The Emerging Role of Visible Light in Melanocyte Biology and Skin Pigmentary Disorders: Friend or Foe?
Abstract
1. Introduction
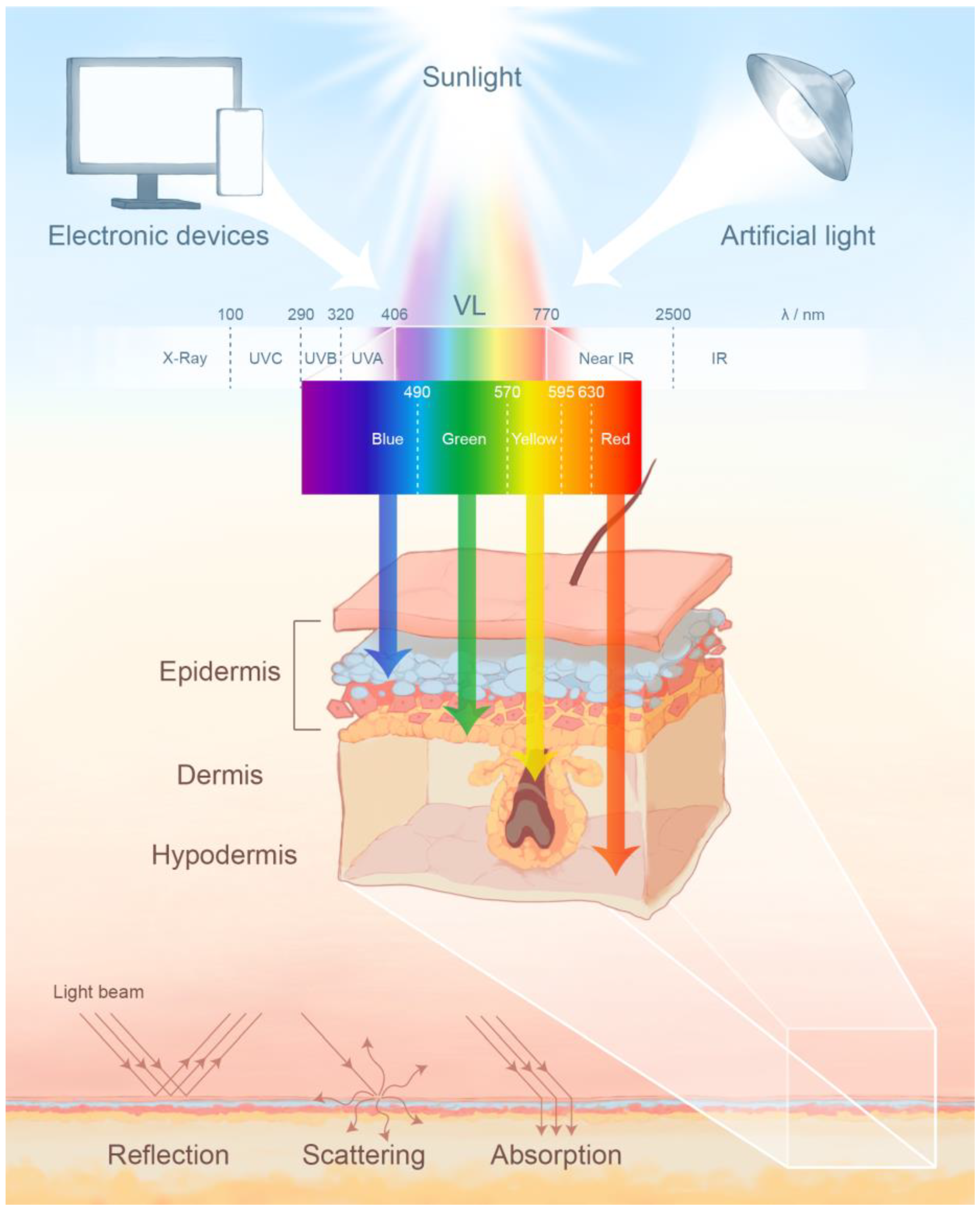
2. The Photobiological Effects of Visible Light on Skin
2.1. The Activation of Chromophores by Visible Light
2.2. The Regulation of Skin Aging by Visible Light
2.3. The Disruption of the Skin Circadian Rhythm by Visible Light
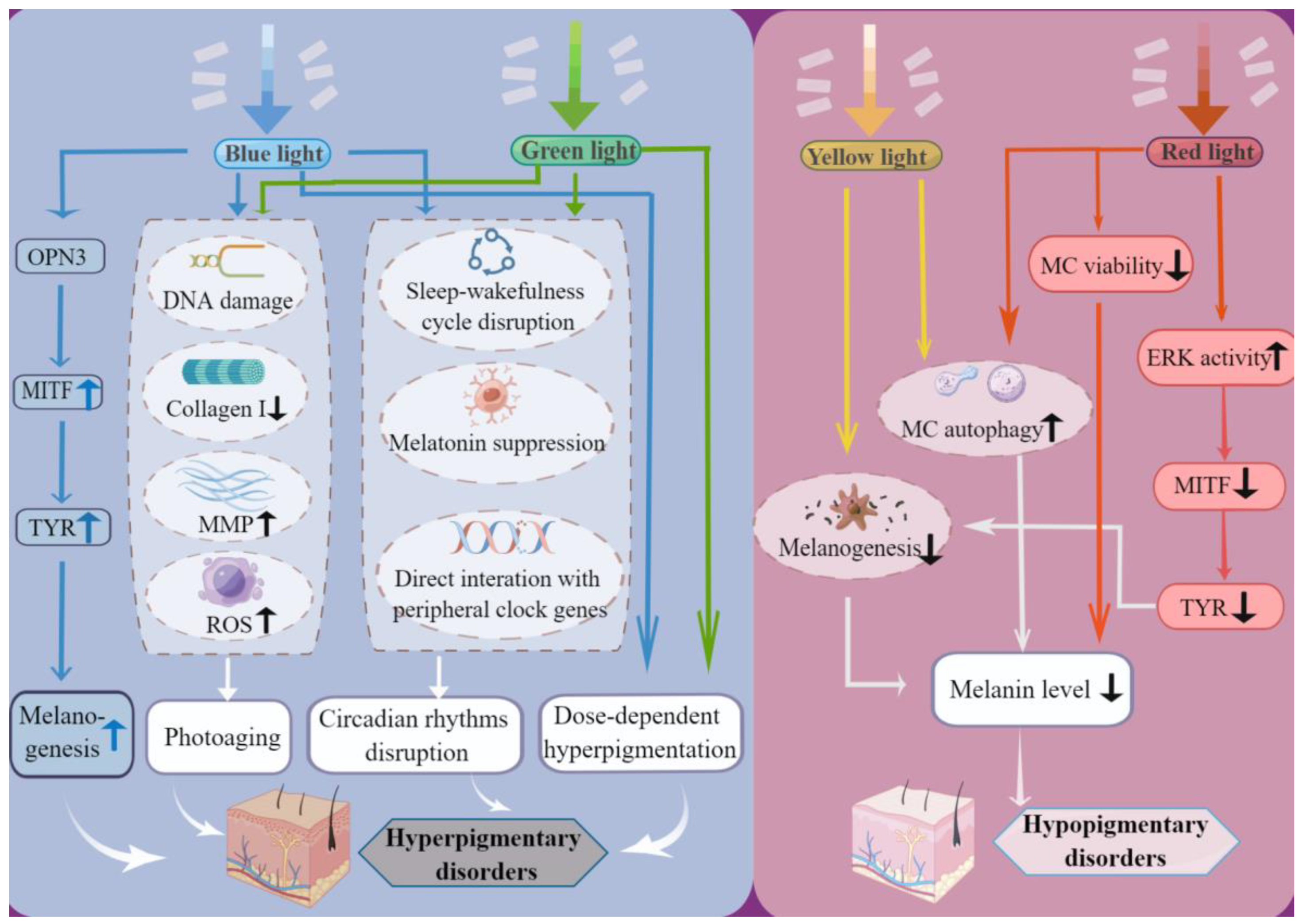
3. Visible Light as an Enhancer of Skin Pigmentary Disorder
3.1. Blue Light and Green Light Induce Hyperpigmentation
3.2. Yellow Light and Red Light Induce or Aggravate Hypopigmentation
3.3. Measures to Protect against Cutaneous Damage by Visible Light
3.3.1. Exposure Reduction
3.3.2. Sunscreens
3.3.3. Antioxidants
| Antioxidants | Mechanisms | Origins | Objects |
|---|---|---|---|
| WH130 | Inhibits melanogenesis by suppressing tyrosinase activity | Extract from Licorice (Wongam); | Murine melanoma B16F10 cells [83] |
| PBE | Reduces VL-induced melanin synthesis by reducing tyrosinase activity and decreasing ED1, and PPAR α, δ, and γ production | Extract from French maritime pine bark (Pinus pinaster) | Human melanocytes [84] |
| Resveratrol | Scavenges ROS induced by blue light (415 nm) LED in human fibroblast | Root extract from Veratrum grandiflorum | Human Skin Fibroblasts [16] |
| Hydroxytyrosol | Protects keratinocytes and fibroblasts from damage induced by blue light through preventing ROS formation, reducing MMP levels, preserving collagen type I production, and decreasing DNA damage | Extract from olive fruits | Human keratinocytes and fibroblasts [18] |
| Licochalcone A | Decreases VL-induced ROS formation in human fibroblast to a level equivalent to unirradiated fibroblast cells, or even below, in vitro, and prevents intradermal carotenoid depletion by VL irradiation in vivo | Root extract from Licorice (Glycyrrhiza inflata); | Human dermal fibroblasts and 10 healthy subjects with Fitzpatrick skin phototype II or III [19] |
| PLE | 1. Decreases PPD and DT; 2. Decreases cyclooxygenase-2 and cell damage; 3. Prevents alterations in morphology, cell survival and cell cycle of human dermal fibroblasts and changes in the expression of MMP-1, CTSK, fibrillins 1 and 2 and elastin, caused by VL | Extract from Polypodium leucotomos; | 22 subjects with Fitzpatrick skin phototype IV–VI [88] Human dermal fibroblasts [90] 7 healthy subjects [89] |
| Carotenoid | Filters out high-energy blue-light rays | Diets | 46 healthy subjects [92] |
| Flavonoid | Decreases photosensitivity of phospholipids to blue-light oxidative damage | Extract from green tea | Langmuir monolayers of 1,2-dipalmitoyl-sn-glycero-3-[phospho-rac-(1-glycerol) (sodium salt) (DPPG) [93] |
| Vitachelox | Protects human keratinocytes by reducing oxidative damage (protein carbonylation) induced by blue-light radiation. | A mixture of three natural extracts: grape (Vitis vinifera) seeds, green tea (Camellia sinensis green) leaves, and oak (Quercus robur) | Human keratinocytes [94] |
4. Visible Light as a Therapeutic Option for Pigmentary Disorders
4.1. Laser-Emitting Lights in the Visible Range
4.2. Intense Pulsed Light (IPL)
4.3. Light-Emitting Diodes (LEDs)
5. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Diffey, B.L. What is light? Photodermatol. Photoimmunol. Photomed. 2002, 18, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Austin, E.; Geisler, A.N.; Nguyen, J.; Kohli, I.; Hamzavi, I.; Lim, H.W.; Jagdeo, J. Visible light. Part I: Properties and cutaneous effects of visible light. J. Am. Acad. Dermatol. 2021, 84, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.; Wang, R.F.; Ozog, D.; Lim, H.W.; Mohammad, T.F. Disorders of hyperpigmentation. Part II. Review of management and treatment options for hyperpigmentation. J. Am. Acad. Dermatol. 2023, 88, 291–320. [Google Scholar] [CrossRef]
- Zanolli, M. The modern paradigm of phototherapy. Clin. Dermatol. 2003, 21, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, B.H.; Hexsel, C.L.; Hamzavi, I.H.; Lim, H.W. Effects of Visible Light on the Skin. Photochem. Photobiol. 2008, 84, 450–462. [Google Scholar] [CrossRef]
- Mahmoud, B.H.; Ruvolo, E.; Hexsel, C.L.; Liu, Y.; Owen, M.R.; Kollias, N.; Lim, H.W.; Hamzavi, I.H. Impact of long-wavelength UVA and visible light on melanocompetent skin. J. Investig. Dermatol. 2010, 130, 2092–2097. [Google Scholar] [CrossRef]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef]
- Setty, S.R. Opsin3-A Link to Visible Light-Induced Skin Pigmentation. J. Investig. Dermatol. 2018, 138, 13–15. [Google Scholar] [CrossRef]
- Regazzetti, C.; Sormani, L.; Debayle, D.; Bernerd, F.; Tulic, M.K.; De Donatis, G.M.; Chignon-Sicard, B.; Rocchi, S.; Passeron, T. Melanocytes Sense Blue Light and Regulate Pigmentation through Opsin-3. Investig. Dermatol. 2018, 138, 171–178. [Google Scholar] [CrossRef]
- Ozdeslik, R.N.; Olinski, L.E.; Trieu, M.M.; Oprian, D.D.; Oancea, E. Human nonvisual opsin 3 regulates pigmentation of epidermal melanocytes through functional interaction with melanocortin 1 receptor. Proc. Natl. Acad. Sci. USA 2019, 116, 11508–11517. [Google Scholar] [CrossRef]
