Predicting Cardiopulmonary Arrest with Digital Biomarkers: A Systematic Review
Abstract
:1. Introduction
- What are the vital sign events or patterns assessed by telemetry that precede IHCAs?
- What single-vital signs patterns detected by telemetry most accurately predict IHCAs?
2. Methods
2.1. Eligibility Criteria
2.1.1. Population and Interventions
2.1.2. Study Types
2.2. Information Sources and Search Strategy
2.3. Selection and Data Collection Process
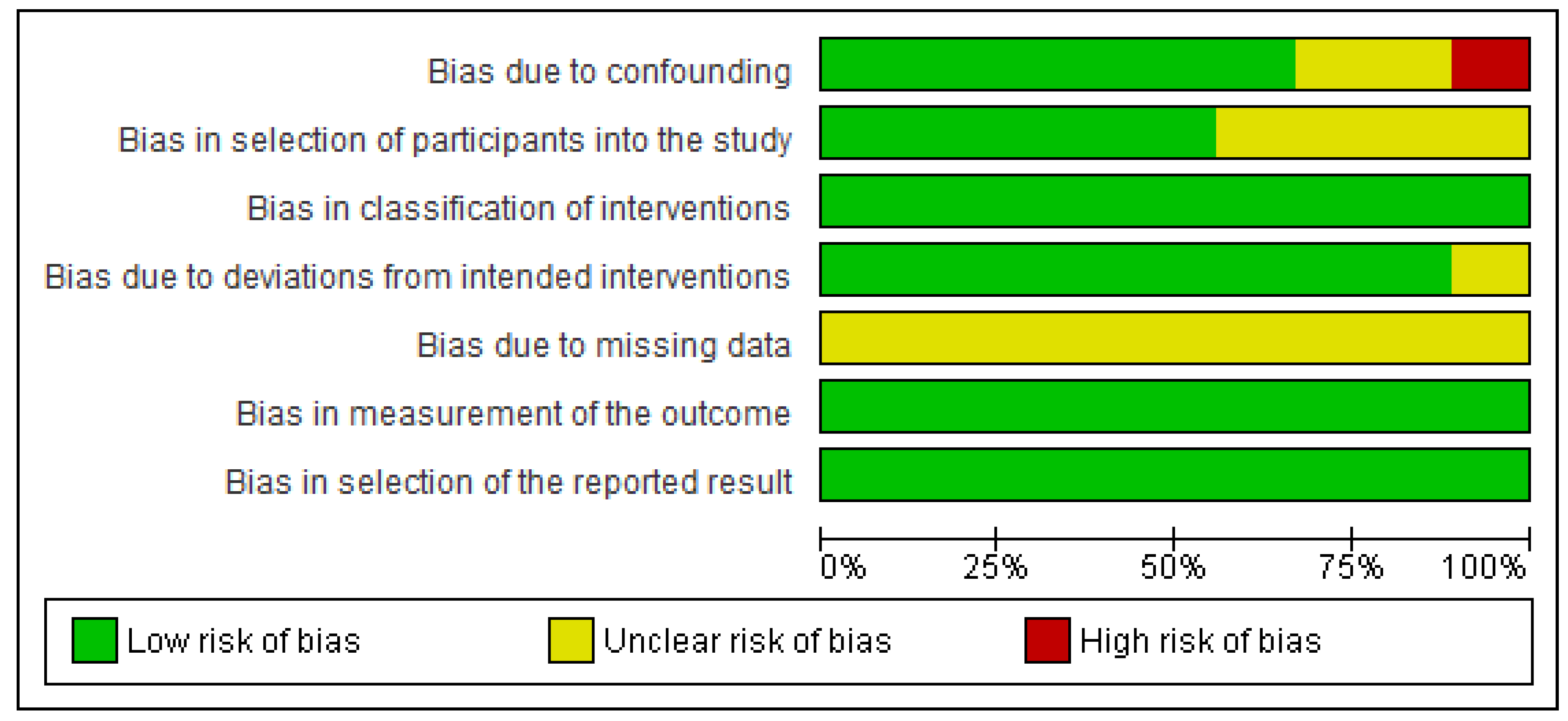
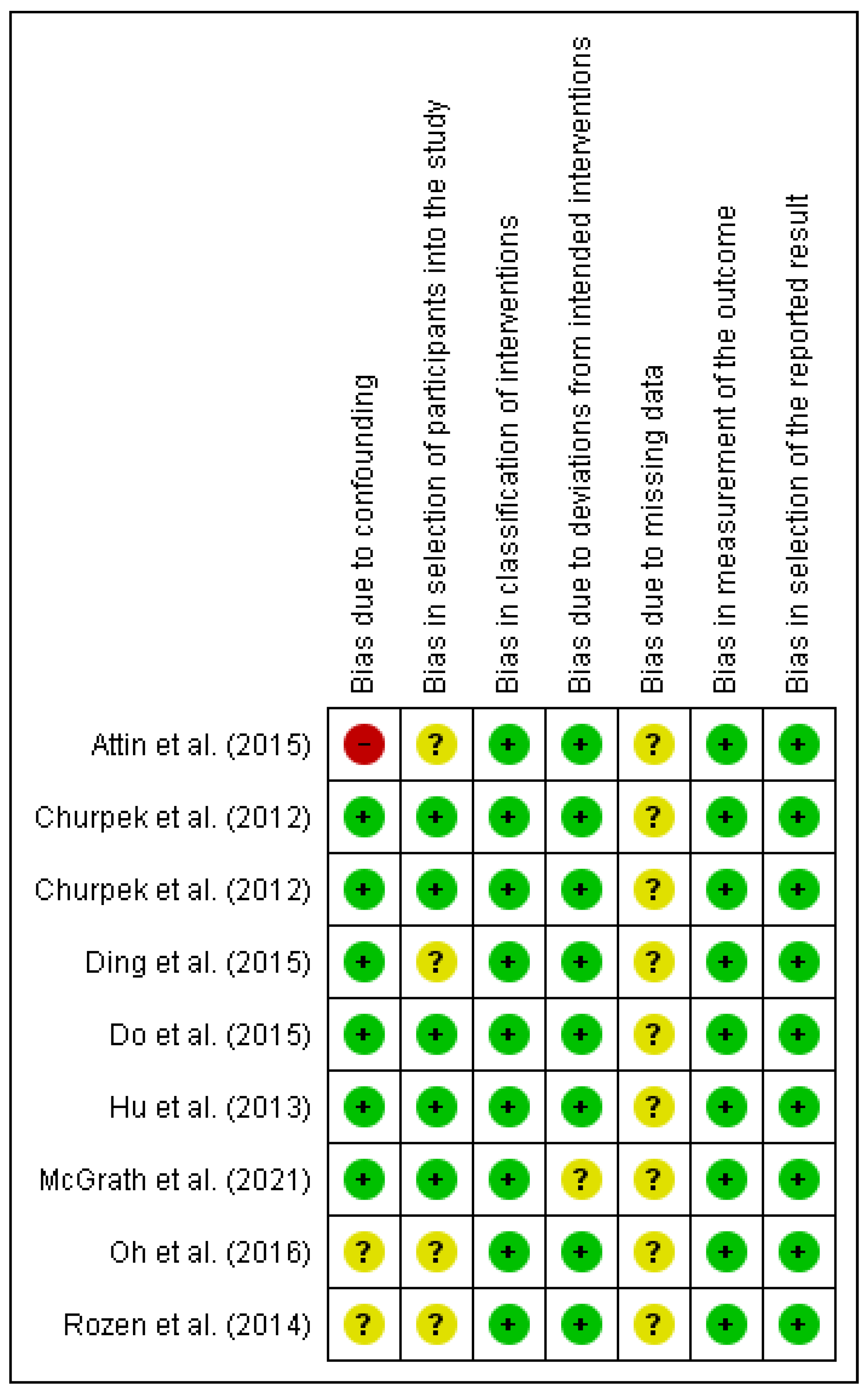
2.4. Risk of Bias of Individual Studies
2.5. Synthesis Methods
3. Results
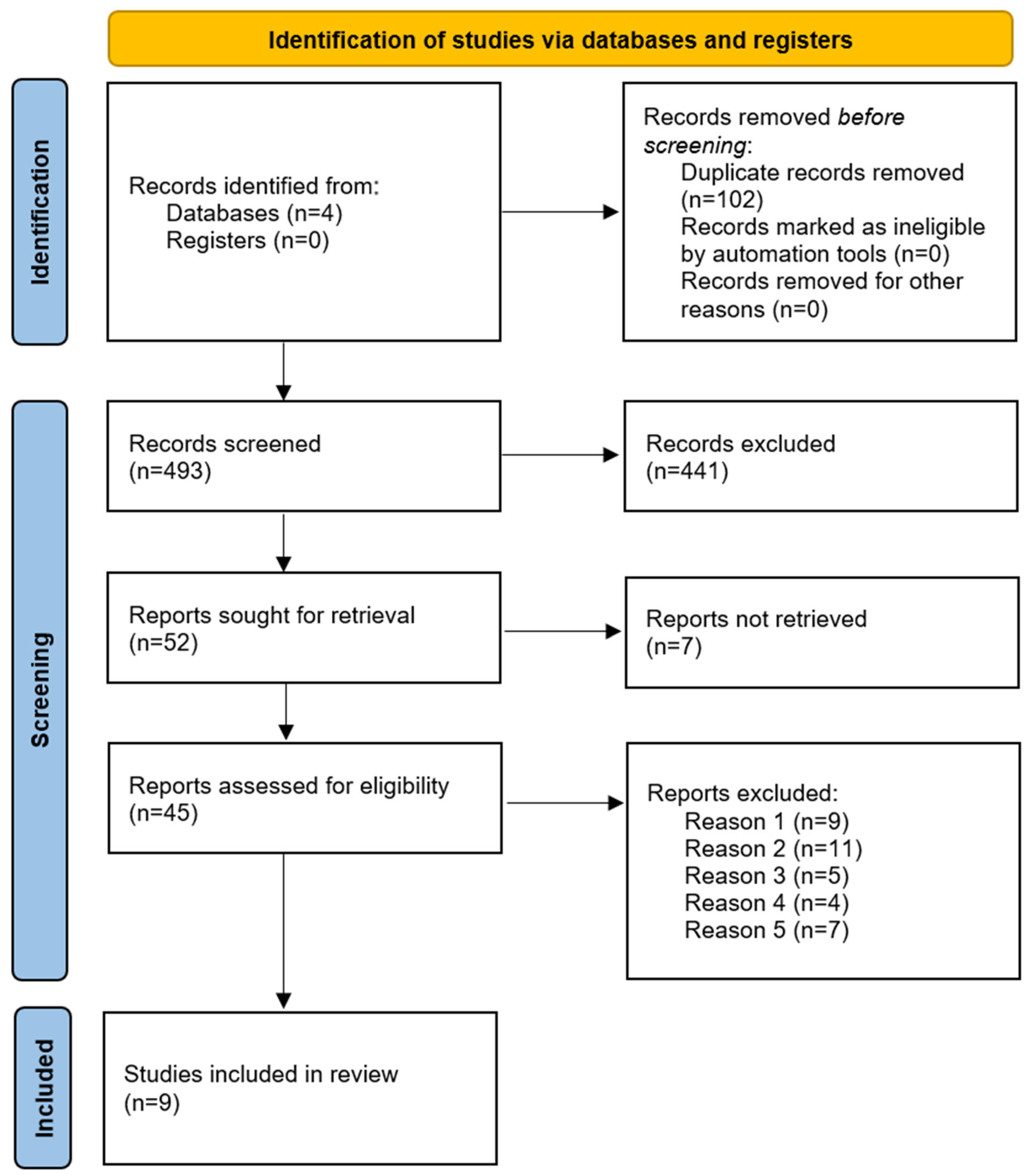
3.1. Study Selection and Characteristics
3.2. Results for Individual Studies
4. Discussion
4.1. Heart Rate
4.2. Blood Pressure
4.3. Respiratory Rate
4.4. Oximetry
4.5. Temperature
4.6. ECG
4.7. Implication of Results
4.8. Early Warning Scores
4.9. Telemetry and AI/ML
4.10. Limitations of This Review
5. Conclusions
Protocol Registration
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andersen, L.W.; Holmberg, M.J.; Berg, K.M.; Donnino, M.W.; Granfeldt, A. In-Hospital Cardiac Arrest: A Review. JAMA 2019, 321, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, V.M.; Larkin, G.L.; Peberdy, M.A.; Carey, S.M.; Kaye, W.; Mancini, M.E.; Nichol, G.; Lane-Truitt, T.; Potts, J.; Ornato, J.P.; et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA 2006, 295, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Peberdy, M.A.; Kaye, W.; Ornato, J.P.; Larkin, G.L.; Nadkarni, V.; Mancini, M.E.; Berg, R.A.; Nichol, G.; Lane-Trultt, T. Cardiopulmonary resuscitation of adults in the hospital: A report of 14,720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation 2003, 58, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Schluep, M.; Gravesteijn, B.Y.; Stolker, R.J.; Endeman, H.; Hoeks, S.E. One-year survival after in-hospital cardiac arrest: A systematic review and meta-analysis. Resuscitation 2018, 132, 90–100. [Google Scholar] [CrossRef]
- Al Awaida, W.; Ahmed, A.A.; Hamza, A.A.; Amber, K.I.; Al-Ameer, H.J.; Jarrar, Y.; Fatima, G.; Maslat, A.O.; Gushchina, Y.; Al Bawareed, O.; et al. Association of KDR rs1870377 genotype with clopidogrel resistance in patients with post percutaneous coronary intervention. Heliyon 2021, 7, e06251. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Saha, A.; Tarver, M.E.; Patel, B. Digital biomarkers: Convergence of digital health technologies and biomarkers. Npj Digit. Med. 2022, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.A. Multiple parameter track and trigger systems. In Textbook of Rapid Response Systems; Springer: Berlin/Heidelberg, Germany, 2017; pp. 87–94. [Google Scholar]
