Post-COVID-19 and Post-COVID-19 Vaccine Arthritis, Polymyalgia Rheumatica and Horton’s Arteritis: A Single-Center Assessment of Clinical, Serological, Genetic, and Ultrasonographic Biomarkers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
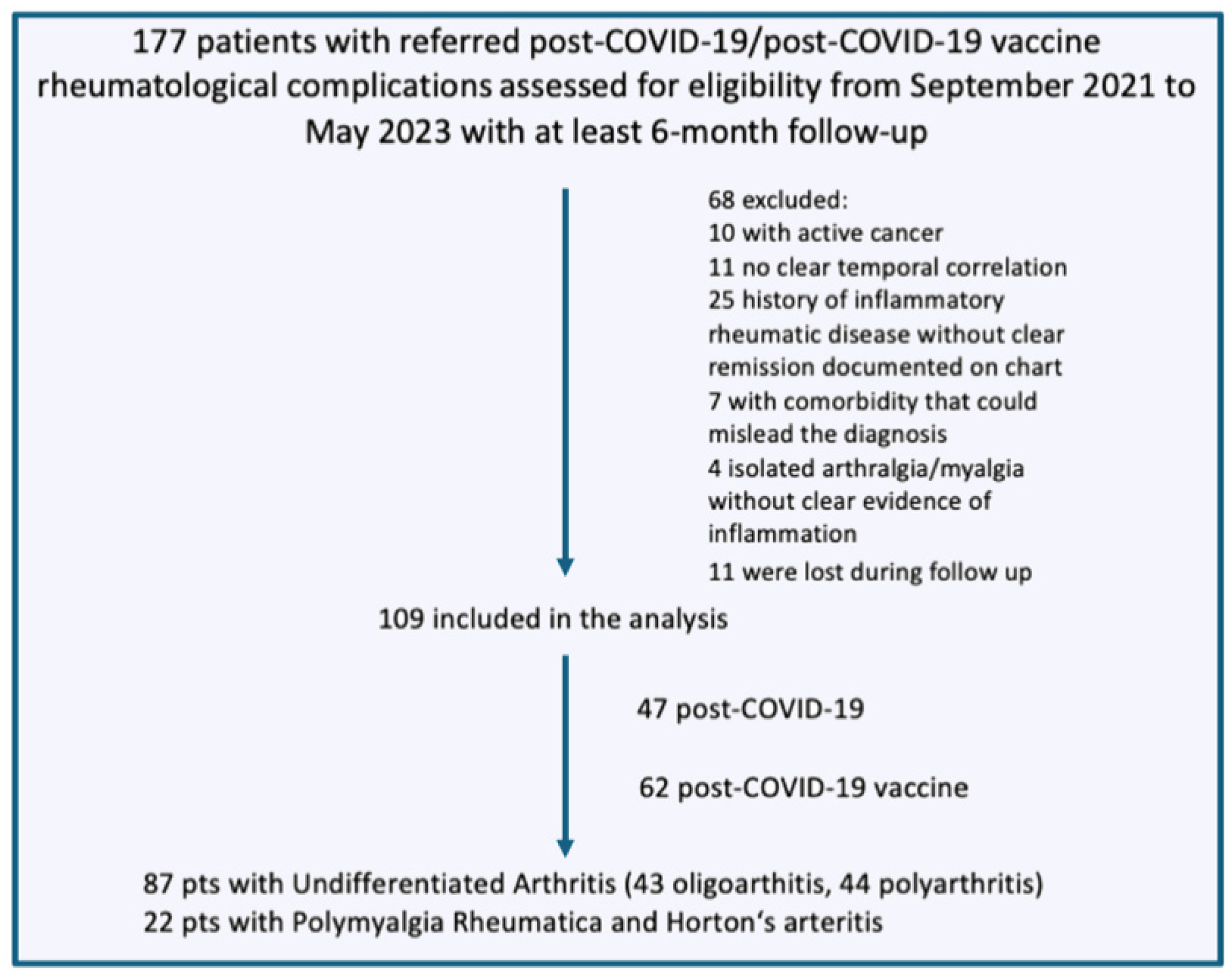
2.2. Participants
2.3. Measurements
- Active synovitis: echogenic, non-compressible, intra-articular synovial vascularization with a PD signal;
- Active tenosynovitis or peri-tendinitis: hypoechoic thickened tissue with or without fluid within the flexor tendon sheath or around the extensor tendons, respectively, seen in two perpendicular planes, displaying a PD signal;
- Pseudo-tenosynovitis (in hands): hypoechoic soft tissue surrounding the flexor tendon showing an intense PD signal;
- Bone erosions: interruptions of the bone profile on two perpendicular scanning planes.
