Real-Life Experience in the Efficacy and Safety of COVID-19 Vaccination in Patients with Advanced Cirrhosis
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.1.1. Ethical Approval
2.1.2. Patient Selection
2.1.3. Patients’ Evaluation
2.1.4. Laboratory Investigations
2.1.5. Plasma Level of Ammonia
2.1.6. Abdominal Ultrasound Evaluation
2.1.7. Upper GI Endoscopy
2.1.8. Statistical Plan
3. Results
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baratella, E.; Ruaro, B.; Marrocchio, C.; Starvaggi, N.; Salton, F.; Giudici, F.; Quaia, E.; Confalonieri, M.; Cova, M.A. Interstitial Lung Disease at High Resolution CT after SARS-CoV-2-Related Acute Respiratory Distress Syndrome According to Pulmonary Segmental Anatomy. J. Clin. Med. 2021, 10, 3985. [Google Scholar] [CrossRef] [PubMed]
- Capone, S.; Fusco, F.M.; Milleri, S.; Borrè, S.; Carbonara, S.; Caputo, S.L.; Leone, S.; Gori, G.; Maggi, P.; Cascio, A.; et al. GRAd-COV2 vaccine provides potent and durable humoral and cellular immunity to SARS-CoV-2 in randomized placebo-controlled phase 2 trial. Cell Rep. Med. 2023, 4, 101084. [Google Scholar] [CrossRef] [PubMed]
- Baron-Franco, B.; Ollero-Baturone, M.; Ternero-Vega, J.E.; Nieto-Martín, M.D.; Moreno-Gaviño, L.; Conde-Guzmán, C.; Gutiérrez-Rivero, S.; Rincón-Gómez, M.; Díaz-Jiménez, P.; Muñoz-Lopez, J.J.; et al. Survival Impact of an On-Site Medicalization Program in the Control of COVID-19 Outbreaks in 11 Nursing Homes. J. Clin. Med. 2023, 12, 6517. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Ma, X.; Yu, H.; Zhang, Z.; Bian, P.; Han, Y.; Sun, J.; Liu, Y.; Yang, C.; Geng, J.; et al. The different clinical characteristics of corona virus disease cases between children and their families in China—The character of children with COVID-19. Emerg. Microbes Infect. 2020, 9, 707–713. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Belouzard, S.; Millet, J.K.; Licitra, B.N.; Whittaker, G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses 2012, 4, 1011–1033. