.; Laureys, S.; Naccache, L.; Ozturk, S.; Rohaut, B.; et al. EAN Panel on Coma, Disorders of Consciousness. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur. J. Neurol. 2020, 27, 741–756. [Google Scholar] [CrossRef]
- De Luca, R.; Calabrò, R.S.; Bramanti, P. Cognitive Rehabilitation after Severe Acquired Brain Injury: Current Evidence and Future Directions. Neuropsychol. Rehabil. 2018, 28, 879–898. [Google Scholar] [CrossRef]
- Thibaut, A.; Schiff, N.; Giacino, J.; Laureys, S.; Gosseries, O. Therapeutic Interventions in Patients with Prolonged Disorders of Consciousness. Lancet Neurol. 2019, 18, 600–614. [Google Scholar] [CrossRef]
- Caliandro, P.; Molteni, F.; Simbolotti, C.; Guanziroli, E.; Iacovelli, C.; Reale, G.; Giovannini, S.; Padua, L. Exoskeleton-Assisted Gait in Chronic Stroke: An EMG and Functional near-Infrared Spectroscopy Study of Muscle Activation Patterns and Prefrontal Cortex Activity. Clin. Neurophysiol. 2020, 131, 1775–1781. [Google Scholar] [CrossRef]
- Barra, A.; Monti, M.; Thibaut, A. Noninvasive Brain Stimulation Therapies to Promote Recovery of Consciousness: Where We Are and Where We Should Go. Semin. Neurol. 2022, 42, 348–362. [Google Scholar] [CrossRef]
- Osińska, A.; Rynkiewicz, A.; Binder, M.; Komendziński, T.; Borowicz, A.; Leszczyński, A. Non-invasive Vagus Nerve Stimulation in Treatment of Disorders of Consciousness—Longitudinal Case Study. Front. Nuerosci. 2022, 16, 834507. [Google Scholar] [CrossRef] [PubMed]
- Fusco, A.; Giovannini, S.; Castelli, L.; Coraci, D.; Gatto, D.M.; Reale, G.; Pastorino, R.; Padua, L. Virtual Reality and Lower Limb Rehabilitation: Effects on Motor and Cognitive Outcome-A Crossover Pilot Study. J. Clin. Med. 2022, 11, 2300. [Google Scholar] [CrossRef] [PubMed]
- Buccino, G.; Vogt, S.; Ritzl, A.; Fink, G.R.; Zilles, K.; Freund, H.J.; Rizzolatti, G. Neural Circuits Underlying Imitation Learning of Hand Actions: An Event-Related FMRI Study. Neuron 2004, 42, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.F.; Weiss, P.L.; Keshner, E.A. Emergence of Virtual Reality as a Tool for Upper Limb Rehabilitation: Incorporation of Motor Control and Motor Learning Principles. Phys. Ther. 2015, 95, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Naef, A.C.; Jeitziner, M.M.; Knobel, S.E.J.; Exl, M.T.; Müri, R.M.; Jakob, S.M.; Nef, T.; Gerber, S.M. Investigating the Role of Auditory and Visual Sensory Inputs for Inducing Relaxation during Virtual Reality Stimulation. Sci. Rep. 2022, 12, 17073. [Google Scholar] [CrossRef]
- Fusco, A.; Tieri, G. Challenges and Perspectives for Clinical Applications of Immersive and Non-Immersive Virtual Reality. J. Clin. Med. 2022, 11, 4540. [Google Scholar] [CrossRef]
- Sanchez-Vives, M.V.; Slater, M. From Presence to Consciousness through Virtual Reality. Nat. Rev. Neurosci. 2005, 6, 332–339. [Google Scholar] [CrossRef]
- Ventura, S.; Brivio, E.; Riva, G.; Baños, R.M. Immersive versus Non-Immersive Experience: Exploring the Feasibility of Memory Assessment Through 360° Technology. Front. Psychol. 2019, 10, 2509. [Google Scholar] [CrossRef]