2.4. Statistical Analysis
3. Results
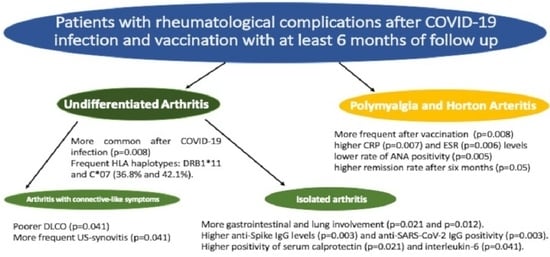
3.1. Comparison of PC and PCV Arthritis to Polymyalgia Rheumatica and Horton’s Arteritis
3.2. Comparison of PC and PCV Isolated Arthritis to “Connective Like” Arthritis
3.3. Comparison of HLA Haplotypes in PC Arthritis vs. PCV Arthritis and in Isolated Arthritis vs. Connective-like Arthritis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahase, E. COVID-19: WHO Declares Pandemic Because of “Alarming Levels” of Spread, Severity, and Inaction. BMJ 2020, 368, m1036. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Map, Johns Hopkins Coronavirus Resource Center 2022. Available online: https://Coronavirus.Jhu.Edu/Map.Html (accessed on 3 October 2023).
- Wei, J.; Matthews, P.C.; Stoesser, N.; Maddox, T.; Lorenzi, L.; Studley, R.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I. Anti-Spike Antibody Response to Natural SARS-CoV-2 Infection in the General Population. Nat. Commun. 2021, 12, 6250. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P. Diverse Functional Autoantibodies in Patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
- Liu, Y.; Sawalha, A.H.; Lu, Q. COVID-19 and Autoimmune Diseases. Curr. Opin. Rheumatol. 2021, 33, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kanduc, D. From Anti-SARS-CoV-2 Immune Responses to COVID-19 via Molecular Mimicry. Antibodies 2020, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Castilla, J.; Stebliankin, V.; Baral, P.; Balbin, C.A.; Sobhan, M.; Cickovski, T.; Mondal, A.M.; Narasimhan, G.; Chapagain, P.; Mathee, K. Potential Autoimmunity Resulting from Molecular Mimicry between SARS-CoV-2 Spike and Human Proteins. Viruses 2022, 14, 1415. [Google Scholar] [CrossRef]
- Segal, Y.; Shoenfeld, Y. Vaccine-Induced Autoimmunity: The Role of Molecular Mimicry and Immune Crossreaction. Cell Mol. Immunol. 2018, 15, 586–594. [Google Scholar] [CrossRef]
- COVID-19 Vaccines, United States Food and Drug Administration (U.S. FDA) 2022. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines-2023-2024 (accessed on 1 April 2023).