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA vaccine against SARS CoV-2—Preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Woodworth, K.R.; Moulia, D.; Collins, J.P.; Hadler, S.C.; Jones, J.M.; Reddy, S.C.; Chamberland, M.; Campos-Outcalt, D.; Morgan, R.L.; Brooks, O.; et al. The Advisory Committee On Immunization Practices’ Interim Recommendation For Use of Pfizer-Biontech COVID-19 Vaccine In Children Aged 5-11 Years-United States, November 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1579–1583. [Google Scholar] [CrossRef]
- Ahammad, I.; Lira, S.S. Designing a novel mRNA vaccine against SARS-CoV-2: An immunoinformatics approach. Int. J. Biol. Macromol. 2020, 162, 820–837. [Google Scholar] [CrossRef]
- Tian, M.; Hua, Z.; Hong, S.; Zhang, Z.; Liu, C.; Lin, L.; Chen, J.; Zhang, W.; Zhou, X.; Zhang, F.; et al. B Cell-Intrinsic MyD88 Signaling Promotes Initial Cell Proliferation and Differentiation To Enhance the Germinal Center Response to a Virus-like Particle. J. Immunol. 2018, 200, 937–948. [Google Scholar] [CrossRef]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and Challenges in the Delivery of mRNA-based Vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef]
- Castells, M.C.; Phillips, E.J. Maintaining safety with SARS-CoV-2 vaccines. N. Engl. J. Med. 2021, 384, 643–649. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Wold, W.S.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013, 13, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatulin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887. [Google Scholar] [CrossRef]
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Xia, S.; Duan, K.; Zhang, Y.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, Y.; Zhang, W.; et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA 2020, 324, 951. [Google Scholar] [CrossRef]
- Munoz, F.M.; Cramer, J.P.; Dekker, C.L.; Dudley, M.Z.; Graham, B.S.; Gurwith, M.; Law, B.; Perlman, S.; Polack, F.P.; Spergel, J.M.; et al. Vaccine-associated enhanced disease: Case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2021, 39, 3053–3066. [Google Scholar] [CrossRef]