- Slater, M.; Sanchez-Vives, M.V. Enhancing Our Lives with Immersive Virtual Reality. Front. Robot. AI 2016, 3, 236866. [Google Scholar] [CrossRef]
- Pizzoli, S.F.M.; Monzani, D.; Mazzocco, K.; Maggioni, E.; Pravettoni, G. The Power of Odor Persuasion: The Incorporation of Olfactory Cues in Virtual Environments for Personalized Relaxation. Perspect. Psychol. Sci. 2022, 17, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Slater, M. Measuring Presence: A Response to the Witmer and Singer Presence Questionnaire. Presence Teleoper. Virtual Environ. 1999, 8, 560–565. [Google Scholar] [CrossRef]
- Suda, M.; Morimoto, K.; Obata, A.; Koizumi, H.; Maki, A. Emotional responses to music: Towards scientific perspectives on music therapy. Neuroreport 2008, 19, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Särkämö, T.; Soto, D. Music listening after stroke: Beneficial effects and potential neural mechanisms. Ann. N. Y. Acad. Sci. 2012, 1252, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.F.; Schellenberg, E.G.; Husain, G. Arousal, mood, and the Mozart effect. Psychol. Sci. 2001, 12, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.E.; LoTemplio, S.B.; McDonnell, A.S.; McNay, G.D.; Greenberg, K.; McKinney, T.; Uchino, B.N.; Strayer, D.L. The Autonomic Nervous System in Its Natural Environment: Immersion in Nature Is Associated with Changes in Heart Rate and Heart Rate Variability. Psychophysiology 2021, 58, e13698. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, D.; Gallese, V. Motion, Emotion and Empathy in Esthetic Experience. Trends Cogn. Sci. 2007, 11, 197–203. [Google Scholar] [CrossRef]
- Naccache, L. Minimally conscious state or cortically mediated state? Brain 2018, 141, 949–960. [Google Scholar] [CrossRef]
- Luauté, J.; Dubois, A.; Heine, L.; Guironnet, C.; Juliat, A.; Gaveau, V.; Tillmann, B.; Perrin, F. Electrodermal Reactivity to Emotional Stimuli in Healthy Subjects and Patients with Disorders of Consciousness. Ann. Phys. Rehabil. Med. 2018, 61, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Espejo, D.; Owen, A.M. Detecting awareness after severe brain injury. Nat. Rev. Neurosci. 2013, 14, 801–809. [Google Scholar] [CrossRef]
- Perez-Marcos, D. Virtual Reality Experiences, Embodiment, Videogames and Their Dimensions in Neurorehabilitation. J. Neuroeng. Rehabil. 2018, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Gidlow, C.J.; Jones, M.V.; Hurst, G.; Masterson, D.; Clark-Carter, D.; Tarvainen, M.P.; Smith, G.R.; Nieuwenhuijsen, M.J. Where to put your best foot forward: Psycho-physiological responses to walking in natural and urban environments. J. Environ. Psychol. 2016, 45, 22–29. [Google Scholar] [CrossRef]
- Coventry, P.A.; Brown, J.E.; Pervin, J.; Brabyn, S.; Pateman, R.; Breedvelt, J.; Gilbody, S.; Stancliffe, R.; McEachan, R.; White, P.L. Nature-based outdoor activities for mental and physical health: Systematic review and meta-analysis. SSM Popul. Health 2021, 16, 100934. [Google Scholar] [CrossRef]
- Annerstedt, M.; Jönsson, P.; Wallergård, M.; Johansson, G.; Karlson, B.; Grahn, P.; Hansen, A.M.; Währborg, P. Inducing physiological stress recovery with sounds of nature in a virtual reality forest—Results from a pilot study. Physiol. Behav. 2013, 118, 240–250. [Google Scholar] [CrossRef]