- Chen, C.; Chen, C. New-onset Inflammatory Arthritis after COVID-19 Vaccination: A Systematic Review. Int. J. Rheum. Dis. 2023, 26, 267–277. [Google Scholar] [CrossRef]
- Salemi, S.; D’Amelio, R. Could Autoimmunity Be Induced by Vaccination? Int. Rev. Immunol. 2010, 29, 247–269. [Google Scholar] [CrossRef]
- Toussirot, É.; Bereau, M. Vaccination and Induction of Autoimmune Diseases. Inflamm. Allergy Drug Targets 2015, 14, 94–98. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.-M.; Shuai, Z.-W.; Ye, D.-Q.; Pan, H.-F. New-Onset Autoimmune Phenomena Post-COVID-19 Vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Waisbren, B.A.S. Acquired Autoimmunity after Viral Vaccination Is Caused by Molecular Mimicry and Antigen Complimentarity in the Presence of an Immunologic Adjuvant and Specific HLA Patterns. Med. Hypotheses 2008, 70, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, F.; O’Neill, S.G. Clinical Aspects of Autoimmune Rheumatic Diseases. Lancet 2013, 382, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Ruscitti, P.; Raimondo, V.; De Angelis, R.; Cacciapaglia, F.; Pigatto, E.; Olivo, D.; Di Cola, I.; Galluccio, F.; Francioso, F.; et al. Spectrum of Short-Term Inflammatory Musculoskeletal Manifestations after COVID-19 Vaccine Administration: A Report of 66 Cases. Ann. Rheum. Dis. 2022, 81, 440–441. [Google Scholar] [CrossRef] [PubMed]
- Nune, A.; Iyengar, K.P.; Goddard, C.; Ahmed, A.E. Multisystem Inflammatory Syndrome in an Adult Following the SARS-CoV-2 Vaccine (MIS-V). BMJ Case Rep. 2021, 14, e243888. [Google Scholar] [CrossRef] [PubMed]
- An, Q.-J.; Qin, D.-A.; Pei, J.-X. Reactive Arthritis after COVID-19 Vaccination. Hum. Vaccin. Immunother. 2021, 17, 2954–2956. [Google Scholar] [CrossRef] [PubMed]
- Mv, P.; Auanassova, A.; Yessirkepov, M.; Zimba, O.; Gasparyan, A.Y.; Kitas, G.D.; Ahmed, S. New-Onset Systemic Vasculitis Following SARS-CoV-2 Infection and Vaccination: The Trigger, Phenotype, and Outcome. Clin. Rheumatol. 2023, 42, 2761–2775. [Google Scholar] [CrossRef] [PubMed]
- Padiyar, S.; Kamath, N.; Mathew, J.; Chandu, A.; Deodhar, D.; Shastry, B.; Shashikala, T.; Ganapati, A. New-Onset Adult-Onset Still’s Disease-like Syndrome after ChAdOx1 nCoV-19 Vaccination—A Case Series with Review of Literature. Clin. Rheumatol. 2022, 41, 1569–1575. [Google Scholar] [CrossRef]
- Gouda, W.; Albasri, A.; Alsaqabi, F.; Al Sabah, H.Y.; Alkandari, M.; Abdelnaby, H. Dermatomyositis Following BNT162b2 mRNA COVID-19 Vaccination. J. Korean Med. Sci. 2022, 37, e32. [Google Scholar] [CrossRef]