- Mantovani, A.; Beatrice, G.; Dalbeni, A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020, 40, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323, e201585. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; Yu, T.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, L.; Wang, F.S. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol. Hepatol. 2020, 5, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Bangash, M.N.; Patel, J.; Parekh, D. COVID-19 and the liver: Little cause for concern. Lancet Gastroenterol. Hepatol. 2020, 5, 529–530. [Google Scholar] [CrossRef] [PubMed]
- Bushberg, J.T.; Seitbert, J.A.; Leidholt, J.E.; Boone, J.M. (Eds.) Ultrasound: The Essential Physics of Medical Imaging, 3rd ed.; Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 500–575. [Google Scholar]
- Boettler, T.; Marjot, T.; Newsome, P.N.; Mondelli, M.U.; Maticic, M.; Cordero, E.; Jalan, R.; Moreau, R.; Cornberg, M.; Berg, T. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep. 2020, 2, 100169. [Google Scholar] [CrossRef] [PubMed]
- Piano, S.; Brocca, A.; Mareso, S.; Angeli, P. Infections complicating cirrhosis. Liver Int. 2018, 38, 126–133. [Google Scholar] [CrossRef]
- Choudhary, N.S.; Dhampalwar, S.; Saraf, N.; Soin, A.S. Outcomes of COVID-19 in Patients with Cirrhosis or Liver Transplantation. J. Clin. Exp. Hepatol. 2021, 11, 713–719. [Google Scholar] [CrossRef]
- Cornberg, M.; Buti, M.; Eberhardt, C.S.; Grossi, P.A.; Shouval, D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J. Hepatol. 2021, 74, 944–951. [Google Scholar] [CrossRef]
- Gartlan, C.; Tipton, T.; Salguero, F.J.; Sattentau, Q.; Gorringe, A.; Carroll, M.W. Vaccine-Associated Enhanced Disease and Pathogenic Human Coronaviruses. Front. Immunol. 2022, 13, 882972. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, C.; Zhao, S.; Sheng, Z.; Xiang, X.; Li, R.; Qian, Z.; Wang, Y.; Chen, B.; Li, Z.; et al. COVID-19 vaccines in patients with decompensated cirrhosis: A retrospective cohort on safety data and risk factors associated with unvaccinated status. Infect. Dis. Poverty 2022, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Chai-Zhen, H.; Kar-Choon, T.; Salmi, A.; Lim-Heng, B.; Haniza, O.; Soek-Siam, T. Severe Hepatocellular Liver Injury After COVID-19 Vaccination Without Autoimmune Hepatitis Features: A Case Series. ACG Case Rep. J. 2022, 9, e00760. [Google Scholar]