- Andersen, L.W.; Kim, W.Y.; Chase, M.; Berg, K.M.; Mortensen, S.J.; Moskowitz, A.; Novack, V.; Cocchi, M.N.; Donnino, M.W. The prevalence and significance of abnormal vital signs prior to in-hospital cardiac arrest. Resuscitation 2016, 98, 112–117. [Google Scholar] [CrossRef]
- Kause, J.; Smith, G.; Prytherch, D.; Parr, M.; Flabouris, A.; Hillman, K. A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom—The ACADEMIA study. Resuscitation 2004, 62, 275–282. [Google Scholar] [CrossRef]
- Moskowitz, A.; Berg, K.M.; Cocchi, M.N.; Chase, M.; Yang, J.X.; Sarge, J.; Grossestreuer, A.V.; Sarge, T.; O’ Donoghue, S.; Donnino, M.W. Cardiac arrest in the intensive care unit: An assessment of preventability. Resuscitation 2019, 145, 15–20. [Google Scholar] [CrossRef]
- Bergum, D.; Haugen, B.O.; Nordseth, T.; Mjølstad, O.C.; Skogvoll, E. Recognizing the causes of in-hospital cardiac arrest—A survival benefit. Resuscitation 2015, 97, 91–96. [Google Scholar] [CrossRef]
- Bellomo, R.; Ackerman, M.; Bailey, M.; Beale, R.; Clancy, G.; Danesh, V.; Hvarfner, A.; Jimenez, E.; Konrad, D.; Lecardo, M.; et al. A controlled trial of electronic automated advisory vital signs monitoring in general hospital wards*. Crit. Care Med. 2012, 40, 2349–2361. [Google Scholar] [CrossRef]
- Julian, D.G.; Valentine, P.A.; Miller, G.G. Disturbances of rate, rhythm and conduction in acute myocardial infarction: A prospective study of 100 consecutive unselected patients with the aid of electrocardiographic monitoring. Am. J. Med. 1964, 37, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Cleverley, K.; Mousavi, N.; Stronger, L.; Ann-Bordun, K.; Hall, L.; Tam, J.W.; Tischenko, A.; Jassal, D.S.; Philipp, R.K. The impact of telemetry on survival of in-hospital cardiac arrests in non-critical care patients. Resuscitation 2013, 84, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Palchaudhuri, S.; Johnson, A.; Trost, J.; Ponor, I.; Zakaria, S. Does this patient need telemetry? An analysis of telemetry ordering practices at an academic medical center. J. Eval. Clin. Pract. 2017, 23, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, F.; Arnold, D.; Baird, A.; Albur, M.; Whiting, P. Early Warning Scores do not accurately predict mortality in sepsis: A meta-analysis and systematic review of the literature. J. Infect. 2018, 76, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.B.; Prytherch, D.R.; Schmidt, P.E.; Featherstone, P.I.; Higgins, B. A review, and performance evaluation, of single-parameter “track and trigger” systems. Resuscitation 2008, 79, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.B.; Chiovaro, J.C.; O’Neil, M.; Kansagara, D.; Quinones, A.; Freeman, M.; Motu’apuaka, M.; Slatore, C.G. VA Evidence-based Synthesis Program Reports. In Early Warning System Scores: A Systematic Review; Department of Veterans Affairs: Washington, DC, USA, 2014. [Google Scholar]