- Ishay, Y.; Kenig, A.; Tsemach-Toren, T.; Amer, R.; Rubin, L.; Hershkovitz, Y.; Kharouf, F. Autoimmune Phenomena Following SARS-CoV-2 Vaccination. Int. Immunopharmacol. 2021, 99, 107970. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Modi, P.; Cascella, M. Diffusing Capacity of the Lungs for Carbon Monoxide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Martinez-Pitre, P.J.; Sabbula, B.R.; Cascella, M. Restrictive Lung Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Marshall, J.C.; Murthy, S.; Diaz, J.; Adhikari, N.; Angus, D.C.; Arabi, Y.M.; Baillie, K.; Bauer, M.; Berry, S.; Blackwood, B. A Minimal Common Outcome Measure Set for COVID-19 Clinical Research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Cimmino, M.A.; Kremers, H.M.; Schmidt, W.A.; Schirmer, M.; Salvarani, C.; Bachta, A.; Dejaco, C.; Duftner, C.; Jensen, H.S. 2012 Provisional Classification Criteria for Polymyalgia Rheumatica: A European League Against Rheumatism/American College of Rheumatology Collaborative Initiative. Arthritis Rheum. 2012, 64, 943–954. [Google Scholar] [CrossRef]
- Ponte, C.; Grayson, P.C.; Robson, J.C.; Suppiah, R.; Gribbons, K.B.; Judge, A.; Craven, A.; Khalid, S.; Hutchings, A.; Watts, R.A.; et al. 2022 American College of Rheumatology/EULAR Classification Criteria for Giant Cell Arteritis. Ann. Rheum. Dis. 2022, 81, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.K.; von Mühlen, C.A.; Fritzler, M.J.; Damoiseaux, J.; Infantino, M.; Klotz, W.; Satoh, M.; Musset, L.; García-De La Torre, I.; Carballo, O.G. The International Consensus on ANA Patterns (ICAP) in 2021—The 6th Workshop and Current Perspectives. J. Appl. Lab. Med. 2022, 7, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Bandinelli, F.; Benucci, M.; Salaffi, F.; Manetti, M.; Infantino, M.; Damiani, A.; Manfredi, M.; Grossi, V.; Matucci, A.; Li Gobbi, F. Do New and Old Biomarkers of Early Undifferentiated Arthritis Correlate with Arthritis Impact Measurement Scales. Clin. Exp. Rheumatol. 2021, 39, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, R.J.; Gibbon, W.W.; Conaghan, P.G.; O’Connor, P.; McGonagle, D.; Pease, C.; Green, M.J.; Veale, D.J.; Isaacs, J.D.; Emery, P. The Value of Sonography in the Detection of Bone Erosions in Patients with Rheumatoid Arthritis: A Comparison with Conventional Radiography. Arthritis Rheum. 2000, 43, 2762–2770. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, S. The STROBE Guidelines. Saudi J. Anaesth 2019, 13, S31–S34. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Martinez-Cajas, J.; Yip, P.M.; Kulasingam, V.; Garland, J.; Holland, D.; Shamseddin, M.K.; Gong, Y. Evaluation and Comparison of Four Quantitative SARS-CoV-2 Serological Assays in COVID-19 Patients and Immunized Healthy Individuals, Cancer Patients, and Patients with Immunosuppressive Therapy. Clin. Biochem. 2023, 116, 1–6. [Google Scholar] [CrossRef]