- Campiche, R.; Curpen, S.J.; Lutchmanen-Kolanthan, V.; Gougeon, S.; Cherel, M.; Laurent, G.; Gempeler, M.; Schuetz, R. Pigmentation effects of blue light irradiation on skin and how to protect against them. Int. J. Cosmet. Sci. 2020, 42, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R.; Parrish, J.A. The Optics of Human Skin. J. Investig. Dermatol. 1981, 77, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Pourang, A.; Tisack, A.; Ezekwe, N.; Torres, A.E.; Kohli, I.; Hamzavi, I.H.; Lim, H.W. Effects of visible light on mechanisms of skin photoaging. Photodermatol. Photoimmunol. Photomed. 2022, 38, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Mamalis, A.; Koo, E.; Jagdeo, J. Resveratrol Prevents Reactive Oxygen Species–Induced Effects of Light-Emitting Diode–Generated Blue Light in Human Skin Fibroblasts. Dermatol. Surg. 2016, 42, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Ohta, S.; Wolf, A.M. Blue light-induced oxidative stress in live skin. Free. Radic. Biol. Med. 2017, 108, 300–310. [Google Scholar] [CrossRef]
- Avola, R.; Graziano, A.C.E.; Pannuzzo, G.; Bonina, F.; Cardile, V. Hydroxytyrosol from olive fruits prevents blue-light-induced damage in human keratinocytes and fibroblasts. J. Cell. Physiol. 2019, 234, 9065–9076. [Google Scholar] [CrossRef]
- Mann, T.; Eggers, K.; Rippke, F.; Tesch, M.; Buerger, A.; Darvin, M.E.; Schanzer, S.; Meinke, M.C.; Lademann, J.; Kolbe, L. High-energy visible light at ambient doses and intensities induces oxidative stress of skin-Protective effects of the antioxidant and Nrf2 inducer Licochalcone A in vitro and in vivo. Photodermatol. Photoimmunol. Photomed. 2020, 36, 135–144. [Google Scholar] [CrossRef]
- Chiarelli-Neto, O.; Ferreira, A.S.; Martins, W.K.; Pavani, C.; Severino, D.; Faião-Flores, F.; Maria-Engler, S.S.; Aliprandini, E.; Martinez, G.R.; Di Mascio, P.; et al. Melanin photosensitization and the effect of visible light on epithelial cells. PLoS ONE 2014, 9, e113266. [Google Scholar] [CrossRef]
- Lorrio, S.; Rodríguez-Luna, A.; Delgado-Wicke, P.; Mascaraque, M.; Gallego, M.; Pérez-Davó, A.; González, S.; Juarranz, Á. Protective Effect of the Aqueous Extract of Deschampsia antarctica (EDAFENCE®) on Skin Cells against Blue Light Emitted from Digital Devices. Int. J. Mol. Sci. 2020, 21, 988. [Google Scholar] [CrossRef] [PubMed]
- Lan CC, E.; Ho, P.Y.; Wu, C.S.; Yang, R.C.; Yu, H.S. LED 590 nm photomodulation reduces UVA-induced metalloproteinase-1 expression via upregulation of antioxidant enzyme catalase. J. Dermatol. Sci. 2015, 78, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.A.; McDaniel, D.H.; Geronemus, R.G.; Weiss, M.A. Clinical trial of a novel non-thermal LED array for reversal of photoaging: Clinical, histologic, and surface profilometric results. Lasers Surg. Med. 2005, 36, 85–91. [Google Scholar] [CrossRef] [PubMed]
- You, H.R.; Kim, S.H.; Yun, S.J.; Lee, S.C.; Lee, J.B. Skin photorejuvenation effects of light-emitting diodes (LEDs): A comparative study of yellow and red LEDs in vitro and in vivo. Clin. Exp. Dermatol. 2016, 41, 798–805. [Google Scholar]
- Barolet, D.; Roberge, C.J.; Auger, F.A.; Boucher, A.; Germain, L. Regulation of skin collagen metabolism in vitro using a pulsed 660 nm LED light source: Clinical correlation with a single-blinded study. J. Investig. Dermatol. 2009, 129, 2751–2759. [Google Scholar] [CrossRef]
- Song, S.; Zhang, Y.; Fong, C.-C.; Tsang, C.-H.; Yang, Z.; Yang, M. cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. J. Investig. Dermatol. 2003, 120, 849–857. [Google Scholar] [CrossRef]
- Wang, Y.; Viennet, C.; Robin, S.; Berthon, J.-Y.; He, L.; Humbert, P. Precise role of dermal fibroblasts on melanocyte pigmentation. J. Dermatol. Sci. 2017, 88, 159–166. [Google Scholar] [CrossRef]
- Passeron, T.; Picardo, M. Melasma, a photoaging disorder. Pigment. Cell Melanoma Res. 2018, 31, 461–465. [Google Scholar] [CrossRef]
- Lyons, A.B.; Moy, L.; Moy, R.; Tung, R. Circadian Rhythm and the Skin: A Review of the Literature. J. Clin. Aesthetic Dermatol. 2019, 12, 42–45. [Google Scholar]
- Gooley, J.J.; Rajaratnam, S.M.W.; Brainard, G.C.; Kronauer, R.E.; Czeisler, C.A.; Lockley, S.W. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci. Transl. Med. 2010, 2, 31ra33. [Google Scholar] [CrossRef]
- Cajochen, C.; Frey, S.; Anders, D.; Späti, J.; Bues, M.; Pross, A.; Mager, R.; Wirz-Justice, A.; Stefani, O. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J. Appl. Physiol. 2011, 110, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Cohen-Zion, M.; Haim, A.; Dagan, Y. Evening light exposure to computer screens disrupts human sleep, biological rhythms, and attention abilities. Chronobiol. Int. 2017, 34, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Sletten, T.L.; Revell, V.L.; Middleton, B.; Lederle, K.A.; Skene, D.J. Age-related changes in acute and phase-advancing responses to monochromatic light. J. Biol. Rhythm. 2009, 24, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Ho Mien, I.; Chua EC, P.; Lau, P.; Tan, L.C.; Lee IT, G.; Yeo, S.C.; Tan, S.S.; Gooley, J.J. Effects of exposure to intermittent versus continuous red light on human circadian rhythms, melatonin suppression, and pupillary constriction. PLoS ONE 2014, 9, e96532. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Goyarts, E.C.; Pelle, E.; Trivero, J.; Pernodet, N. Blue light disrupts the circadian rhythm and create damage in skin cells. Int. J. Cosmet. Sci. 2019, 41, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Hardman, J.A.; Tobin, D.J.; Haslam, I.S.; Farjo, N.; Farjo, B.; Al-Nuaimi, Y.; Grimaldi, B.; Paus, R. The peripheral clock regulates human pigmentation. J. Investig. Dermatol. 2015, 135, 1053–1064. [Google Scholar] [CrossRef]