- Anderson, A.P.; Mayer, M.D.; Fellows, A.M.; Cowan, D.R.; Hegel, M.T.; Buckey, J.C. Relaxation with Immersive Natural Scenes Presented Using Virtual Reality. Aerosp. Med. Hum. Perform. 2017, 88, 520–526. [Google Scholar] [CrossRef]
- Tronstad, C.; Amini, M.; Bach, D.R.; Martinsen, Ø.G. Current Trends and Opportunities in the Methodology of Electrodermal Activity Measurement. Physiol. Meas. 2022, 43, 02TR01. [Google Scholar] [CrossRef] [PubMed]
- Bodien, Y.G.; Chatelle, C.; Taubert, A.; Uchani, S.; Giacino, J.T.; Ehrlich-Jones, L. Updated measurement characteristics and clinical utility of the Coma Recovery Scale-Revised among individuals with acquired brain injury. Arch. Phys. Med. Rehabil. 2021, 102, 169–171. [Google Scholar] [CrossRef]
- Hagen, C.; Malkmus, D.; Durham, P. Levels of Cognitive Functioning; Rancho Los Amigos Hospital: Downey, CA, USA, 1972. [Google Scholar]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S. The Restorative Benefits of Nature: Toward an Integrative Framework. J. Environ. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- Shanahan, D.F.; Bush, R.; Gaston, K.J.; Lin, B.B.; Dean, J.; Barber, E.; Fuller, R.A. Health Benefits from Nature Experiences Depend on Dose. Sci. Rep. 2016, 6, 28551. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.; Kannis-Dymand, L.; Schaffer, V.; Millear, P.; Allen, A.; Stallman, H.; Mason, J.; Wood, A.; Atkinson-Nolte, J. Virtual Immersion in Nature and Psychological Well-Being: A Systematic Literature Review. J. Environ. Psychol. 2022, 80, 101765. [Google Scholar] [CrossRef]
- Browning, M.H.E.M.; Mimnaugh, K.J.; van Riper, C.J.; Laurent, H.K.; LaValle, S.M. Can Simulated Nature Support Mental Health? Comparing Short, Single-Doses of 360-Degree Nature Videos in Virtual Reality With the Outdoors. Front. Psychol. 2020, 10, 2667. [Google Scholar] [CrossRef]
- Schellenberg, E.G. Music and nonmusical abilities. Ann. N. Y. Acad. Sci. 2001, 930, 355–371. [Google Scholar] [CrossRef]
- Jäncke, L.; Cheetham, M.; Baumgartner, T. Virtual Reality and the Role of the Prefrontal Cortex in Adults and Children. Front. Neurosci. 2009, 3, 52–59. [Google Scholar] [CrossRef]
- Mukamel, R.; Ekstrom, A.D.; Kaplan, J.; Iacoboni, M.; Fried, I. Single-Neuron Responses in Humans during Execution and Observation of Actions. Curr. Biol. 2010, 20, 750–756. [Google Scholar] [CrossRef]
- Vecchiato, G.; Tieri, G.; Jelic, A.; De Matteis, F.; Maglione, A.G.; Babiloni, F. Electroencephalographic Correlates of Sensorimotor Integration and Embodiment during the Appreciation of Virtual Architectural Environments. Front. Psychol. 2015, 6, 1944. [Google Scholar] [CrossRef]
- Gerber, S.M.; Jeitziner, M.-M.; Sänger, S.D.; Knobel, S.E.J.; Marchal-Crespo, L.; Müri, R.M.; Schefold, J.C.; Jakob, S.M.; Nef, T. Comparing the Relaxing Effects of Different Virtual Reality Environments in the Intensive Care Unit: Observational Study. JMIR Perioper. Med. 2019, 2, e15579. [Google Scholar] [CrossRef]