- Bai, Z.; Cao, Y.; Liu, W.; Li, J. The SARS-CoV-2 Nucleocapsid Protein and Its Role in Viral Structure, Biological Functions, and a Potential Target for Drug or Vaccine Mitigation. Viruses 2021, 13, 1115. [Google Scholar] [CrossRef]
- Shenoy, P.; Ahmed, S.; Shanoj, K.C.; Shenoy, V.; Damodaran, D.; Menon, A.R.; Alias, B.; SanjoSaijan; Devakumar, D.; Babu, A.S.S. Antibody Responses after Documented COVID-19 Disease in Patients with Autoimmune Rheumatic Disease. Clin. Rheumatol. 2021, 40, 4665–4670. [Google Scholar] [CrossRef] [PubMed]
- Lumley, S.F.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; Warren, F.; et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N. Engl. J. Med. 2021, 384, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Freund, O.; Breslavsky, A.; Fried, S.; Givoli-Vilensky, R.; Cohen-Rubin, S.; Zacks, N.; Kleinhendler, E.; Unterman, A.; Frydman, S.; Wand, O.; et al. Interactions and Clinical Implications of Serological and Respiratory Variables 3 Months after Acute COVID-19. Clin. Exp. Med. 2023, 23, 3729–3736. [Google Scholar] [CrossRef] [PubMed]
- Freund, O.; Breslavsky, A.; Givoli-Vilensky, R.; Zacks, N.; Gershman, E.; Melloul, A.; Wand, O.; Bilenko, N.; Bar-Shai, A. Assessment of a Close Respiratory Follow-up Schedule at 3 and 6 Months after Acute COVID-19 and Its Related Investigations. Respir. Med. 2023, 217, 107367. [Google Scholar] [CrossRef] [PubMed]
- Zafarani, A.; Razizadeh, M.H.; Pashangzadeh, S.; Amirzargar, M.R.; Taghavi-Farahabadi, M.; Mahmoudi, M. Natural Killer Cells in COVID-19: From Infection, to Vaccination and Therapy. Future Virol. 2023, 18, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Pountain, G.; Keogan, M.; Brown, D.; Hazleman, B. Circulating T Cell Subtypes in Polymyalgia Rheumatica and Giant Cell Arteritis: Variation in the Percentage of CD8+ Cells with Prednisolone Treatment. Ann. Rheum. Dis. 1993, 52, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Luppi, F.; Sebastiani, M.; Salvarani, C.; Bendstrup, E.; Manfredi, A. Acute Exacerbation of Interstitial Lung Disease Associated with Rheumatic Disease. Nat. Rev. Rheumatol. 2022, 18, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Lui, R.N.; Sung, J.J. COVID-19 and the Digestive System. J. Gastroenterol. Hepatol. 2020, 35, 744–748. [Google Scholar] [CrossRef]
- Bandinelli, F.; Manetti, M.; Ibba-Manneschi, L. Occult Spondyloarthritis in Inflammatory Bowel Disease. Clin. Rheumatol. 2016, 35, 281–289. [Google Scholar] [CrossRef]
- Ojetti, V.; Saviano, A.; Covino, M.; Acampora, N.; Troiani, E.; Franceschi, F.; Abbate, V.; Addolorato, G.; Agostini, F.; Ainora, M.E. COVID-19 and Intestinal Inflammation: Role of Fecal Calprotectin. Dig. Liver Dis. 2020, 52, 1231–1233. [Google Scholar] [CrossRef]