- Poletini, M.O.; de Assis, L.V.M.; Moraes, M.N.; Castrucci, A.M.d.L. Estradiol differently affects melanin synthesis of malignant and normal melanocytes: A relationship with clock and clock-controlled genes. Mol. Cell. Biochem. 2016, 421, 29–39. [Google Scholar] [CrossRef]
- Sarkar, S.; Porter, K.I.; Dakup, P.P.; Gajula, R.P.; Koritala, B.S.C.; Hylton, R.; Kemp, M.G.; Wakamatsu, K.; Gaddameedhi, S. Circadian clock protein BMAL1 regulates melanogenesis through MITF in melanoma cells. Pigment. Cell Melanoma Res. 2021, 34, 955–965. [Google Scholar] [CrossRef]
- Duan, J.; Greenberg, E.N.; Karri, S.S.; Andersen, B. The circadian clock and diseases of the skin. FEBS Lett. 2021, 595, 2413–2436. [Google Scholar] [CrossRef]
- Suitthimeathegorn, O.; Yang, C.; Ma, Y.; Liu, W. Direct and Indirect Effects of Blue Light Exposure on Skin: A Review of Published Literature. Ski. Pharmacol. Physiol. 2022, 35, 305–318. [Google Scholar] [CrossRef]
- Kohli, I.; Chaowattanapanit, S.; Mohammad, T.; Nicholson, C.; Fatima, S.; Jacobsen, G.; Kollias, N.; Lim, H.; Hamzavi, I. Synergistic effects of long-wavelength ultraviolet A1 and visible light on pigmentation and erythema. Br. J. Dermatol. 2018, 178, 1173–1180. [Google Scholar] [CrossRef]
- Kohli, I.; Braunberger, T.L.; Nahhas, A.F.; Mirza, F.N.; Mokhtari, M.; Lyons, A.B.; Kollias, N.; Ruvolo, E.; Lim, H.W.; Hamzavi, I.H. Long-wavelength Ultraviolet A1 and Visible Light Photoprotection: A Multimodality Assessment of Dose and Response. Photochem. Photobiol. 2020, 96, 208–214. [Google Scholar] [CrossRef]
- Duteil, L.; Cardot-Leccia, N.; Queille-Roussel, C.; Maubert, Y.; Harmelin, Y.; Boukari, F.; Ambrosetti, D.; Lacour, J.; Passeron, T. Differences in visible light-induced pigmentation according to wavelengths: A clinical and histological study in comparison with UVB exposure. Pigment. Cell Melanoma Res. 2014, 27, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramaniam, R.; Roy, A.; Sharma, B.; Nagalakshmi, S. Are there mechanistic differences between ultraviolet and visible radiation induced skin pigmentation? Photochem. Photobiol. Sci. 2011, 1, 1887–1893. [Google Scholar] [CrossRef]
- Sklar, L.R.; Almutawa, F.; Lim, H.W.; Hamzavi, I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: A review. Photochem. Photobiol. Sci. 2013, 12, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Schutz, R. Blue Light and the Skin. Curr. Probl. Dermatol. 2021, 55, 354–373. [Google Scholar] [PubMed]
- Randhawa, M.; Seo, I.; Liebel, F.; Southall, M.D.; Kollias, N.; Ruvolo, E. Visible Light Induces Melanogenesis in Human Skin through a Photoadaptive Response. PLoS ONE 2015, 10, e0130949. [Google Scholar] [CrossRef]
- Schütz, R.; Vollhardt, J.; Rudolph, T. clinical effects of LED blue light on skin pigmentation and carotenoid depletion. J. Investig. Dermatol. 2019, 5, 130. [Google Scholar] [CrossRef][Green Version]
- Kleinpenning, M.M.; Otero, M.E.; Van Erp PE, J.; Gerritsen MJ, P.; Van De Kerkhof PC, M. Efficacy of blue light vs. red light in the treatment of psoriasis: A double-blind, randomized comparative study. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 219–225. [Google Scholar] [CrossRef]
- Jo, H.L.; Jung, Y.; Suh, B.; Cho, E.; Kim, K.; Kim, E. Clinical evaluation method for blue light (456 nm) protection of skin. J. Cosmet. Dermatol. 2020, 19, 2438–2443. [Google Scholar] [CrossRef]
- Kleinpenning, M.M.; Smits, T.; Frunt, M.H.A.; van Erp, P.E.J.; van de Kerkhof, P.C.M.; Gerritsen, R.M.J.P. Clinical and histological effects of blue light on normal skin. Photodermatol. Photoimmunol. Photomed. 2010, 26, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Falcone, D.; Uzunbajakava, N.E.; van Abeelen, F.; Oversluizen, G.; Peppelman, M.; van Erp, P.E.J.; van de Kerkhof, P.C.M. Effects of blue light on inflammation and skin barrier recovery following acute perturbation. Pilot study results in healthy human subjects. Photodermatol. Photoimmunol. Photomed. 2018, 34, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Moreiras, H.; O’Connor, C.; Bell, M.; Tobin, D.J. Visible light and human skin pigmentation: The importance of skin phototype. Exp. Dermatol. 2021, 30, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Lyons, A.B.; Trullas, C.; Kohli, I.; Hamzavi, I.H.; Lim, H.W. Photoprotection beyond ultraviolet radiation: A review of tinted sunscreens. J. Am. Acad. Dermatol. 2021, 84, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Sheth, V.M.; Pandya, A.G. Melasma: A comprehensive update: Part I. J. Am. Acad. Dermatol. 2011, 65, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Rivas, S.; Pandya, A.G. Treatment of melasma with topical agents, peels and lasers: An evidence-based review. Am. J. Clin. Dermatol. 2013, 14, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, G.P.; Esposito, A.C.C.; Olivatti, T.O.F.; Yoshida, M.M.; Miot, H.A. Evaluation of ex vivo melanogenic response to UVB, UVA, and visible light in facial melasma and unaffected adjacent skin. An. Bras. Dermatol. 2020, 95, 684–690. [Google Scholar] [CrossRef]
- Duteil, L.; Queille-Roussel, C.; Lacour, J.-P.; Montaudié, H.; Passeron, T. Short-term exposure to blue light emitted by electronic devices does not worsen melasma. J. Am. Acad. Dermatol. 2020, 83, 913–914. [Google Scholar] [CrossRef]