- Morgan, R.J.; Lloyd-Williams, F.; Wright, M.M.; Morgan-Warren, R. An early warning scoring system for detecting developing critical illness. Clin. Intensive Care 1997, 8, 100. [Google Scholar]
- Downey, C.L.; Tahir, W.; Randell, R.; Brown, J.M.; Jayne, D.G. Strengths and limitations of early warning scores: A systematic review and narrative synthesis. Int. J. Nurs. Stud. 2017, 76, 106–119. [Google Scholar] [CrossRef]
- Philip, K.E.; Pack, E.; Cambiano, V.; Rollmann, H.; Weil, S.; O’Beirne, J. The accuracy of respiratory rate assessment by doctors in a London teaching hospital: A cross-sectional study. J. Clin. Monit. Comput. 2015, 29, 455–460. [Google Scholar] [CrossRef]
- Convertino, V.A.; Koons, N.J. The compensatory reserve: Potential for accurate individualized goal-directed whole blood resuscitation. Transfusion 2020, 60, S150–S157. [Google Scholar] [CrossRef]
- Zhang, J.; Han, C.; Yang, F.; Xu, S.; Hu, H.; Chen, E. Application Value of Vital Signs Telemetry System for 2019 Novel Coronavirus Disease Suspected Cases in Isolation Wards. Infect. Drug Resist. 2020, 13, 2971–2977. [Google Scholar] [CrossRef]
- Akel, M.A.; Carey, K.A.; Winslow, C.J.; Churpek, M.M.; Edelson, D.P. Less is more: Detecting clinical deterioration in the hospital with machine learning using only age, heart rate, and respiratory rate. Resuscitation 2021, 168, 6–10. [Google Scholar] [CrossRef]
- Muralitharan, S.; Nelson, W.; Di, S.; McGillion, M.; Devereaux, P.J.; Barr, N.G.; Petch, J. Machine Learning–Based Early Warning Systems for Clinical Deterioration: Systematic Scoping Review. J. Med. Internet Res. 2021, 23, e25187. [Google Scholar] [CrossRef]
- Romero-Brufau, S.; Whitford, D.; Johnson, M.G.; Hickman, J.; Morlan, B.W.; Therneau, T.; Naessens, J.; Huddleston, J.M. Using machine learning to improve the accuracy of patient deterioration predictions: Mayo Clinic Early Warning Score (MC-EWS). J. Am. Med. Inf. Assoc. 2021, 28, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Churpek, M.M.; Yuen, T.C.; Huber, M.T.; Park, S.Y.; Hall, J.B.; Edelson, D.P. Predicting cardiac arrest on the wards: A nested case-control study. Chest 2012, 141, 1170–1176. [Google Scholar] [CrossRef]
- Churpek, M.M.; Yuen, T.C.; Park, S.Y.; Meltzer, D.O.; Hall, J.B.; Edelson, D.P. Derivation of a cardiac arrest prediction model using ward vital signs*. Crit. Care Med. 2012, 40, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Bai, Y.; Tinoco, A.; Mortara, D.; Do, D.; Boyle, N.G.; Pelter, M.M.; Hu, X. Developing new predictive alarms based on ECG metrics for bradyasystolic cardiac arrest. Physiol. Meas. 2015, 36, 2405–2422. [Google Scholar] [CrossRef]