- Andrade, R.J.; Aithal, G.P.; Björnsson, E.S.; Kaplowitz, N.; Kullak-Ublick, G.A.; Larrey, D.; Karlsen, T.H. EASL clinical practice guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef] [PubMed]
- Läubli, H.; Balmelli, C.; Kaufmann, L.; Stanczak, M.; Syedbasha, M.; Vogt, D.; Hertig, A.; Müller, B.; Gautschi, O.; Stenner, F.; et al. Influenza vaccination of cancer patients during PD-1 blockade induces protection but may raise the risk for immune-related adverse events. J. Immunother. Cancer 2018, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Giovanellla, L.; Campennì, A. SARS-CoV-2 vaccine may trigger thyroid autoimmunity: Real-life experience and review of the literature. J. Endocrinol. Investig. 2022, 45, 2283–2289. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Nemati, M.; Jafarzadeh, S.; Nozari, P.; Mortazavi, S.M.J. Thyroid dysfunction following vaccination with COVID-19 vaccines: A basic review of the preliminary evidence. J. Endocrinol. Investig. 2022, 45, 1835–1863. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zeng, J.; Jiang, Y.; Xu, Y.W.; Wang, Y.; Zhong, G.; Liu, N.; Wang, Y.; Zhang, Z.; Li, Y.; et al. Thyroid function and associated mood changes after COVID-19 vaccines in patients with Hashimoto thyroiditis. Front. Immunol. 2023, 14, 1129746. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E.; Kharrazian, D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: Implications for autoimmune diseases. Front. Immunol. 2020, 11, 617089. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Gao, F.; Wang, L.; Meng, Y.; Ageno, W.; Qi, X. Incidence and outcomes of splanchnic vein thrombosis after diagnosis of COVID-19 or COVID-19 vaccination: A systematic review and meta-analysis. J. Thromb. Thrombolysis 2022, 55, 18–31. [Google Scholar] [CrossRef]
- Schulz, J.B.; Berlit, P.; Diener, H.C.; Gerloff, C.; Greinacher, A.; Klein, C.; Petzold, G.C.; Piccininni, M.; Poli, S.; Röhrig, R.; et al. COVID-19 vaccine-associated cerebral venous thrombosis in Germany. Ann. Neurol. 2021, 90, 627–639. [Google Scholar] [CrossRef]