- Ursini, F.; Ruscitti, P.; Raimondo, V.; De Angelis, R.; Cacciapaglia, F.; Pigatto, E.; Olivo, D.; Di Cola, I.; Galluccio, F.; Francioso, F. Systemic Syndromes of Rheumatological Interest with Onset after COVID-19 Vaccine Administration: A Report of 30 Cases. Clin. Rheumatol. 2022, 41, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Abu Hussein, N.; Machahua, C.; Ruchti, S.; Horn, M.; Piquilloud, L.; Prella, M.; Geiser, T.; von Garnier, C.; Funke-Chambour, M. Circulating Calprotectin Levels Four Months after Severe and Non-Severe COVID-19. BMC Infect. Dis. 2023, 23, 650. [Google Scholar] [CrossRef] [PubMed]
- Bandinelli, F.; Denaro, V.; Prignano, F.; Collaku, L.; Ciancio, G.; Matucci-Cerinic, M. Ultrasonographic Wrist and Hand Abnormalities in Early Psoriatic Arthritis Patients: Correlation with Clinical, Dermatological, Serological and Genetic Indices. Clin. Exp. Rheumatol. 2015, 33, 330–335. [Google Scholar]
- Viatte, S.; Plant, D.; Han, B.; Fu, B.; Yarwood, A.; Thomson, W.; Symmons, D.P.; Worthington, J.; Young, A.; Hyrich, K.L. Association of HLA-DRB1 Haplotypes with Rheumatoid Arthritis Severity, Mortality, and Treatment Response. JAMA 2015, 313, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.; Sandor, C.; Stahl, E.A.; Freudenberg, J.; Lee, H.-S.; Jia, X.; Alfredsson, L.; Padyukov, L.; Klareskog, L.; Worthington, J. Five Amino Acids in Three HLA Proteins Explain Most of the Association between MHC and Seropositive Rheumatoid Arthritis. Nat. Genet. 2012, 44, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Benucci, M.; Damiani, A.; Li Gobbi, F.; Bandinelli, F.; Infantino, M.; Grossi, V.; Manfredi, M.; Noguier, G.; Meacci, F. Correlation between HLA Haplotypes and the Development of Antidrug Antibodies in a Cohort of Patients with Rheumatic Diseases. Biol. Targets Ther. 2018, 12, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Talotta, R. Molecular Mimicry and HLA Polymorphisms May Drive Autoimmunity in Recipients of the BNT-162b2 mRNA Vaccine: A Computational Analysis. Microorganisms 2023, 11, 1686. [Google Scholar] [CrossRef]
- Velasteguí, E.; Vera, E.; Muñoz, M.S.; Vanden Berghe, W.; Orellana-Manzano, A. HLA-C: Evolution, Epigenetics, and Pathological Implications in the Major Histocompatibility Complex. Front. Genet. 2023, 14, 1206034. [Google Scholar] [CrossRef]
| Arthritis (N = 87) | Polymyalgia Rheumatica—Horton’s Arteritis (N = 22) | p-Value | |
|---|---|---|---|
| Age, years (median, IQR) | 58.00 (47.00–74.00) | 71.00 (54.75–79.00) | 0.069 |
| Female Gender (N, %) | 54 (62.1%) | 18 (81.8%) | 0.129 |
| Post COVID-19 (N, %) | 43 (49.4%) | 4 (18.2%) | 0.008 |
| Post COVID-19 vaccine (N, %) | 44 (50.6%) | 18 (81.8%) | 0.008 |
| Specific vaccine administered (N, %) | BNT162b2 35(79.6%) mRNA-1273 6 (13.6%) AZD1222 1 (2.3%) Heterologous 2 (4.6%) | BNT162b2 13 (72.2%) mRNA-1273 2 (11.1%) AZD1222 3 (16.7%) Heterologous 0 (0%) | 0.524 0.070 0.999 0.999 |