- Verallo-Rowell, V.M.; Pua, J.M.; Bautista, D. Visible light photopatch testing of common photocontactants in female filipino adults with and without melasma: A cross-sectional study. J. Drugs Dermatol. 2008, 7, 149–156. [Google Scholar]
- Boukari, F.; Jourdan, E.; Fontas, E.; Montaudié, H.; Castela, E.; Lacour, J.-P.; Passeron, T. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: A prospective randomized comparative trial. J. Am. Acad. Dermatol. 2015, 72, 189–190.e1. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Z.; Jiang, M.; Zhang, C.; Wang, X.; Xiang, L. Light-emitting diode 585nm photomodulation inhibiting melanin synthesis and inducing autophagy in human melanocytes. J. Dermatol. Sci. 2018, 89, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; You, C.E.; Park, M.Y. Blue and red light combination LED phototherapy for acne vulgaris in patients with skin phototype IV. Lasers Surg. Med. 2007, 39, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.T.; Kwon, T.R.; Choi, E.J.; Kim, S.R.; Seok, J.; Mun, S.K.; Yoo, K.H.; Choi, Y.S.; Choi, S.Y.; Kim, B.J. Inhibitory effect of 660-nm LED on melanin synthesis in in vitro and in vivo. Photodermatol. Photoimmunol. Photomed. 2017, 33, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.U.; Roh, M.R.; Lee, J.H. Vitiligo following intense pulsed light treatment. J. Dermatol. 2010, 37, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.; Sheehan-Dare, R.A. The Koebner phenomenon in vitiligo following treatment of a port-wine stain naevus by pulsed dye laser. Br. J. Dermatol. 1998, 138, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, F.; Bonifazi, E.; Greco, I. Koebner phenomenon in vitiligo after treatment with dye laser. Eur. J. Pediatr. Dermatol. 2006, 3, 169. [Google Scholar]
- van Geel, N.; Speeckaert, R.; Mollet, I.; De Schepper, S.; De Wolf, J.; Tjin, E.P.; Luiten, B.M.; Lambert, J.; Brochez, L. In vivo vitiligo induction and therapy model: Double-blind, randomized clinical trial. Pigment. Cell Melanoma Res. 2012, 25, 57–65. [Google Scholar] [CrossRef]
- Alkhalifah, A. A Case Report of Vitiligo Induced by Alexandrite Hair Removal Laser. Case Rep. Dermatol. 2021, 13, 521–524. [Google Scholar] [CrossRef]
- Kumari, J.; Das, K.; Babaei, M.; Rokni, G.R.; Goldust, M. The impact of blue light and digital screens on the skin. J. Cosmet. Dermatol. 2023, 22, 1185–1190. [Google Scholar] [CrossRef]
- Geisler, A.N.; Austin, E.; Nguyen, J.; Hamzavi, I.; Jagdeo, J.; Lim, H.W. Visible light. Part II: Photoprotection against visible and ultraviolet light. J. Am. Acad. Dermatol. 2021, 84, 1233–1244. [Google Scholar] [CrossRef]
- Linos, E.; Keiser, E.; Fu, T.; Colditz, G.; Chen, S.; Tang, J.Y. Hat, shade, long sleeves, or sunscreen? Rethinking US sun protection messages based on their relative effectiveness. Cancer Causes Control 2011, 22, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Almutawa, F.; Vandal, R.; Wang, S.Q.; Lim, H.W. Current status of photoprotection by window glass, automobile glass, window films, and sunglasses. Photodermatol. Photoimmunol. Photomed. 2013, 29, 65–72. [Google Scholar] [CrossRef]
- Tuchinda, C.; Srivannaboon, S.; Lim, H.W. Photoprotection by window glass, automobile glass, and sunglasses. J. Am. Acad. Dermatol. 2006, 54, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, E.; Fair, M.; Hutson, A.; Liebel, F. Photoprotection against visible light-induced pigmentation. Int. J. Cosmet. Sci. 2018, 40, 589–595. [Google Scholar] [CrossRef]
- Martini, A.P.M.; Campos, P.M.B.G.M. Influence of visible light on cutaneous hyperchromias: Clinical efficacy of broad-spectrum sunscreens. Photodermatol. Photoimmunol. Photomed. 2018, 34, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.F.; Sarkas, H.W.; Ba, P.B.; Bouche, D. Beyond sun protection factor: An approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J. Cosmet. Dermatol. 2020, 19, 407–415. [Google Scholar] [CrossRef]
- Castanedo-Cazares, J.P.; Hernandez-Blanco, D.; Carlos-Ortega, B.; Fuentes-Ahumada, C.; Torres-Álvarez, B. Near-visible light and UV photoprotection in the treatment of melasma: A double-blind randomized trial. Photodermatol. Photoimmunol. Photomed. 2014, 30, 35–42. [Google Scholar] [CrossRef]
- Dumbuya, H.; Grimes, P.; Lynch, S.; Ji, K.; Brahmachary, M.; Zheng, Q.; Bouez, C.; Wangari-Talbot, J. Impact of Iron-Oxide Containing Formulations Against Visible Light-Induced Skin Pigmentation in Skin of Color Individuals. J. Drugs Dermatol. 2020, 19, 712–717. [Google Scholar] [CrossRef]
- Moyal, D.; Seite, S. Prevention of melasma intensification with sunscreen. J. Am. Acad. Dermatol. 2020, 6, AB20. [Google Scholar] [CrossRef]
- Lim, H.W.; Kohli, I.; Ruvolo, E.; Kolbe, L.; Hamzavi, I.H. Impact of visible light on skin health: The role of antioxidants and free radical quenchers in skin protection. J. Am. Acad. Dermatol. 2022, 86, S27–S37. [Google Scholar] [CrossRef]
- Zastrow, L.; Lademann, J. Light—Instead of UV Protection: New Requirements for Skin Cancer Prevention. Anticancer Res. 2016, 36, 1389–1393. [Google Scholar] [PubMed]
- Callender, V.D.; St Surin-Lord, S.; Davis, E.C.; Maclin, M. Postinflammatory hyperpigmentation: Etiologic and therapeutic considerations. Am. J. Clin. Dermatol. 2011, 12, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.H.; Jang, G.Y.; Ji, Y.-J.; Lee, J.H.; Choi, S.J.; Hyun, T.K.; Kim, H.D. Antioxidant and Anti-Melanogenic Activities of Heat-Treated Licorice (Wongam, Glycyrrhiza glabra × G. uralensis) Extract. Curr. Issues Mol. Biol. 2021, 43, 1171–1187. [Google Scholar] [CrossRef] [PubMed]