- Hu, X.; Do, D.; Bai, Y.; Boyle, N.G. A case-control study of non-monitored ECG metrics preceding in-hospital bradyasystolic cardiac arrest: Implication for predictive monitor alarms. J. Electrocardiol. 2013, 46, 608–615. [Google Scholar] [CrossRef]
- McGrath, S.P.; Perreard, I.M.; MacKenzie, T.; Blike, G.T. Patterns in continuous pulse oximetry data prior to pulseless electrical activity arrest in the general care setting. J. Clin. Monit. Comput. 2021, 35, 537–545. [Google Scholar] [CrossRef]
- Oh, H.; Lee, K.; Seo, W. Temporal patterns of change in vital signs and Cardiac Arrest Risk Triage scores over the 48 hours preceding fatal in-hospital cardiac arrest. J. Adv. Nurs. 2016, 72, 1122–1133. [Google Scholar] [CrossRef]
- Rozen, T.H.; Mullane, S.; Kaufman, M.; Hsiao, Y.F.F.; Warrillow, S.; Bellomo, R.; Jones, D.A. Antecedents to cardiac arrests in a teaching hospital intensive care unit. Resuscitation 2014, 85, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Attin, M.; Feld, G.; Lemus, H.; Najarian, K.; Shandilya, S.; Wang, L.; Sabouriazad, P.; Lin, C.-D. Electrocardiogram characteristics prior to in-hospital cardiac arrest. J. Clin. Monit. Comput. 2015, 29, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Do, D.H.; Hayase, J.; Tiecher, R.D.; Bai, Y.; Hu, X.; Boyle, N.G. ECG changes on continuous telemetry preceding in-hospital cardiac arrests. J. Electrocardiol. 2015, 48, 1062–1068. [Google Scholar] [CrossRef]
- Bhalala, U.S.; Bonafide, C.P.; Coletti, C.M.; Rathmanner, P.E.; Nadkarni, V.M.; Berg, R.A.; Witzke, A.K.; Kasprzak, M.S.; Zubrow, M.T. Antecedent bradycardia and in-hospital cardiopulmonary arrest mortality in telemetry-monitored patients outside the ICU. Resuscitation 2012, 83, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- George, A.L.; Folk, B.P.; Crecelius, P.L.; Campbell, W.B. Pre-arrest morbidity and other correlates of survival after in-hospital cardiopulmonary arrest. Am. J. Med. 1989, 87, 28–34. [Google Scholar] [CrossRef]
- Rich, K. Inhospital Cardiac Arrest: Pre-Event Variables and Nursing Response. Clin. Nurse Spec. 1999, 13, 147–153. [Google Scholar] [CrossRef]
- Kim, W.Y.; Shin, Y.J.; Lee, J.M.; Huh, J.W.; Koh, Y.; Lim, C.M.; Hong, S.B. Modified Early Warning Score Changes Prior to Cardiac Arrest in General Wards. PLoS ONE 2015, 10, e0130523. [Google Scholar] [CrossRef]
- Royal College of Physicians. National Early Warning Score (NEWS) 2: Standardising the Assessment of Acute-Illness Severity in the NHS. Updated Report of a Working Party; RCP: London, UK, 2017; Available online: www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2 (accessed on 20 December 2022).