- Gerber, S.M.; Jeitziner, M.M.; Wyss, P.; Chesham, A.; Urwyler, P.; Müri, R.M.; Jakob, S.M.; Nef, T. Visuo-Acoustic Stimulation That Helps You to Relax: A Virtual Reality Setup for Patients in the Intensive Care Unit. Sci. Rep. 2017, 7, 13228. [Google Scholar] [CrossRef] [PubMed]
- Ersin, K.; Gürlek, E.; Güler, H.; Ertugay, Ç.K.; Şerbetçioǧlu, M.B. Appropriate Image Selection with Virtual Reality in Vestibular Rehabilitation: Cross-Sectional Study. JMIR Serious Games 2023, 11, e40806. [Google Scholar] [CrossRef] [PubMed]
- Alvarsson, J.J.; Wiens, S.; Nilsson, M.E. Stress Recovery during Exposure to Nature Sound and Environmental Noise. Int. J. Environ. Res. Public Health 2010, 7, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Micarelli, A.; Viziano, A.; Micarelli, B.; Augimeri, I.; Alessandrini, M. Vestibular Rehabilitation in Older Adults with and without Mild Cognitive Impairment: Effects of Virtual Reality Using a Head-Mounted Display. Arch. Gerontol. Geriatr. 2019, 83, 246–256. [Google Scholar] [CrossRef]
- White, M.P.; Yeo, N.L.; Vassiljev, P.; Lundstedt, R.; Wallergård, M.; Albin, M.; Lõhmus, M. A Prescription for “Nature”—The Potential of Using Virtual Nature in Therapeutics. Neuropsychiatr. Dis. Treat. 2018, 14, 3001–3013. [Google Scholar] [CrossRef] [PubMed]
- Calogiuri, G.; Litleskare, S.; Fagerheim, K.A.; Rydgren, T.L.; Brambilla, E.; Thurston, M. Experiencing Nature through Immersive Virtual Environments: Environmental Perceptions, Physical Engagement, and Affective Responses during a Simulated Nature Walk. Front. Psychol. 2018, 8, 2321. [Google Scholar] [CrossRef]
- Kiryu, T.; So, R.H.Y. Sensation of Presence and Cybersickness in Applications of Virtual Reality for Advanced Rehabilitation. J. Neuroeng. Rehabil. 2007, 4, 34. [Google Scholar] [CrossRef]
- Jäger, M.; Gruber, N.; Müri, R.; Mosimann, U.P.; Nef, T. Manipulations to Reduce Simulator-Related Transient Adverse Health Effects during Simulated Driving. Med. Biol. Eng. Comput. 2014, 52, 601–610. [Google Scholar] [CrossRef]
- Imbimbo, I.; Coraci, D.; Santilli, C.; Loreti, C.; Piccinini, G.; Ricciardi, D.; Castelli, L.; Fusco, A.; Bentivoglio, A.R.; Padua, L. Parkinson’s Disease and Virtual Reality Rehabilitation: Cognitive Reserve Influences the Walking and Balance Outcome. Neurol. Sci. 2021, 42, 4615–4621. [Google Scholar] [CrossRef]
- Basagni, B.; Di Rosa, E.; Bertoni, D.; Mondini, S.; De Tanti, A. Long Term Effects of Severe Acquired Brain Injury: A Follow-up Investigation on the Role of Cognitive Reserve on Cognitive Outcomes. Appl. Neuropsychol. Adult 2023, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dick, T.E.; Hsieh, Y.H.; Dhingra, R.R.; Baekey, D.M.; Galán, R.F.; Wehrwein, E.; Morris, K.F. Cardiorespiratory coupling: Common rhythms in cardiac, sympathetic, and respiratory activities. Prog. Brain Res. 2014, 209, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Usoh, M.; Catena, E.; Arman, S.; Slater, M. Using Presence Questionnaires in Reality. Presence Teleoper. Virtual Environ. 2000, 9, 497–503. [Google Scholar] [CrossRef]

| ID | Sex | Age (Years) | Etiology | DoC | CRS-r | GCS | LCF | Days from Acute Event |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | Male | 69 | Non-traumatic | MCS− | 14 | 7 | 2 | 108 |