- Walsh, K.A.; Lewis, D.A.; Clifford, T.M.; Hundley, J.C.; Gokun, Y.; Angulo, P.; Davis, G.A. Risk factors for venous thromboembolism in patients with chronic liver disease. Ann. Pharmacother. 2013, 47, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Primignani, M.; Lemma, L.; Chantarangkul, V.; Dell’Era, A.; Iannuzzi, F.; Aghemo, A.; Mannucci, P.M. Detection of the imbalance of procoagulant versus anticoagulant factors in cirrhosis by a simple laboratory method. Hepatology 2010, 52, 249–255. [Google Scholar] [CrossRef]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.; et al. Risk of Myocarditis After Sequential Doses of COVID-19 Vaccine and SARS-CoV-2 Infection by Age and Sex. Circulation 2022, 146, 743–754. [Google Scholar] [CrossRef]
- Kaur, H.; Premkumar, M. Diagnosis and Management of Cirrhotic Cardiomyopathy. J. Clin. Exp. Hepatol. 2022, 12, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Fix, O.K.; Blumberg, E.A.; Chang, K.M.; Chu, J.; Chung, R.T.; Goacher, E.K.; Hameed, B.; Kaul, D.R.; Kulik, L.M.; Kwok, R.M.; et al. AASLD Expert Panel Consensus Statement: Vaccines to Prevent COVID-19 Infection in Patients with Liver Disease. Hepatology 2021, 72, 1049–1064. [Google Scholar] [CrossRef] [PubMed]
- FDA Briefing Document: Johnson & Johnson/Janssen COVID-19 Vaccine. Vaccines and Related Biological Products Advisory Committee Meeting. Available online: www.fda.gov/media/146217/download (accessed on 2 July 2021).
| Parameter | COVID-19 Vaccine Complications | Total | p | |||
|---|---|---|---|---|---|---|
| Absent | Present | SW, p | ||||
| N = 50 | N = 20 | N = 70 | ||||
| Age (years) | 54 ± 11 | 57 ± 12 | 55 (29–87) IQR 15 | 0.583 | 0.335 | |
| Sex | 0 | 19 (38.8%) | 7 (33.3%) | 26 (37.1%) | 0.666 | |
| 1 | 30 (61.2%) | 14 (66.7%) | 44 (62.9%) | |||
| DM | 0 | 33 (67.3%) | 15 (71.4%) | 48 (68.6%) | 0.736 | |
| 1 | 16 (32.7%) | 6 (28.6%) | 22 (31.4%) | |||
| WBC (×109/L) | 5.49 ± 2.35 | 5.29 ± 1.67 | 4.83 (0.93–13.2) IQR 2.67 | 0.003 | 0.953 | |
| HB (gm/dL) | 12.2 ± 1.6 | 12.3 ± 1.1 | 12 (10–17) IQR 2.23 | 0.003 | 0.398 | |