| Signs and symptoms | |||
| Headache (N, %) | 15 (17.2%) | 6 (27.3%) | 0.363 |
| Mandibular claudication (N, %) | 0 (0%) | 4 (18.2%) | 0.001 |
| Gastro-intestinal symptoms (N, %) | 17 (19.5%) | 0 (0%) | 0.021 |
| HRCT ILD (N, %) | 20 (23%) | 0 (0%) | 0.010 |
| 3-month remission (N, %) | 23 (26.4%) | 10 (45.4%) | 0.118 |
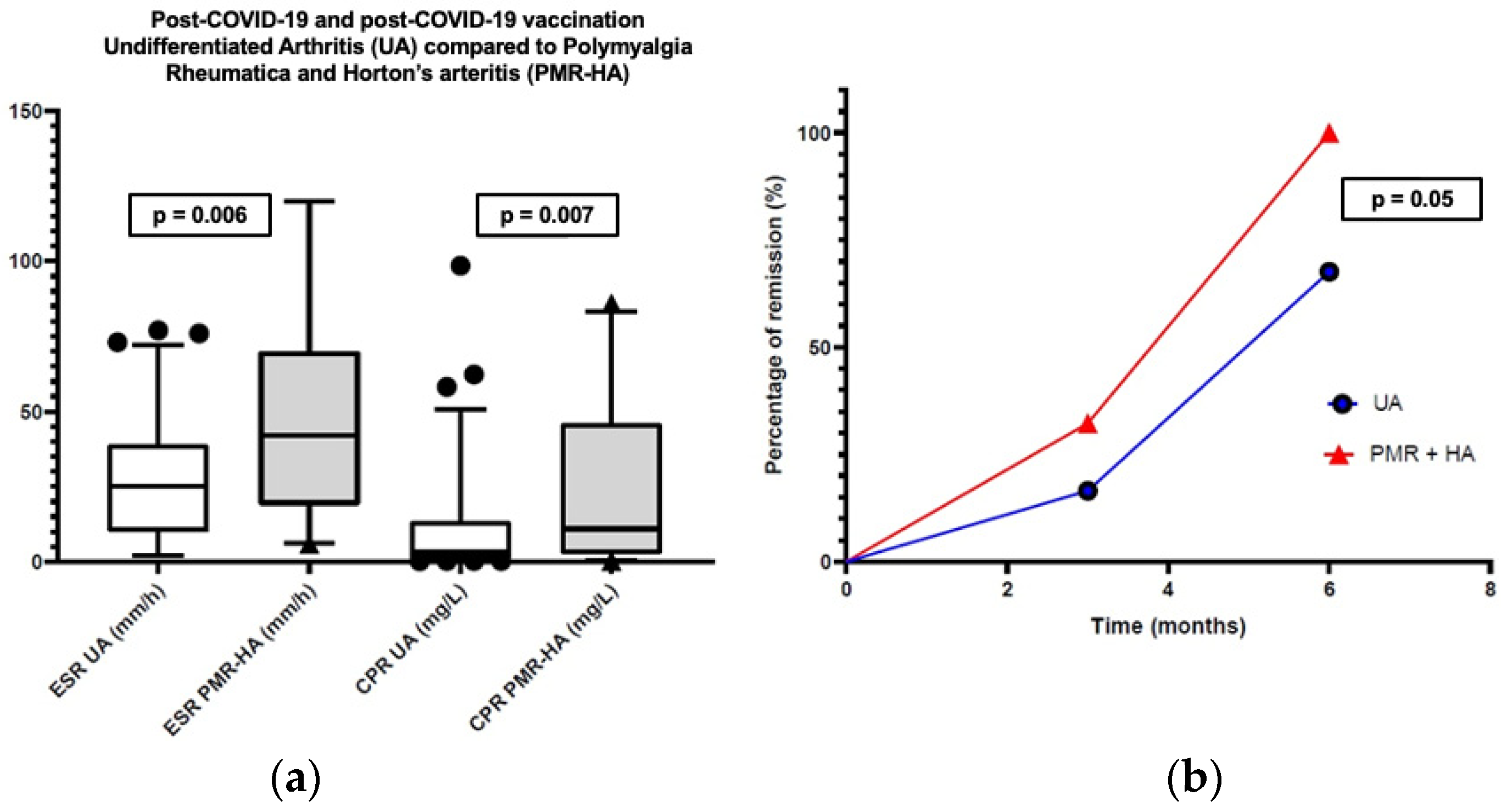
| 6-month remission (N, %) | 52 (59.8%) | 20 (90.9%) | 0.050 |
| Treatments | |||
| Hydroxychloroquin N (%) | 59 (67.8%) | 1 (4.5%) | 0.001 |
| Steroids N (%) | 77 (88.5%) | 22 (100%) | 0.207 |
| Methotrexate N (%) | 10 (11.5%) | 1 (4.55%) | 0.457 |
| Salazopyrin N (%) | 32 (36.8%) | 0 (0%) | 0.001 |
| Anti-TNF alpha N (%) | etanercept (1.1%) | 0% | 0.999 |
| Pregabalin | 36 (41.4%) | 6 (27.3%) | 0.327 |
| L-carnitine and alpha-lipoic acid | 11 (12.6%) | 1 (4.5%) | 0.453 |
| Vitamin D | 59 (67.8%) | 13 (59.1%) | 0.459 |
| Prostaglandin E1 | 1 (1.2%) | 0 (0%) | 0.999 |
| Immunoglobuline | 1 (1.12%) | 0 (0%) | 0.999 |
| Mycophenolate | 3 (3.5%) | 0 (0%) | 0.999 |
| Arthritis (N = 87) | Polymyalgia Rheumatica and Horton’s Arteritis (N = 22) | p-Value | |
|---|---|---|---|
| CRP (mg/dL) (median, IQR) cut-off < 0.5 mg/dL | 0.32 (0.12–1.35) | 1.28 (0.37–4.8) | 0.007 |
| ESR (mm/h) (median, IQR) cut-off: 0–25 | 25 (10–39) | 43 (25–70) | 0.006 |
| RF positive (N, %) | 10 (11.5%) | 0 (0%) | 0.208 |
| ACPA positive (N, %) | 5 (5.8%) | 0 (0%) | 0.581 |
| RF and ACPA positive (N, %) | 13 (14.8%) | 0 (0%) | 0.067 |
| ANA positive (≥1:160) (N, %) | 62 (71.3%) | 8 (36.4%) | 0.005 |
| Serum calprotectin positive (N, %) | 18 (20.7%) | 4 (18.2%) | 0.999 |
| IL-6 positive (N, %) | 16 (18.4%) | 7 (31.8%) | 0.240 |
| Anti-spike protein antibody BAU Who/mL (median, IQR) | 1632 (296–1632) | 256 (83–776) | 0.003 |
| Anti-SARS-CoV-2 antibody IgG, AU/mL (median, IQR) | 10.10 (0.50–41.05) | 0.55 (0.20–6.85) | 0.064 |
| Anti SARS-CoV-2 antibody IgG, positive (N, %) | 39 (44.8%) | 2 (9.1%) | 0.003 |
| NK (cells/mcl) (median, IQR) cut-off: 200–400 | 233.9 (162–382.3) | 141.5 (93.68–248.7) | 0.037 |
| “Connective Like” Arthritis (N = 30) | Isolated Arthritis (N = 57) | p-Value | |
|---|---|---|---|
| Laboratory features | |||
| CRP (mg/dL) (median, IQR) cut-off < 0.5 mg/dL | 0.27 (0.13–0.64) | 0.39 (0.12–2.57) | 0.154 |
| ESR (mm/h) (median, IQR) cut-off: 0–25 | 22.50 (10.50–35) | 28.00 (9.50–44.50) | 0.412 |
| RF positive (N, %) | 2 (6.7%) | 8 (14.0%) | 0.483 |
| ACPA positive (N, %) | 2 (6.7%) | 3 (5.3%) | 0.999 |
| RF and ACPA positive (N, %) | 4 (13.3%) | 9 (15.8%) | 0.999 |
| ANA positive (≥1:160): N, %, median, IQR | 23 (76.7%) 1:160 (1:160–1:320) Speckled: 21 (70.0%) | 39 (68.4%) 1:160 (1:80–1:240) Speckled: 28 (48.1%) | 0.471 0.133 0.073 |