- Leis Ayres, E.; dos Santos Silva, J.; Eberlin, S.; Facchini, G.; Vasconcellos, C.; Da Costa, A. Invitro effect of pine bark extract on melanin synthesis, tyrosinase activity, production of endothelin-1, and PPAR in cultured melanocytes exposed to Ultraviolet, Infrared, and Visible light radiation. J. Cosmet. Dermatol. 2022, 21, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, E.; Boothby-Shoemaker, W.; Kumar, N.; Hamzavi, I.H.; Lim, H.W.; Kohli, I. Evaluation of efficacy of antioxidant-enriched sunscreen prodcuts against long wavelength ultraviolet A1 and visible light. Int. J. Cosmet. Sci. 2022, 44, 394–402. [Google Scholar] [CrossRef]
- Lyons, A.B.; Zubair, R.; Kohli, I.; Nahhas, A.F.; Braunberger, T.L.; Mokhtari, M.; Ruvolo, E.; Lim, H.W.; Hamzavi, I.H. Mitigating Visible Light and Long Wavelength UVA1-induced Effects with Topical Antioxidants. Photochem. Photobiol. 2022, 98, 455–460. [Google Scholar] [CrossRef]
- Kern, J.; Wood, E.; Almukhtar, R.; Angra, K.; Lipp, M.; Goldman, M. Evaluation of an SPF50 Sunscreen Containing Photolyase and Antioxidants for its Anti-Photoaging Properties and Photoprotection. J. Drugs Dermatol. 2022, 21, 517–520. [Google Scholar] [CrossRef]
- Mohammad, T.F.; Kohli, I.; Nicholson, C.L.; Treyger, G.; Chaowattanapanit, S.; Nahhas, A.F.; Braunberger, T.L.; Lim, H.W.; Hamzavi, I.H. Oral Polypodium Leucotomos Extract and Its Impact on Visible Light-Induced Pigmentation in Human Subjects. J. Drugs Dermatol. 2019, 18, 1198–1203. [Google Scholar]
- Truchuelo, M.T.; Jiménez, N.; Días, I.J.; Gallego-Rentero, M.; Alonso-Juarranz, M.; Gonzalez, S. A Pilot Study to Assess the Effects of an Oral Photo Protector of Botanical Origin against Visible and Infrared Radiations in Human Volunteers. Dermatol. Dermatol. Dis. 2019, 6, 2. [Google Scholar]
- Zamarrón, A.; Lorrio, S.; González, S.; Juarranz, Á. Fernblock Prevents Dermal Cell Damage Induced by Visible and Infrared A Radiation. Int. J. Mol. Sci. 2018, 19, 2250. [Google Scholar] [CrossRef]
- Nahhas, A.F.; Abdel-Malek, Z.A.; Kohli, I.; Braunberger, T.L.; Lim, H.W.; Hamzavi, I.H. The potential role of antioxidants in mitigating skin hyperpigmentation resulting from ultraviolet and visible light-induced oxidative stress. Photodermatol. Photoimmunol. Photomed. 2019, 35, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V.; Bowman, J.P.; Deshpande, J. Overall skin tone and skin-lightening-improving effects with oral supplementation of lutein and zeaxanthin isomers: A double-blind, placebo-controlled clinical trial. Clin. Cosmet. Investig. Dermatol. 2016, 9, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Pires, F.; Geraldo, V.P.; Antunes, A.; Marletta, A.; Oliveira, O.N., Jr.; Raposo, M. Effect of blue light irradiation on the stability of phospholipid molecules in the presence of epigallocatechin-3-gallate. Colloids Surf. B Biointerfaces 2019, 177, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Togni, S.; Maramaldi, G.; Cavagnino, A.; Corti, A.; Giacomelli, L. Vitachelox Protection of the Skin Against Blue Light-Induced Protein Carbonylation. Cosmetics 2019, 3, 49. [Google Scholar] [CrossRef]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar] [PubMed]
- Ross, E.V. Laser versus intense pulsed light: Competing technologies in dermatology. Lasers Surg. Med. 2006, 38, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R.; Parrish, J.A. Selective photothermolysis: Precise microsurgery by selective absorption of pulsed radiation. Science 1983, 4569, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Galeckas, K.J.; Collins, M.; Ross, E.V.; Uebelhoer, N.S. Split-face treatment of facial dyschromia: Pulsed dye laser with a compression handpiece versus intense pulsed light. Dermatol. Surg. 2008, 34, 672–680. [Google Scholar] [CrossRef]
- Chern, P.L.; Domankevitz, Y.; Ross, E.V. Pulsed dye laser treatment of pigmented lesions: A randomized clinical pilot study comparison of 607- and 595-nm wavelength lasers. Lasers Surg. Med. 2010, 42, 865–869. [Google Scholar] [CrossRef]
- Fateh, S.; Farnaghi, F.; Ehsani, A.H.; Noormohammadpour, P.; Seirafi, H. Efficacy and safety of long-pulse pulsed dye laser delivered with compression versus cryotherapy for treatment of solar lentigines. Indian J. Dermatol. 2011, 56, 48–51. [Google Scholar] [CrossRef]
- Ghaninejhadi, H.; Ehsani, A.; Edrisi, L.; Gholamali, F.; Akbari, Z.; Noormohammadpour, P. Solar Lentigines: Evaluating Pulsed Dye Laser (PDL) as an Effective Treatment Option. J. Lasers Med. Sci. 2013, 4, 33–38. [Google Scholar] [PubMed]
- Allemanna, I.B.; Goldberg, D.J. (Eds.) Benign Pigmented Lesions. In Basics in Dermatological Laser Applications; Part of: Current Problems in Dermatology; S. Karger Publishing: Basel, Switzerland, 2011; pp. 81–96. [Google Scholar]
- Abd El-Naby, N.; Mostafa Ali, M.; Hawwam, S.A.; Sarhan, N. The clinical and electron microscopic evaluation of the impact of pulsed dye laser techniques on solar lentigines (randomized clinical trial). J. Dermatolog. Treat. 2022, 33, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Labadie, J.G.; Krunic, A.L. Long pulsed dye laser with a back-to-back double-pulse technique and compression for the treatment of epidermal pigmented lesions. Lasers Surg. Med. 2019, 51, 136–140. [Google Scholar] [CrossRef]