| PLT (×109/L) | 109 ± 22 | 110 ± 19 | 109 ± 21 | 0.09 | 0.851 | |
| ALT (IU/L) | 45 ± 11 | 39 ± 10 | 43 ± 11 | 0.282 | 0.045 | |
| AST (IU/L) | 51 ± 10 | 55 ± 13 | 52 ± 11 | 0.361 | 0.256 | |
| PC% | 56 ± 10 | 57 ± 11 | 56 ± 10 | 0.54 | 0.645 | |
| INR | 1.58 ± 0.24 | 1.66 ± 0.27 | 1.61 ± 0.25 | 0.414 | 0.226 | |
| Total Bilirubin (mg/dL) | 1.42 ± 0.31 | 1.54 ± 0.31 | 1.4 (0.88–2.2) IQR 0.45 | 0.041 | 0.083 | |
| Albumin (gm/dL) | 3.39 ± 0.48 | 3.32 ± 0.40 | 3.37 ± 0.45 | 0.885 | 0.576 | |
| Ammonia (ug/dL) | 74.1 ± 17.4 | 79.1 ± 19.2 | 75.6 ± 18.0 | 0.409 | 0.287 | |
| Sodium (mEq/L) | 140 ± 4 | 137 ± 5 | 139 (130–147) IQR 7.3 | 0.038 | 0.006 | |
| Creatinine (mg/dL) | 1.32 ± 0.25 | 1.49 ± 0.25 | 1.37 ± 0.26 | 0.937 | 0.041 | |
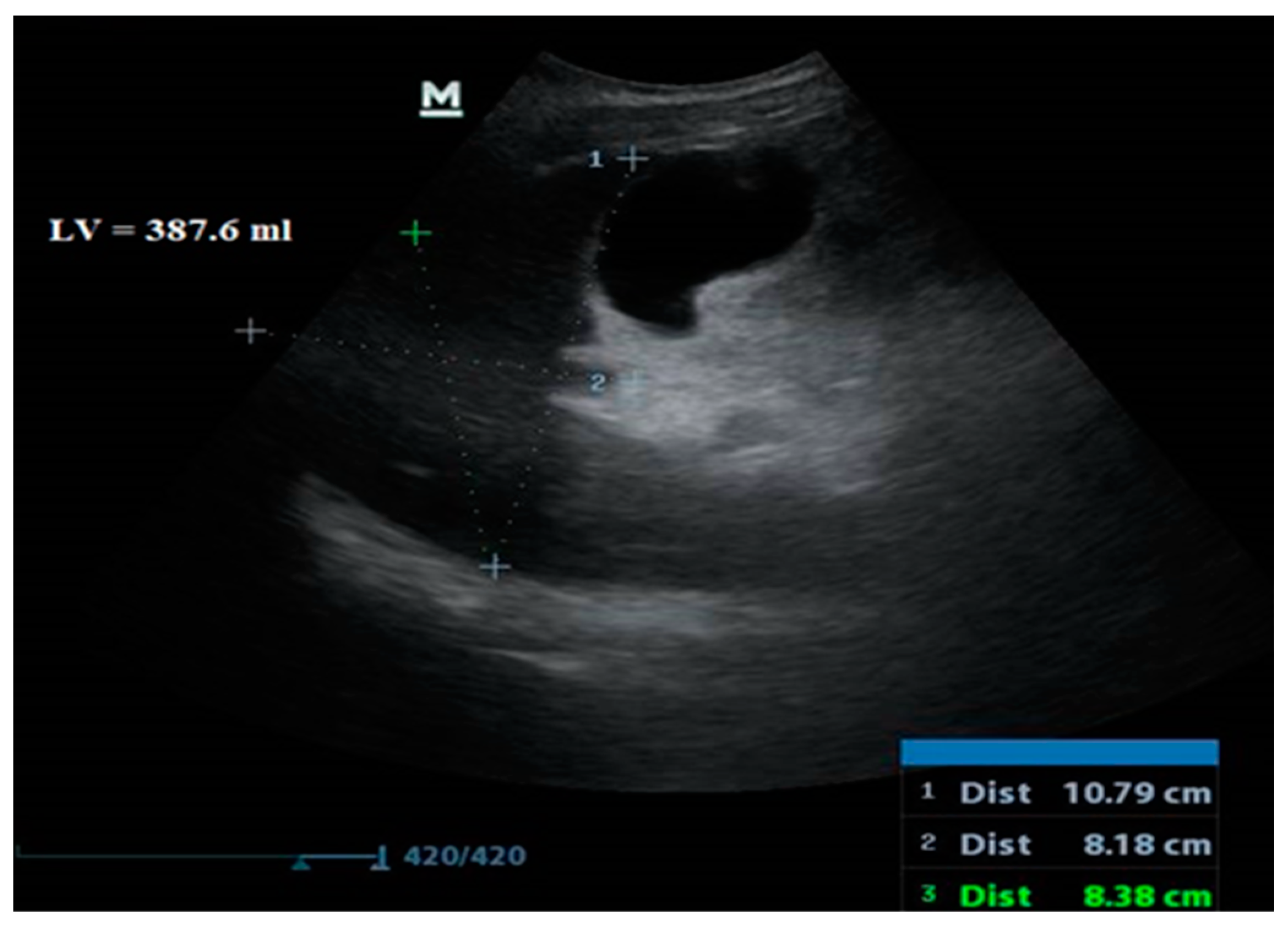
| Liver Volume (mL) | 808.3 ± 147.8 | 593.5 ± 122.0 | 726 (403–1280) IQR 211 | 0.01 | <0.001 | |
| MELD | 16 ± 3 | 19 ± 3 | 17 ± 3 | 0.265 | 0.001 | |
| CTP | 5 | 17 (34.7%) | 4 (19.0%) | 21 (30.0%) | 0.309 | |
| 6 | 17 (34.7%) | 7 (33.3%) | 24 (34.3%) | |||
| 7 | 13 (26.5%) | 7 (33.3%) | 20 (28.6%) | |||
| 8 | 2 (4.1%) | 3 (14.3%) | 5 (7.1%) | |||
| FIB-4 | 3.96 ± 1.31 | 4.61 ± 1.69 | 3.8 (1.4–7.7) IQR 1.64 | 0.000 | 0.123 | |
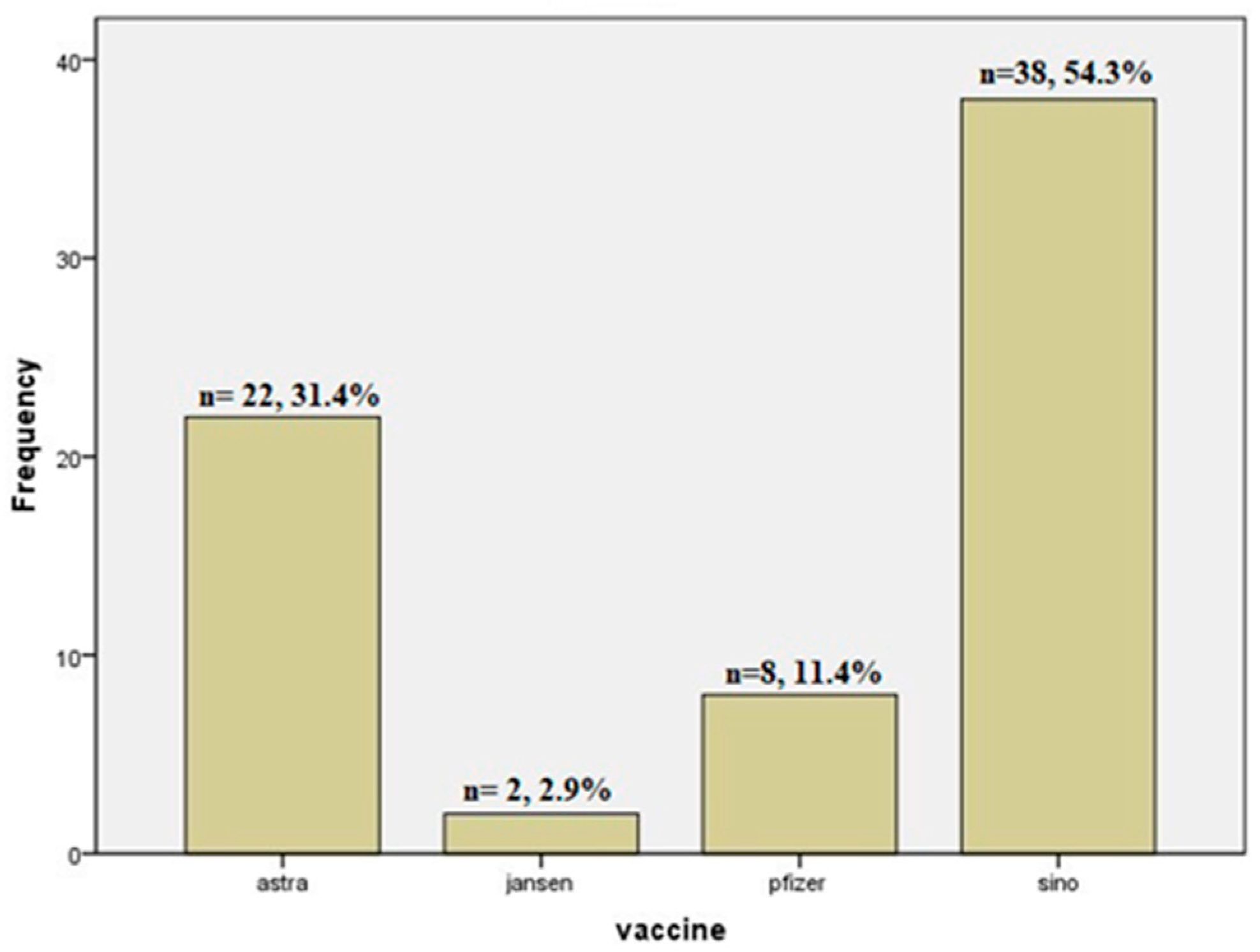
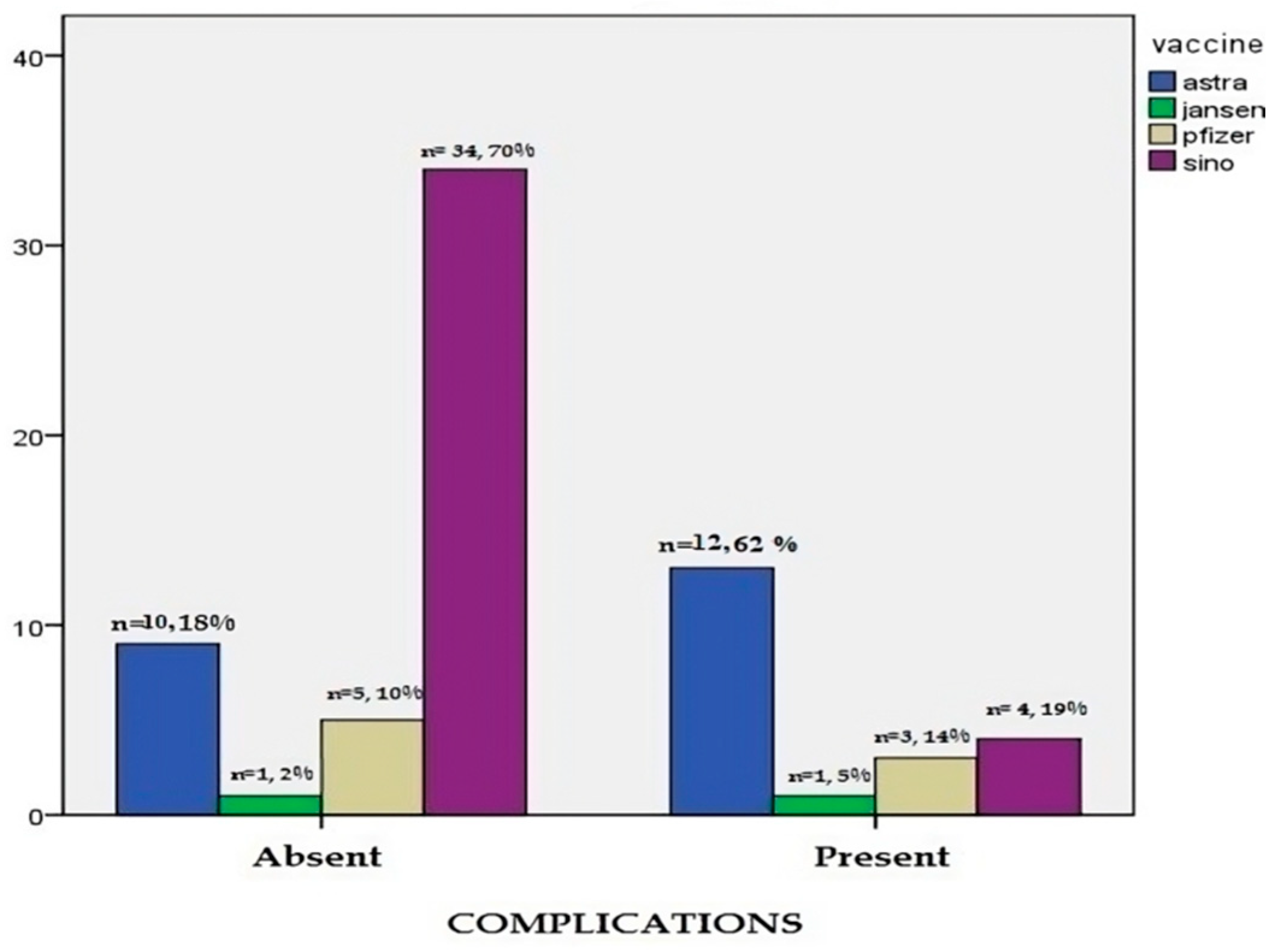
| Vaccine Type | Astra® | 10 (18.4%) | 12 (61.9%) | 22 (31.4%) | 0.001 | |
| Jansen® | 1 (2.0%) | 1 (4.8%) | 2 (2.9%) | |||
| Pfizer® | 5 (10.2%) | 3 (14.3%) | 8 (11.4%) | |||
| Sino® | 34 (69.4%) | 4 (19.0%) | 38 (54.3%) | |||
| Complications (n = 20) | Age | Sex | Diabetes | ALT | Sodium | Creatinine | MELD | Liver Volume (LV) | Correlation (p) |
|---|---|---|---|---|---|---|---|---|---|
| Decompensation (n = 20, 100%) | 56.1 ± 10.3 | 14M/6F | 6 (30%) | 38.7 ± 9.3 | 136.5 ± 4.61 | 1.5 ± 0.25 | 19.1 ± 2.5 | 571.7 ± 71.6 | -ALT (0.019) |
| -Sodium (0.005) | |||||||||
| -Creatinine (0.01) | |||||||||
| -LV (0.000) | |||||||||
| -MELD (0.000) | |||||||||
| Hypothyroidism (n = 11, 55%) | 59.4 ± 6.9 | 5M/6F | 1 (9.1%) | 43.1 ± 8.8 | 139 ± 4.58 | 1.44 ± 0.37 | 17.5 ± 3.5 | 614.3 ± 192 | -LV (0.005) |