| Serum calprotectin positive (N, %) | 2 (6.7%) | 16 (28.1%) | 0.021 |
| IL-6 positive (N, %) | 2 (6.7%) | 14 (24.6%) | 0.041 |
| Anti-spike protein antibody, BAU Who/mL (median, IQR) | 1316 (207–1632) | 1632 (380–1632) | 0.330 |
| Anti SARS-CoV-2 antibody IgG, AU/mL (median, IQR) | 19.57 (0.62–57.10) | 4.10 (0.35–34.10) | 0.075 |
| Anti SARS-CoV-2 antibody IgG, positive (N, %) | 21 (70.0%) | 18 (31.6%) | 0.008 |
| NK (cells/mcl) Median (IQR) cut-off: 200–400 | 229.9 (150.0–364.8) | 229.0 (161.8–380.4) | 0.593 |
| HRCT interstitial lung fibrosis (N, %) | 5 (16.7%) Post infection 3 (10%) Post-vaccine 2 (6.6%) Subpleural fibrotic nodules 3 (10%) Traction bronchiectasis 4 (13.3%) Ground glass 3 (10%) Micro-honey combing 1 (3.3%) Reticulation 1 (3.3%) | 15 (26.3%) Post infection 6 (10.5%) Post-vaccine 9 (15.8%) Subpleural nodules 6 (10.5%) Traction bronchiectasis 14 (24.5%) Ground glass 2 (3.5%) Micro-honey combing 1 (1.7%) Reticulation 2 (3.5%) | 0.186 |
| Decreased DLCO (N, %) (<60% or <75% with low FVC) | 5 (16.7%) Post infection 3 (10%) Post-vaccine 2 (6.6%) | 2 (3.5%) Post infection 1 (1.8%) Post-vaccine 1 (1.8%) | 0.041 |
| US synovitis (*) | 27 (90.0%) | 47 (70.2%) | 0.041 |
| US pseudo tenosynovitis (*) | 21 (70.0%) | 32 (55.2%) | 0.251 |
| US tenosynovitis (*) | 17 (56.7%) | 40 (70.2%) | 0.240 |
| US erosions | 1 (3.3%) | 4 (7.0%) | 0.656 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandinelli, F.; Pagano, M.; Vallecoccia, M.S. Post-COVID-19 and Post-COVID-19 Vaccine Arthritis, Polymyalgia Rheumatica and Horton’s Arteritis: A Single-Center Assessment of Clinical, Serological, Genetic, and Ultrasonographic Biomarkers. J. Clin. Med. 2023, 12, 7563. https://doi.org/10.3390/jcm12247563
Bandinelli F, Pagano M, Vallecoccia MS. Post-COVID-19 and Post-COVID-19 Vaccine Arthritis, Polymyalgia Rheumatica and Horton’s Arteritis: A Single-Center Assessment of Clinical, Serological, Genetic, and Ultrasonographic Biomarkers. Journal of Clinical Medicine. 2023; 12(24):7563. https://doi.org/10.3390/jcm12247563
Chicago/Turabian StyleBandinelli, Francesca, Mario Pagano, and Maria Sole Vallecoccia. 2023. "Post-COVID-19 and Post-COVID-19 Vaccine Arthritis, Polymyalgia Rheumatica and Horton’s Arteritis: A Single-Center Assessment of Clinical, Serological, Genetic, and Ultrasonographic Biomarkers" Journal of Clinical Medicine 12, no. 24: 7563. https://doi.org/10.3390/jcm12247563
APA StyleBandinelli, F., Pagano, M., & Vallecoccia, M. S. (2023). Post-COVID-19 and Post-COVID-19 Vaccine Arthritis, Polymyalgia Rheumatica and Horton’s Arteritis: A Single-Center Assessment of Clinical, Serological, Genetic, and Ultrasonographic Biomarkers. Journal of Clinical Medicine, 12(24), 7563. https://doi.org/10.3390/jcm12247563