- Beane, A.; De Silva, A.P.; De Silva, N.; Sujeewa, J.A.; Rathnayake, R.M.D.; Sigera, P.C.; Athapattu, P.L.; Mahipala, P.G.; Rashan, A.; Munasinghe, S.B.; et al. Evaluation of the feasibility and performance of early warning scores to identify patients at risk of adverse outcomes in a low-middle income country setting. BMJ Open 2018, 8, e019387. [Google Scholar] [CrossRef]
- Bedell, S.E.; Deitz, D.C.; Leeman, D.; Delbanco, T.L. Incidence and Characteristics of Preventable latrogenic Cardiac Arrests. JAMA 1991, 265, 2815–2820. [Google Scholar] [CrossRef] [PubMed]
- Fieselmann, J.F.; Hendryx, M.S.; Helms, C.M.; Wakefield, D.S. Respiratory rate predicts cardiopulmonary arrest for internal medicine inpatients. J. Gen. Intern. Med. 1993, 8, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Spångfors, M.; Molt, M.; Samuelson, K. In-hospital cardiac arrest and preceding National Early Warning Score (NEWS): A retrospective case-control study. Clin. Med. 2020, 20, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.; Djärv, T. Preceding national early warnings scores among in-hospital cardiac arrests and their impact on survival. Am. J. Emerg. Med. 2017, 35, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Lott, C.; Truhlár, A. Cardiac arrest in special circumstances. Curr. Opin. Crit. Care 2021, 27, 642–648. [Google Scholar] [CrossRef]
- Cuthbertson, B.H.; Boroujerdi, M.; McKie, L.; Aucott, L.; Prescott, G. Can physiological variables and early warning scoring systems allow early recognition of the deteriorating surgical patient? Crit. Care Med. 2007, 35, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Abdelghani, S.A.; Rosenthal, T.M.; Morin, D.P. Surface Electrocardiogram Predictors of Sudden Cardiac Arrest. Ochsner J. 2016, 16, 280. [Google Scholar]
- Kwon, J.-m.; Kim, K.-H.; Jeon, K.-H.; Lee, S.Y.; Park, J.; Oh, B.-H. Artificial intelligence algorithm for predicting cardiac arrest using electrocardiography. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 98. [Google Scholar] [CrossRef]
- Bell, L. Alarm fatigue linked to patient’s death. Interview by Laura Wallis. Am. J. Nurs. 2010, 110, 16. [Google Scholar] [CrossRef]
- Borowski, M.; Görges, M.; Fried, R.; Such, O.; Wrede, C.; Imhoff, M. Medical device alarms. Biomed. Tech. 2011, 56, 73–83. [Google Scholar] [CrossRef]
- Kenny, P.E. Alarm fatigue and patient safety. Pa Nurse 2011, 66, 22. [Google Scholar] [PubMed]
- Helfand, M.; Christensen, V.; Anderson, J. Technology Assessment: Early Sense for Monitoring Vital Signs in Hospitalized Patients; Department of Veterans Affairs: Washington, DC, USA, 2016.
- Gerry, S.; Bonnici, T.; Birks, J.; Kirtley, S.; Virdee, P.S.; Watkinson, P.J.; Collins, G.S. Early warning scores for detecting deterioration in adult hospital patients: Systematic review and critical appraisal of methodology. BMJ 2020, 369, m1501. [Google Scholar] [CrossRef]
- Kyriacos, U.; Jelsma, J.; James, M.; Jordan, S. Monitoring Vital Signs: Development of a Modified Early Warning Scoring (Mews) System for General Wards in a Developing Country. PLoS ONE 2014, 9, e87073. [Google Scholar] [CrossRef] [PubMed]
- Churpek, M.M.; Adhikari, R.; Edelson, D.P. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation 2016, 102, 1–5. [Google Scholar] [CrossRef]
- Cho, K.-J.; Kwon, O.; Kwon, J.-m.; Lee, Y.; Park, H.; Jeon, K.-H.; Kim, K.-H.; Park, J.; Oh, B.-H. Detecting Patient Deterioration Using Artificial Intelligence in a Rapid Response System. Crit. Care Med. 2020, 48, e285–e289. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.; Gil, H.-W.; Cho, N.-J.; Lee, H. Machine Learning-Based Cardiac Arrest Prediction for Early Warning System. Mathematics 2022, 10, 2049. [Google Scholar] [CrossRef]
- Kwon, J.m.; Lee, Y.; Lee, Y.; Lee, S.; Park, J. An Algorithm Based on Deep Learning for Predicting In-Hospital Cardiac Arrest. J. Am. Heart Assoc. 2018, 7, e008678. [Google Scholar] [CrossRef]
- Wu, T.T.; Lin, X.Q.; Mu, Y.; Li, H.; Guo, Y.S. Machine learning for early prediction of in-hospital cardiac arrest in patients with acute coronary syndromes. Clin. Cardiol. 2021, 44, 349–356. [Google Scholar] [CrossRef]
- Wadden, J.J. Defining the undefinable: The black box problem in healthcare artificial intelligence. J. Med. Ethics 2022, 48, 764–768. [Google Scholar] [CrossRef]
- Petch, J.; Di, S.; Nelson, W. Opening the Black Box: The Promise and Limitations of Explainable Machine Learning in Cardiology. Can J Cardiol 2022, 38, 204–213. [Google Scholar] [CrossRef]
- Khalid, S.; Khalil, T.; Nasreen, S. A survey of feature selection and feature extraction techniques in machine learning. In Proceedings of the 2014 Science and Information Conference, London, UK, 27–29 August 2014; pp. 372–378. [Google Scholar]
- Pudjihartono, N.; Fadason, T.; Kempa-Liehr, A.W.; O’Sullivan, J.M. A Review of Feature Selection Methods for Machine Learning-Based Disease Risk Prediction. Front. Bioinform. 2022, 2, 927312. [Google Scholar] [CrossRef] [PubMed]
- Hira, Z.M.; Gillies, D.F. A Review of Feature Selection and Feature Extraction Methods Applied on Microarray Data. Adv. Bioinform. 2015, 2015, 198363. [Google Scholar] [CrossRef] [PubMed]
- Ladha, L. Feature Selection Methods and Algorithms. 2011. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwi_9KiWmeiCAxUQCYgKHUHxC9sQFnoECBQQAQ&url=http%3A%2F%2Fwww.enggjournals.com%2Fijcse%2Fdoc%2FIJCSE11-03-05-051.pdf&usg=AOvVaw0Wb4jnyb78pwOobDc_iLhI&opi=89978449 (accessed on 15 September 2023).