| Splanchnic Thrombosis (n = 5, 25%) | 56.5 ± 8.7 | 3M/2F | 3 (60%) | 38 ± 9.2 | 136.2 ± 4.4 | 1.7 ± 0.33 | 19.4 ± 3.3 | 539.1 ± 89.5 | -Creatinine (0.003) |
| -LV (0.005) | |||||||||
| Cardiomyopathy (n = 5, 25%) | 58 ± 8.75 | 3M/2F | 0 (0%) | 38.4 ± 8.7 | 137.4 ± 5.9 | 1.53 ± 0.3 | 18.8 ± 1.8 | 509.7 ± 42.2 | -LV (0.001) |
| Vaccine-associated enhanced disease (n = 2, 10%) | 59.5 ± 10.6 | 2F | 0 (0%) | 30 ± 5.7 | 137 ± 5.65 | 1.33 ± 0.01 | 18.5 ± 0.71 | 531.5 ± 114.8 | None |
| p | 0.884 | - | 0.029 | 0.392 | 0.693 | 0.5 | 0.61 | 0.49 | - |
| Variable | COVID-19 Vaccine Complications | p | |
|---|---|---|---|
| Before Vaccine | After Vaccine | ||
| N = 20 | N = 20 | ||
| WBC (×109/L) | 5.32 ± 1.7 | 4.81 ± 0.76 | 0.324 |
| Hemoglobin (gm/L) | 12.36 ± 1.07 | 11.6 ± 1.2 | 0.038 |
| Platelets (×109/L) | 108.9 ± 19.2 | 109.1 ± 8.67 | 0.97 |
| INR | 1.67 ± 0.28 | 1.92 ± 0.42 | 0.037 |
| Total Bilirubin (mg/dL) | 1.56 ± 0.31 | 2.73 ± 0.5 | 0.000 |
| Albumin (gm/dL) | 3.33 ± 0.41 | 2.66 ± 0.29 | 0.000 |
| Ammonia (ug/dL) | 79.2 ± 19.7 | 96 ± 18.8 | 0.02 |
| Sodium (mEq/L) | 136.45 ± 4.6 | 135.6 ± 4.9 | 0.613 |
| Creatinine (mg/dL) | 1.49 ± 0.25 | 1.57 ± 0.36 | 0.413 |
| CTP | 6.45 ± 1 | 9.65 ± 0.75 | 0.000 ** |
| MELD | 19.1 ± 2.5 | 22.9 ± 3.91 | 0.001 |
| COMPLICATIONS | ||
|---|---|---|
| Pearson Correlation | Sig. (2-Tailed) | |
| Age | 0.096 | 0.427 |
| Sex | 0.093 | 0.441 |
| DM | −0.019 | 0.873 |
| WBC | −0.030 | 0.805 |
| HB | −0.046 | 0.704 |
| PLT | −0.008 | 0.947 |
| ALT | 0.279 | 0.019 |
| AST | 0.105 | 0.385 |
| PC% | 0.032 | 0.795 |
| INR | 0.153 | 0.205 |
| Total Bilirubin | 0.204 | 0.091 |
| Albumin | −0.058 | 0.634 |
| Ammonia | 0.129 | 0.288 |
| Sodium | −0.330 | 0.005 |
| Creatinine | 0.302 | 0.011 |
| Liver Volume | −0.640 | 0.000 |
| MELD | 0.439 | 0.000 |
| CTP | 0.220 | 0.067 |
| FIB-4 | 0.227 | 0.058 |
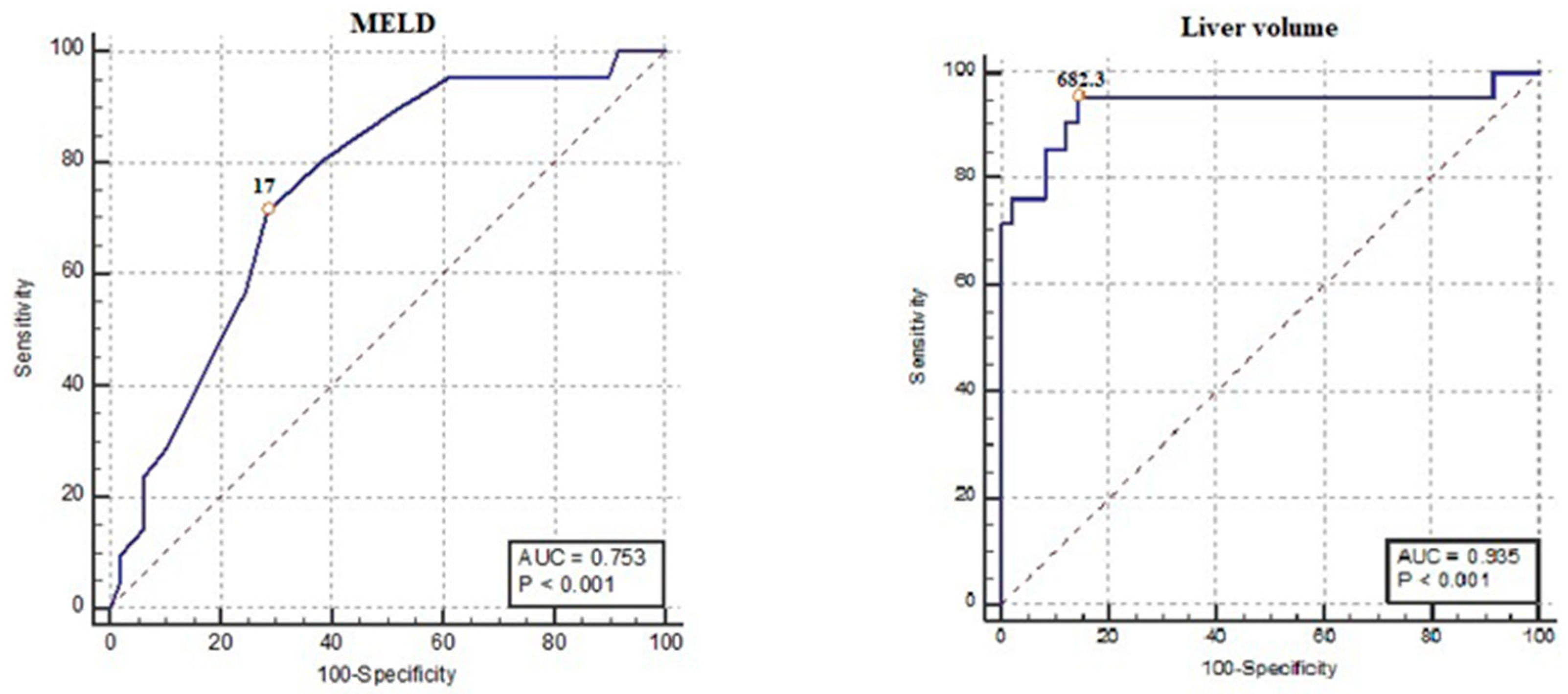
| Cut-off | Sensitivity % | Specificity % | PPV | NPV | AUC | J Value | p | |
|---|---|---|---|---|---|---|---|---|
| MELD Score | >17 | 71.4 47.8–88.7 | 70 56.7–83.4 | 51.7 38.9–64.3 | 85.4 74.4–92.1 | 0.753 0.636–0.848 | 0.43 | <0.001 |