| Patient 2 | Male | 22 | Non-traumatic | UWS | 9 | 7 | 2 | 173 |
| Patient 3 | Male | 81 | Non-traumatic | UWS | 4 | 6 | 2 | 108 |
| Patient 4 | Male | 29 | Traumatic | MCS− | 12 | 8 | 2 | 122 |
| Patient 5 | Male | 52 | Non-traumatic | MCS− | 13 | 10 | 2 | 103 |
| Patient 6 | Female | 66 | Non-traumatic | MCS− | 12 | 11 | 2 | 48 |
| Patient 7 | Female | 70 | Non-traumatic | MCS− | 13 | 10 | 2 | 89 |
| Patient 8 | Male | 21 | Traumatic | MCS− | 13 | 8 | 2 | 56 |
| Patient 9 | Female | 66 | Non-traumatic | UWS | 4 | 6 | 2 | 24 |
| Patient 10 | Female | 23 | Traumatic | MCS+ | 16 | 6 | 3 | 50 |
| Patient 11 | Male | 27 | Traumatic | MCS+ | 18 | 12 | 3 | 35 |
| Patient 12 | Male | 21 | Traumatic | MCS+ | 16 | 11 | 3 | 26 |
| ID | Auditory | Visual | Motor | Oromotor/ Verbal | Communication | Arousal | Total Score |
|---|---|---|---|---|---|---|---|
| Patient 1 | 4 | 4 | 3 | 0 | 0 | 3 | 14 |
| Patient 2 | 2 | 1 | 2 | 2 | 0 | 2 | 9 |
| Patient 3 | 1 | 1 | 1 | 0 | 0 | 1 | 4 |
| Patient 4 | 2 | 3 | 4 | 1 | 0 | 2 | 12 |
| Patient 5 | 3 | 2 | 5 | 1 | 0 | 2 | 13 |
| Patient 6 | 2 | 3 | 2 | 2 | 1 | 2 | 12 |
| Patient 7 | 2 | 3 | 4 | 2 | 0 | 2 | 13 |
| Patient 8 | 2 | 3 | 5 | 1 | 0 | 2 | 13 |
| Patient 9 | 1 | 1 | 1 | 0 | 0 | 1 | 4 |
| Patient 10 | 3 | 4 | 5 | 1 | 1 | 2 | 16 |
| Patient 11 | 4 | 4 | 5 | 1 | 1 | 3 | 18 |
| Patient 12 | 4 | 3 | 5 | 1 | 1 | 2 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reale, G.; Fusco, A.; Calciano, R.; Vallario, N.; Vagnarelli, G.; Caliandro, P.; Castelli, L.; Moci, M.; Tieri, G.; Iasevoli, L.; et al. The Immediate Effects of Immersive Virtual Reality on Autonomic Nervous System Function in Patients with Disorders of Consciousness after Severe Acquired Brain Injury: A Pilot Study. J. Clin. Med. 2023, 12, 7639. https://doi.org/10.3390/jcm12247639
Reale G, Fusco A, Calciano R, Vallario N, Vagnarelli G, Caliandro P, Castelli L, Moci M, Tieri G, Iasevoli L, et al. The Immediate Effects of Immersive Virtual Reality on Autonomic Nervous System Function in Patients with Disorders of Consciousness after Severe Acquired Brain Injury: A Pilot Study. Journal of Clinical Medicine. 2023; 12(24):7639. https://doi.org/10.3390/jcm12247639
Chicago/Turabian StyleReale, Giuseppe, Augusto Fusco, Rossella Calciano, Noemi Vallario, Gabriele Vagnarelli, Pietro Caliandro, Letizia Castelli, Marco Moci, Gaetano Tieri, Luigi Iasevoli, and et al. 2023. "The Immediate Effects of Immersive Virtual Reality on Autonomic Nervous System Function in Patients with Disorders of Consciousness after Severe Acquired Brain Injury: A Pilot Study" Journal of Clinical Medicine 12, no. 24: 7639. https://doi.org/10.3390/jcm12247639
APA StyleReale, G., Fusco, A., Calciano, R., Vallario, N., Vagnarelli, G., Caliandro, P., Castelli, L., Moci, M., Tieri, G., Iasevoli, L., & Padua, L. (2023). The Immediate Effects of Immersive Virtual Reality on Autonomic Nervous System Function in Patients with Disorders of Consciousness after Severe Acquired Brain Injury: A Pilot Study. Journal of Clinical Medicine, 12(24), 7639. https://doi.org/10.3390/jcm12247639