- Knight, B.P.; Pelosi, F.; Michaud, G.F.; Strickberger, S.A.; Morady, F. Clinical Consequences of Electrocardiographic Artifact Mimicking Ventricular Tachycardia. N. Engl. J. Med. 1999, 341, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Javadi, G.; Jamzad, A.; Hamilton, A.; Sibley, S.; Abolmaesumi, P.; Maslove, D.; Mousavi, P. Detecting Atrial Fibrillation in ICU Telemetry data with Weak Labels. In Proceedings of the 6th Machine Learning for Healthcare Conference, Proceedings of Machine Learning Research, Virtual, 6–7 August 2021; pp. 176–195. [Google Scholar]
- Alamgir, A.; Mousa, O.; Shah, Z. Artificial Intelligence in Predicting Cardiac Arrest: Scoping Review. JMIR Med. Inf. 2021, 9, e30798. [Google Scholar] [CrossRef]
- Maslove, D.M.; Elbers, P.W.G.; Clermont, G. Artificial intelligence in telemetry: What clinicians should know. Intensive Care Med. 2021, 47, 150–153. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Author (Year) | Cases | Telemetry System | Physiological Markers Assessed | Time Assessed | Participant Characteristics | Controls | Types of CA |
|---|---|---|---|---|---|---|---|
| Do et al. (2015) [37] | 81 | General Electric monitoring systems (GE Healthcare, Waukesha, WI, USA), | ECG (5 metrics) •PR interval •QRS duration •ST segment elevation or depression •New onset arrhythmias •QTc (only for VT/VF/TdP arrests) | 24 h | •Mean age (SD): 62 (19) •Male: 56% •ICU at the time of IHCA: 74% | No | •VT/VF/TdP: 18% •Bradyasystolic: 26% •PEA: 56% |
| Attin et al. (2015) [36] | 39 | Not reported | ECG (5 metrics) •Heart rate •QRS duration •ST segment elevation or depression •QRS morphology •New onset arrhythmias | 8 h | •Mean age (SD): 69.5 (13) •Male: 64% •ICU at the time of IHCA: 44% •Structural heart conditions: 100% | No | •PEA and asystole: 100% (not specified) •VT/VF: excluded from the study |
| Churpek et al. (2012) [29] | 88 | Not reported | •Heart rate •Blood pressure (SBP and DBP) •Respiratory rate •Temperature •SatO2 | 48 h | •Mean age (SD): 64 (16) * •Female: 57% † •Prior ICU admission: 41% * | Yes | All ᵃ |
| Churpek et al. (2012) [30] | 88 | Not reported | •Heart rate •Blood pressure (SBP and DBP) •Respiratory rate •Temperature •SatO2 | 48 h | •Age, mean (SD): 64 (16) * •Female: 57% † •Prior ICU admission: 41% * | Yes, matched | All ᵃ |
| Hu et al. (2013) [32] | 22 | General Electric bedside monitors (GE Healthcare, Waukesha, WI, USA) | ECG (8 metrics) •Heart rate •PRp •pAmp •QTpc •tAmp •QRS width •rAmp •relJAmp | 24 h | Mean age (SD): 63 (19) ‡ Male: 68% ‡ ICU at the time of CA: 77% ‡ | Yes, matched | Bradysystolic CA |
| Rozen et al. (2014) [35] | 36 | Not reported | •Heart rate •Blood pressure (SBP and MAP) •Respiratory rate •SatO2 | 12 h | Age, median (IQR): 67 (52.5–75.0) † Male: 58.3% † ICU at the time of CA: 100% † | Yes, matched | •PEA: 38.9% •Severe bradycardia: 22% •Asystole: 11.1% •VT: 25% •Vfib: 2.8% |
| Ding et al. (2015) [31] | 27 | General Electric bedside monitors (GE Healthcare, Waukesha, WI, USA) | ECG (17 metrics) •PR interval •P-wave duration •QRS duration •RR interval •QT interval •SerumK2 •T-wave complexity •ST segment for leads I, II, and V •SDNN •HRV total power •HRV very low, low, and high •HRV normalized low and normalized high-frequency power | 24 h | Mean age (SD): 61.4 (20.7) ‡ Male: 18 (66.7%) ‡ ICU at the time of CA: not reported ‡ | Yes, matched | Bradyasystolic CA |
| Oh et al. (2016) [34] | 46 | GE Marquette Solar 8000M Patient Monitor (General Electric, Windsor, CT, USA) | •Heart rate •Blood pressure (SBP and DBP) •Respiratory rate •Temperature •SatO2 | 48 h | Mean age (SD): 61 (18) † Male: 72% † ICU at the time of CA: 100% † | Yes | All ᵃ ᵇ |
| McGrath et al. (2021) [33] | 38 | Masimo Root® monitor (Masimo Corp., Irvine, CA, USA) | •Pulse rate •SatO2 | 12 h | Mean age (SD): 65.3 (12.9) † Male: 71.1% † ICU at the time of CA: 0% | Yes | PEA |
| Study | SBP (mmHg) | DBP (mmHg) | MAP (mmHg) | Heart Rate (bpm) | Respiratory Rate (rpm) | Temperature (°C) | SatO2 (%) | Change from Baseline |
|---|---|---|---|---|---|---|---|---|