- Geddes, E.R.; Stout, A.B.; Friedman, P.M. Retrospective analysis of the treatment of melasma lesions exhibiting increased vascularity with the 595-nm pulsed dye laser combined with the 1927-nm fractional low-powered diode laser. Lasers Surg. Med. 2017, 49, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.; Elfar, N.N.; Rizk, O.M.; Eissa, N.Y. Pulsed dye laser versus intense pulsed light in melasma: A split-face comparative study. J. Dermatolog. Treat. 2018, 29, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.H.; Suh, H.S.; Choi, Y.S. Treatment of Melasma with Pulsed-Dye Laser and 1,064-nm Q-Switched Nd:YAG Laser: A Split-Face Study. Ann. Dermatol. 2018, 30, 1. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, H.H.; Boukari, F.; Fontas, E.; Montaudié, H.; Bahadoran, P.; Lacour, J.P.; Passeron, T. Copper Bromide Laser vs Triple-Combination Cream for the Treatment of Melasma: A Randomized Clinical Trial. JAMA Dermatol. 2015, 151, 791–792. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, I.V.; Topchiy, S.B.; Pushkareva, A.E.; Klyuchareva, S.V.; Andrusenko, Y.N. Treatment of Congenital Melanocytic Nevi With a Dual-Wavelengths Copper Vapor Laser: A Case Series. J. Lasers Med. Sci. 2021, 12, e5. [Google Scholar] [CrossRef]
- Eimpunth, S.; Wanitphakdeedecha, R.; Triwongwaranat, D.; Varothai, S.; Manuskiatti, W. Therapeutic outcome of melasma treatment by dual-wavelength (511 and 578 nm) laser in patients with skin phototypes III-V. Clin. Exp. Dermatol. 2014, 39, 292–297. [Google Scholar] [CrossRef]
- Lee, H.I.; Lim, Y.Y.; Kim, B.J.; Kim, M.N.; Min, H.J.; Hwang, J.H.; Song, K.Y. Clinicopathologic efficacy of copper bromide plus/yellow laser (578 nm with 511 nm) for treatment of melasma in Asian patients. Dermatol. Surg. 2010, 36, 885–893. [Google Scholar] [CrossRef]
- Guss, L.; Goldman, M.P.; Wu, D.C. Picosecond 532 nm Neodymium-Doped Yttrium Aluminium Garnet Laser for the Treatment of Solar Lentigines in Darker Skin Types: Safety and Efficacy. Dermatol. Surg. 2017, 43, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Negishi, K.; Akita, H.; Matsunaga, Y. Prospective study of removing solar lentigines in Asians using a novel dual-wavelength and dual-pulse width picosecond laser. Lasers Surg. Med. 2018, 50, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Vachiramon, V.; Iamsumang, W.; Triyangkulsri, K. Q-switched double frequency Nd:YAG 532-nm nanosecond laser vs. double frequency Nd:YAG 532-nm picosecond laser for the treatment of solar lentigines in Asians. Lasers Med. Sci. 2018, 33, 1941–1947. [Google Scholar] [CrossRef] [PubMed]
- Hom, D.B.; Ingraffea, A. Efficacy of Q-switched Nd:YAG Frequency Doubling 532nm Laser Combined with Photorejuvenation on Freckles. Med. Aesthet. Beauty 2020, 21, 41–45. [Google Scholar]
- Kerkar, S.; Shilpa, K.; Revathi, T.N. Efficacy of 532-nm Q-switched Nd:YAG Laser in the Treatment of Lip Melanosis. J. Cutan. Aesthet. Surg. 2021, 14, 203–207. [Google Scholar]
- Altalhab, S.; Aljamal, M.; Mubki, T.; AlNomair, N.; Algoblan, S.; Alalola, A.; Alissa, I.; Alissa, A. Q-switched 532 nm Nd:YAG laser therapy for physiological lip hyperpigmentation: Novel classification, efficacy, and safety. J. Dermatol. Treat. 2022, 33, 1324–1328. [Google Scholar] [CrossRef]
- Alabdulrazzaq, H.; Brauer, J.A.; Bae, Y.-S.; Geronemus, R.G. Clearance of yellow tattoo ink with a novel 532-nm picosecond laser. Lasers Surg. Med. 2015, 47, 285–288. [Google Scholar] [CrossRef]
- Bernstein, E.F.; Schomacker, K.T.; Basilavecchio, L.D.; Plugis, J.M.; Bhawalkar, J.D. A novel dual-wavelength, Nd:YAG, picosecond-domain laser safely and effectively removes multicolor tattoos. Lasers Surg. Med. 2015, 47, 542–548. [Google Scholar] [CrossRef]
- Yu, H.-S.; Wu, C.-S.; Kao, Y.-H.; Chiou, M.-H.; Yu, C.-L. Helium-neon laser irradiation stimulates migration and proliferation in melanocytes and induces repigmentation in segmental-type vitiligo. J. Investig. Dermatol. 2003, 120, 56–64. [Google Scholar] [CrossRef]
- Lan, C.-C.E.; Wu, S.-B.; Wu, C.-S.; Shen, Y.-C.; Chiang, T.-Y.; Wei, Y.-H.; Yu, H.-S. Induction of primitive pigment cell differentiation by visible light (helium–neon laser): A photoacceptor-specific response not replicable by UVB irradiation. J. Mol. Med. 2012, 90, 321–330. [Google Scholar] [CrossRef]
- Lan, C.C.; Wu, C.S.; Chiou, M.H.; Hsieh, P.C.; Yu, H.S. Low-energy helium-neon laser induces locomotion of the immature melanoblasts and promotes melano genesis of the more differentiated melanoblasts: Recapitulation of vitiligo repigmentation in vitro. J. Investig. Dermatol. 2006, 126, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.-C.; Wu, C.-S.; Chiou, M.-H.; Chiang, T.-Y.; Yu, H.-S. Low-energy helium—Neon laser induces melanocyte proliferation via interaction with type IV collagen: Visible light as a therapeutic option for vitiligo. Br. J. Dermatol. 2009, 2, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Boen, M.; Wilson, M.J.V.; Wu, D.C.; Goldman, M.P. Laser_Depigmentation_in_Extensive_Vitiligo.26. Dermatol. Surg. 2019, 4, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.R.; Anderson, R.R. Treatment of Benign Pigmented Epidermal Lesions by Q-Switched Ruby Laser. Int. J. Dermatol. 1993, 32, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.R.; Flotte, T.J.; Gange, R.W.; Anderson, R.R. Treatment of nevus of Ota by Q-switched ruby laser. J. Am. Acad. Dermatol. 1994, 30 Pt 1, 743–751. [Google Scholar] [CrossRef]