| Liver Volume | ≤682.3 | 95.24 76.2–99.9 | 85.71 72.8–94.1 | 74.1 58.8–85.1 | 97.7 86.1–99.7 | 0.935 0.849–0.980 | 0.81 | <0.001 |
| Covariate | B | SE | Wald | Sig. | RR | 95%CI for RR |
|---|---|---|---|---|---|---|
| ALT (IU/L) | 0.000 | 0.038 | 0.000 | 0.995 | 1.00 | 0.93–1.08 |
| Sodium (mEq/L) | −0.107 | 0.107 | 1.009 | 0.315 | 0.90 | 0.73–1.11 |
| Creatinine (mg/dL) | −1.025 | 2.346 | 0.191 | 0.662 | 0.36 | 0.00–35.62 |
| Liver Volume (mL) | −0.020 | 0.006 | 10.485 | 0.001 | 0.98 | 0.97–0.99 |
| MELD | −0.019 | 0.198 | 0.010 | 0.922 | 0.98 | 0.67–1.45 |
| FIB-4 | 0.265 | 0.269 | 0.972 | 0.324 | 1.30 | 0.77–2.21 |
| Constant | 28.035 | |||||
| Variable(s) entered: ALT, Sodium, Creatinine, Liver Volume, MELD, FIB-4. | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanafy, A.S.; Embaby, A.; Salem, S.M.; Behiry, A.; Ebrahim, H.A.; Elkattawy, H.A.; Abed, S.Y.; Almadani, M.E.; El-Sherbiny, M. Real-Life Experience in the Efficacy and Safety of COVID-19 Vaccination in Patients with Advanced Cirrhosis. J. Clin. Med. 2023, 12, 7578. https://doi.org/10.3390/jcm12247578
Hanafy AS, Embaby A, Salem SM, Behiry A, Ebrahim HA, Elkattawy HA, Abed SY, Almadani ME, El-Sherbiny M. Real-Life Experience in the Efficacy and Safety of COVID-19 Vaccination in Patients with Advanced Cirrhosis. Journal of Clinical Medicine. 2023; 12(24):7578. https://doi.org/10.3390/jcm12247578
Chicago/Turabian StyleHanafy, Amr Shaaban, Ahmed Embaby, Sara Mohamed Salem, Ahmed Behiry, Hasnaa Ali Ebrahim, Hany Ahmed Elkattawy, Sally Yussef Abed, Moneer E. Almadani, and Mohamad El-Sherbiny. 2023. "Real-Life Experience in the Efficacy and Safety of COVID-19 Vaccination in Patients with Advanced Cirrhosis" Journal of Clinical Medicine 12, no. 24: 7578. https://doi.org/10.3390/jcm12247578
APA StyleHanafy, A. S., Embaby, A., Salem, S. M., Behiry, A., Ebrahim, H. A., Elkattawy, H. A., Abed, S. Y., Almadani, M. E., & El-Sherbiny, M. (2023). Real-Life Experience in the Efficacy and Safety of COVID-19 Vaccination in Patients with Advanced Cirrhosis. Journal of Clinical Medicine, 12(24), 7578. https://doi.org/10.3390/jcm12247578