| Churpek et al. (2012) [29] | Not reported by authors † | * Minimum DBP achieved: Sen (95% CI) •<50: 45% (33–55) •<45: 29% (17–38) •<40: 16% (7–28) Spec (95% CI) •<50: 77% (57–84) •<45: 90% (79–96) •<40: 96% (92–99) | Not assessed | * Maximum HR achieved: Sen (95% CI) •>110: 44% (29–57) •>120: 28% (18–40) •>130: 19% (9–29) Spec (95% CI) •>110: 80% (70–88) •>120: 92% (81–98) •>130: 97% (92–99) | * Maximum RR achieved: Sen (95% CI) •>20: 67% (56–77) •>22: 49% (38–60) •>24: 32% (20–43) •>26: 25% (13–37) Spec (95% CI) •>20: 70% (51–80) •>22: 86% (78–92) •>24: 94% (89–97) •>26: 96% (92–99) | Not reported by authors † | Not reported by authors † | Change within case group from admission to 30 min prior to CA: •Heartrate: increased 9.6% (SD = 27) * •Respiratory rate: increased 9.3% (SD = 28) * Other vital signs not reported by authors † |
| Churpek et al. (2012) [30] | Not reported | Minimum achieved: CA group: •>49: 48% ‡ •40–49: 32% ‡ •35–39: 7% ‡ •<35: 14% ‡ Controls: •>49: 76% •40–49: 20% •35–39: 2% •<35: 2% | Not assessed | Maximum achieved: CA group: •<110: 47% ‡ •110–139: 36% ‡ •>139: 17% ‡ Controls: •<110: 76% •110–139: 22% •>139: 2% | Maximum achieved: CA group: •<21: 24% ‡ •21–23: 22% ‡ •24–25: 19% ‡ •26–29: 14% ‡ •>29: 22% ‡ Controls: •<21: 67% •21–23: 18% •24–25: 8% •26–29: 4% •>29: 2% | Not reported | Not reported | Not assessed |
| Rozen et al. (2014) [35] | Lowest achieved: median (IQR) •CA: 97 (80–106) * •Controls: 106 (90–115) | Not assessed | Lowest achieved: median (IQR) •CA: 65 (56–70) * •Controls: 70 (65–80) | Highest achieved: median (IQR) •CA: 110 (90–130) † •Controls: 100 (85–110) | Lowest achieved: median (IQR) •CA: 16 (12–20) * •Controls: 12 (10–16) Highest achieved: median (IQR) •CA: 22 (19–34) * •Controls: 18 (14–20) | Not assessed | Lowest oxygen saturation achieved: not reported by authors † | Not assessed |
| Oh et al. (2016) [34] | Mean value during 24 h before CA (SD) •Case group: 116.61 (15.08) ᵇ •Control 1 group: 104.85 (17.31) •Control 2 group: 132.82 (15.56) Mean value 1 h before the event (SD) •Case group: 75 (38.43) ‡ •Control 1 group: 60 (22.27) •Control 2 group: consistently higher | Mean value during 24 h before CA (SD) •Case group: 62.51 (10.99) ᵇ •Control 1 group: 58.02 (11.06) •Control 2 group: 77.17 (9.59) Mean value 1 h before the event (SD) •Case group: 43 (21.43) ‡ •Control 1 group: 35 (13.89) •Control 2 group: consistently higher | Not assessed | Mean value during 24 h before CA (SD) •Case group: 108.54 (19.98) ᵇ •Control 1 group: 103.63 (22.05) •Control 2 group: 84.33 (15.63) Mean value 1 h before the event (SD) •Case group: 83 (27.88) ‡ •Control 1 group: 62 (34.30) •Control 2 group: consistent, no prominent changes | Mean value during 24 h before CA (SD) •Case group: 24.64 (4.99) ᵇ •Control 1 group: 24.00 (5.36) •Control 2 group: 19.32 (3.76) Common pattern 1 h before CA: •Case group: wide fluctuations until shortly before cardiac arrest •Control 1 group: sudden drops from 2 h before death. •Control 2 group: consistent | Mean value during 24 h before CA (SD) •Case group: 36.25 (5.45) † •Control 1 group: 36.63 (0.62) •Control 2 group: 37.00 (0.39) Mean value 1 h before the event (SD) •Case group: 36 (5.40) ‡ •Control 1 group: maintained •Control 2 group: consistently higher | Not assessed | Not assessed |
| McGrath et al. (2021) [33] | Not assessed | Not assessed | Not assessed | (Pulse rate) Percent difference between the CA group and controls during the same interval: 0–5 min prior to CA: •Mean: 10.8% † •Range: 94.9% * 21–25 min prior to CA: •Mean: 7.6% † •Range: 20.9% † | Not assessed | Not assessed | Percent difference between the PEA group and controls during the same interval: 0–5 min prior to CA: •Mean: −7.2% * •Range: 185.9% * 21–25 min prior to CA: •Mean: −0.7% † •Range: 42.5% † | Percent difference within PEA group, 1 h vs. 7 h prior to CA Pulse rate: •Mean: −5.24% * •Range: −28.31% SatO2: •Mean: 2.7% * •Range: −33% * |
| Study | ECG Reading | Earliest Change | Heart Rate | Arrhythmias | PR Interval | QRS Duration | QRS Morphology | ST Segment | QT Interval | Other Parameters |
|---|---|---|---|---|---|---|---|---|---|---|
| Do et al. (2015) [37] | Manual | >1 h prior to CA for QRS prolongation, atrial tachy-arrhythmias, isorhythmic dissociation, and PR prolongation ≥50% when present | Not evaluated | Bradyarrhythmia: 28% of cases Non-sustained VT: 6% of cases Atrial tachyarrhythmia: 21% of cases (baseline Afib not included) | Shortening: 5% of cases Prolongation: 17% of cases | Prolongation: 19% of cases | Not evaluated | Elevation or depression: 31% of cases | QTc Prolongation: 50% of the VT/VF/TdP arrests Prolongation: 77% of the polymorphic VT/TdP arrests | N/A |