- Watanabe, S.; Takahashi, H. Treatment of nevus of ota with the q-switched rubylaser. N. Engl. J. Med. 1994, 26, 1745–1750. [Google Scholar] [CrossRef]
- Rathod, S.; Munshi, A.; Agarwal, J. Skin markings methods and guidelines: A reality in image guidance radiotherapy era. South Asian J. Cancer 2020, 1, 27–29. [Google Scholar] [CrossRef]
- Ma, S.-Y.; Gong, Y.-Q.; Zhang, W.J.; Liang, B.-H.; Li, Y.-M.; Xie, Z.-M.; Zhu, H.-L. Split-face comparison of the efficacy of picosecond 532 nm Nd:YAG laser and Q-switched 755 nm Alexandrite laser for treatment of freckles. J. Cosmet. Laser Ther. 2022, 24, 22–27. [Google Scholar] [CrossRef]
- Yamada-Kanazawa, S.; Jinnin, M.; Fukushima, S. Nevus of Ota on the auricle successfully treated with Q-switched ruby laser. Drug Discov. Ther. 2022, 16, 254–255. [Google Scholar] [CrossRef]
- Taylor, C.R.; Anderson, R.R. Ineffective treatment of refractory melasma and postinflammatory hyperpigmentation by Q-switched ruby laser. J. Dermatol. Surg. Oncol. 1994, 20, 592–597. [Google Scholar] [CrossRef]
- Jang, W.S.; Lee, C.K.; Kim, B.J.; Kim, M.N. Efficacy of 694-nm Q-Switched Ruby Fractional Laser Treatment of Melasma in Female Korean Patients. Dermatol. Surg. 2011, 37, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Hilton, S.; Heise, H.; Buhren, B.A.; Schrumpf, H.; Bölke, E.; Gerber, P.A. Treatment of melasma in Caucasian patients using a novel 694-nm Q-switched ruby fractional laser. Eur. J. Med. Res. 2013, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.L.; Hu, B.; Zhang, C. Efficacy of 694-nm fractional Q-switched ruby laser (QSRL) combined with sonophoresis on levorotatory vitamin C for treatment of melasma in Chinese patients. Lasers Med. Sci. 2016, 31, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tong, X.; Yang, J.; Yang, L.; Tao, J.; Tu, Y. Q-switched alexandrite laser treatment of facial and labial lentigines associated with Peutz-Jeghers syndrome. Photodermatol. Photoimmunol. Photomed. 2012, 28, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Wattanakrai, P.; Mornchan, R.; Eimpunth, S. A randomized, split-face clinical trial of low-fluence Q-switched neodymium-doped yttrium aluminum garnet (1064 nm) laser versus low-fluence Q-switched alexandrite laser (755 nm) for the treatment of facial melasma. Lasers Surg. Med. 2014, 46, 531–537. [Google Scholar]
- Zhang, B.; Chu, Y.; Xu, Z.; Sun, Y.; Li, L.; Han, X.; Wang, C.; Wei, L.; Liu, Y.; Ma, L. Treatment of Café-Au-Lait Spots Using Q-Switched Alexandrite Laser: Analysis of Clinical Characteristics of 471 Children in Mainland China. Lasers Surg. Med. 2019, 51, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Manuskiatti, W.; Yan, C.; Tantrapornpong, P.; Cembrano, K.A.; Techapichetvanich, T.; Wanitphakdeedecha, R. A Prospective, Split-Face, Randomized Study Comparing a 755-nm Picosecond Laser With and Without Diffractive Lens Array in the Treatment of Melasma in Asians. Lasers Surg. Med. 2021, 53, 95–103. [Google Scholar] [CrossRef]
- Vachiramon, V.; Namasondhi, A.; Anuntrangsee, T.; Jurairattanaporn, N. Randomized, evaluator-blinded comparative study of a potassium titanyl phosphate (KTP) 532-nm picosecond laser and an alexandrite 755-nm picosecond laser for the treatment of solar lentigines in Asians. J. Cosmet. Dermatol. 2022, 21, 4370–4377. [Google Scholar] [CrossRef]
- Raulin, C.; Greve, B.; Grema, H. IPL technology: A review. Lasers Surg. Med. 2003, 32, 78–87. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Choi, H. Intense Pulsed Light Attenuates UV-Induced Hyperimmune Response and Pigmentation in Human Skin Cells. Int. J. Mol. Sci. 2021, 22, 3173. [Google Scholar] [CrossRef]
- Bjerring, P.; Christiansen, K. Intense pulsed light source for treatment of small melanocytic nevi and solar lentigines. J. Cutan. Laser Ther. 2000, 2, 177–181. [Google Scholar] [CrossRef]
- Kawada, A.; Shiraishi, H.; Asai, M.; Kameyama, H.; Sangen, Y.; Aragane, Y.; Tezuka, T. Clinical improvement of solar lentigines and ephelides with an intense pulsed light source. Dermatol. Surg. 2002, 28, 504–508. [Google Scholar] [PubMed]
- Sasaya, H.; Kawada, A.; Wada, T.; Hirao, A.; Oiso, N. Clinical effectiveness of intense pulsed light therapy for solar lentigines of the hands. Dermatol. Ther. 2011, 24, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, D.P.; Peterson, J.D. Efficacy and safety of intense pulsed light with a KTP filter for the treatment of solar lentigines. Lasers Surg. Med. 2019, 51, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Amornpetkul, W.; Kanokrungsee, S.; Kamanamool, N.; Udompataikul, M.; Rojhirunsakool, S. Comparison between the use of intense pulsed light and Q-switched neodymium-doped yttrium aluminum garnet laser for the treatment of axillary hyperpigmentation. J. Cosmet. Dermatol. 2021, 20, 2785–2793. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Huang, L.; Chen, Y.; Yan, T.; Yang, B.; Man, M.Q. The efficacy of intense pulsed light for Becker’s nevus: A retrospective analysis of 45 cases. J. Cosmet. Dermatol. 2021, 20, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Babilas, P.; Schreml, S.; Szeimies, R.-M.; Landthaler, M. Intense pulsed light (IPL): A review. Lasers Surg. Med. 2010, 42, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Chen, J.Z.; Wei, H.C.; Wu, Y.; Liu, M.; Xu, Y.Y.; Dong, G.H.; Chen, H.D. Efficacy and Safety of Intense Pulsed Light in Treatment of Melasma in Chinese Patients. Dermatol. Surg. 2008, 34, 693–701. [Google Scholar]