| Attin et al. (2015) [36] | Manual | Median time of QRS morphology changes: 121 min prior CA | •82% had decreased heart rate prior to CA. •The heart rate during the last hour before CA significantly differed from previous hours (p < 0.05). •Mean heart rate decreased by 3 bpm within the last hour (p < 0.01), 5 bpm within 10–5 min prior to CA (p < 0.01), and 7.6 bpm within the last 5 min (p < 0.01). | Observed during periods of decreased heart rate: •Sinus bradycardia: 47% •Junctional rhythm: 28% •Afib: 19% •3rd-degree AV block: 6% | Not evaluated | Prolongation in 51% of cases. More often (p < 0.01) in the last hour vs. 8 h before CA. | QRS morphology changes: •Overall: 54% of cases •Asystole arrests: 80% •PEA arrests: 38% | Elevation: 51% of cases Depression: 59% of cases | Not evaluated | N/A |
| Hu et al. (2013) [32] | Automated | Longest lead time was 20.4 h (SD = 1.6) for QRS width ≥184.8 ms or ≤73.8 ms with a TPR = 9.1% and FPR = 0% | Metric value: •≥149.3 bpm: TPR = 4.6% FPR = 0% •≤38.8 bpm: TPR = 4.6% FPR = 0% Absolute slope of the trending (1-h window) •TPR = 9.1% FPR = 0% | Not evaluated | PRp Metric value: •≥196.6 ms: TPR = 27.3% FPR = 0% Absolute slope of the trending (0.5-h window) •TPR = 13.6% FPR = 0% | Metric value: •≥184.8 ms: TPR = 13.6% FPR = 0% •≤73.8 ms: TPR = 9.1% FPR = 0% Absolute slope of the trending (2-h window) •TPR = 13.6% FPR = 2.3% | Not evaluated | Not evaluated | QTpc Metric value: •≥533.8 ms: TPR = 9.1% FPR = 0% •≤229.9 ms: TPR = 9.1% FPR = 2.3% Absolute slope of the trending (1-h window) • TPR = 13.6% FPR = 2.3% | Metric value: •relJAmp ≥ 20%: TPR = 22.7% FPR = 0% •tAmp ≥ 3.9 au: TPR = 9.1% FPR = 0% •rAmp ≥ 9.3 au: TPR = 4.6% FPR = 2.2% •rAmp ≤ 0.9 au: TPR = 4.6% FPR = 0% •pAmp ≥ 2.7 au: TPR = 4.6% FPR = 2.3% •pAmp ≤ 0.1 au: TPR = 4.6% FPR = 0% Absolute slope of the trending (2-h window): •relJAmp: TPR = 18.2% FPR = 2.3% •tAmp: TPR = 4.6% FPR = 0% •rAmp: TPR = 27.3% FPR = 2.3% |
| Ding et al. (2015) [31] | Automated | Longest lead time was 16.1 h (SD = 8.1) for ST II ≥ 143 μV with a TPR = 7.4% ᵃ | Not evaluated | Not evaluated | Trend duration: •2.8 h: TPR = 48.2% ᵃ Slope: •−8.8 ms/h: TPR = 18.5% ᵃ •17.5 ms/h: TPR = 18.5% ᵃ | Trend duration: •2.7 h: TPR = 40.7% ᵃ Slope: •7.4 ms/h: TPR = 14.8% ᵃ | Not evaluated | Trend duration: •ST I (3.0 h): TPR = 51.9% ᵃ •ST II (3.3 h): TPR = 18.5% ᵃ •ST V (3.1 h): TPR = 33.3% ᵃ Terminal value: •ST I ≤ −56 μV: TPR = 22.2% ᵃ Slope: •ST I = 8.8 ms/h: TPR= 14.8% ᵃ •ST II = −14.6 ms/h: TPR = 14.8% ᵃ •ST II = 37.6 ms/h: TPR = 14.8% ᵃ •ST V = 32.6 ms/h: TPR = 14.8% ᵃ | Trend duration: •3.3 h: TPR = 33.3% ᵃ Terminal value: •≤313 ms: TPR = 11.1% ᵃ •≥492 ms: TPR = 11.1% ᵃ Slope: •−23.5 ms/h: TPR = 11.1% ᵃ •19.4 ms/h: TPR = 25.9% ᵃ | Trend duration: •RR (3.5 h): TPR = 51.9% ᵃ •T Complex (2.9 h): TPR = 40.7% ᵃ •SDNN (3.6 h): TPR = 40.7% ᵃ •SerumK2 (3.2 h): TPR = 29.6% ᵃ •Pdur (2.7 h): TPR = 25.9% ᵃ •HRV (3.0 h): TPR = 25.9% ᵃ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Sario Velasquez, G.D.; Forte, A.J.; McLeod, C.J.; Bruce, C.J.; Pacheco-Spann, L.M.; Maita, K.C.; Avila, F.R.; Torres-Guzman, R.A.; Garcia, J.P.; Borna, S.; et al. Predicting Cardiopulmonary Arrest with Digital Biomarkers: A Systematic Review. J. Clin. Med. 2023, 12, 7430. https://doi.org/10.3390/jcm12237430
De Sario Velasquez GD, Forte AJ, McLeod CJ, Bruce CJ, Pacheco-Spann LM, Maita KC, Avila FR, Torres-Guzman RA, Garcia JP, Borna S, et al. Predicting Cardiopulmonary Arrest with Digital Biomarkers: A Systematic Review. Journal of Clinical Medicine. 2023; 12(23):7430. https://doi.org/10.3390/jcm12237430
Chicago/Turabian StyleDe Sario Velasquez, Gioacchino D., Antonio J. Forte, Christopher J. McLeod, Charles J. Bruce, Laura M. Pacheco-Spann, Karla C. Maita, Francisco R. Avila, Ricardo A. Torres-Guzman, John P. Garcia, Sahar Borna, and et al. 2023. "Predicting Cardiopulmonary Arrest with Digital Biomarkers: A Systematic Review" Journal of Clinical Medicine 12, no. 23: 7430. https://doi.org/10.3390/jcm12237430
APA StyleDe Sario Velasquez, G. D., Forte, A. J., McLeod, C. J., Bruce, C. J., Pacheco-Spann, L. M., Maita, K. C., Avila, F. R., Torres-Guzman, R. A., Garcia, J. P., Borna, S., Felton, C. L., Carter, R. E., & Haider, C. R. (2023). Predicting Cardiopulmonary Arrest with Digital Biomarkers: A Systematic Review. Journal of Clinical Medicine, 12(23), 7430. https://doi.org/10.3390/jcm12237430