- Yi, J.; Hong, T.; Zeng, H.; Li, P.; Li, P.; Wang, S.; Chen, J.; Li, P.; Zhou, J. A Meta-analysis-Based Assessment of Intense Pulsed Light for Treatment of Melasma. Aesthetic Plast. Surg. 2020, 44, 947–952. [Google Scholar] [CrossRef]
- Dierickx, C.C.; Anderson, R.R. Visible light treatment of photoaging. Dermatol. Ther. 2005, 18, 191–208. [Google Scholar] [CrossRef]
- Weiss, R.A.; McDaniel, D.H.; Geronemus, R.G.; Margaret, A.W.; Karen, L.B.; Munavalli, G.M.; Bellew, S.G. Clinical experience with light-emitting diode (LED) photomodulation. Dermatol. Surg. 2005, 31 Pt 2, 1199–1205. [Google Scholar] [CrossRef]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs. light emitting diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef]
- Jin, S.; Chen, L.; Xu, Z.; Xing, X.; Zhang, C.; Xiang, L. 585 nm light-emitting diodes inhibit melanogenesis through upregulating H19/miR-675 axis in LEDs-irradiated keratinocytes by paracrine effect. J. Dermatol. Sci. 2020, 98, 102–108. [Google Scholar] [CrossRef]
- Dai, X.; Jin, S.; Xuan, Y.; Yang, Y.; Lu, X.; Wang, C.; Chen, L.; Xiang, L.; Zhang, C. 590 nm LED Irradiation Improved Erythema through Inhibiting Angiogenesis of Human Microvascular Endothelial Cells and Ameliorated Pigmentation in Melasma. Cells 2022, 11, 3949. [Google Scholar] [CrossRef]
- Mpofana, N.; Abrahamse, H. The Management of Melasma on Skin Types V and VI Using Light Emitting Diode Treatment. Photomed. Laser Surg. 2018, 36, 522–529. [Google Scholar] [CrossRef]
- Xuan, Y.J.; Dai, X.X.; Chen, L.; Xiang, L.H.; Jin, S.L.; Zhang, C.F. Efficacy and safety of home-based 590 nm light-emitting diodes and in-hospital 1064 nm Q-switched Nd:YAG laser in the treatment of facial melasma: A single-centre, prospective, randomized clinical trial. J. Eur. Acad. Dermatol. Venereol. 2023; ahead of print. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, K.-H.; Choi, J.-W.; Kwon, J.-K.; Lee, D.R.; Shin, M.S.; Lee, J.S.; You, C.E.; Park, M.Y. A prospective, randomized, placebo-controlled, double-blinded, and split-face clinical study on LED phototherapy for skin rejuvenation: Clinical, profilometric, histologic, ultrastructural, and biochemical evaluations and comparison of three different treatment settings. J. Photochem. Photobiol. B 2007, 88, 51–67. [Google Scholar]
- A Weiss, R.; A Weiss, M.; Geronemus, R.G.; McDaniel, D.H. A novel non-thermal non-ablative full panel LED photomodulation device for reversal of photoaging: Digital microscopic and clinical results in various skin types. J. Drugs Dermatol. 2004, 3, 605–610. [Google Scholar]
- Cho, H.; Kim, B.; Kim, O.S.; Kim, Y.; Yang, Y.; Song, J.; Liu, D.; Jeon, S.; Kim, O. Photochemical reaction to increase melanogenesis using Buddleja officinalis and blue light-emitting diode irradiation in B16F10. Photodiagn. Photodyn. Ther. 2021, 35, 102456. [Google Scholar] [CrossRef]
- Lodi, G.; Del Re, C.; Nisticò, S.P.; Bennardo, L.; Cannarozzo, G.; Sannino, M. Blue light-emitting diodes for the treatment of localized vitiligo: A retrospective study. J. Cosmet. Dermatol. 2023, 22, 1273–1278. [Google Scholar] [CrossRef]

| Laser | IPL | LED | |
|---|---|---|---|
| Intensity | High | High | Low |
| Pulse width | Unadjustable | Continuous and adjustable | Continuous and adjustable |
| Coherence | Coherent | Incoherent | Incoherent |
| Directionality | High/Single | Low/Multiple | Low/Multiple |
| Wavelength and Chromaticity | Monochromatic | 500–1200 nm, Polychromatic | 400–800 nm Polychromatic |
| Spot Size | Small point: <1 cm × 1 cm | Medium: 5 cm × 2 cm | Large: 30 cm × 30 cm |
| Mechanism | Selective Photothermalmolysis | Selective Photothermalmolysis | Photobiomodulation |
| Indications for pigmentary disorders | Benign epidermal pigmented lesions (ephelides, lentigo, PLH, café au lait macules, pigmented seborrheic keratoses…); Benign dermal pigmented lesions (CMN, nevus of Ota/Ito…); Mixed (epidermal/ dermal) pigmented lesions (Becker’s nevus, melasma, PIH); Tattoos; Vitiligo | Benign epidermal pigmented lesions (ephelides, lentigo, café au lait macules, …); mixed (epidermal/dermal) pigmented lesions (Becker’s nevus, melasma, PIH, poikiloderma of Civatte) | Melasma; Vitiligo |
| Adverse effects | Relatively common, mainly hyperpigmentation, sometimes scarring | Infrequent, sometimes erythema and hyperpigmentation | Rarely seen |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Jin, S.; Dai, X.; Chen, L.; Xiang, L.; Zhang, C. The Emerging Role of Visible Light in Melanocyte Biology and Skin Pigmentary Disorders: Friend or Foe? J. Clin. Med. 2023, 12, 7488. https://doi.org/10.3390/jcm12237488
He X, Jin S, Dai X, Chen L, Xiang L, Zhang C. The Emerging Role of Visible Light in Melanocyte Biology and Skin Pigmentary Disorders: Friend or Foe? Journal of Clinical Medicine. 2023; 12(23):7488. https://doi.org/10.3390/jcm12237488
Chicago/Turabian StyleHe, Xuanxuan, Shanglin Jin, Xiaoxi Dai, Li Chen, Leihong Xiang, and Chengfeng Zhang. 2023. "The Emerging Role of Visible Light in Melanocyte Biology and Skin Pigmentary Disorders: Friend or Foe?" Journal of Clinical Medicine 12, no. 23: 7488. https://doi.org/10.3390/jcm12237488
APA StyleHe, X., Jin, S., Dai, X., Chen, L., Xiang, L., & Zhang, C. (2023). The Emerging Role of Visible Light in Melanocyte Biology and Skin Pigmentary Disorders: Friend or Foe? Journal of Clinical Medicine, 12(23), 7488. https://doi.org/10.3390/jcm12237488







