Spinal Involvement in Patients with Chronic Non-Bacterial Osteomyelitis (CNO): An Analysis of Distinctive Imaging Features
Abstract
1. Introduction
2. Materials and Methods
- the number and location of affected vertebrae and continuity or non-continuity of involvement; continuous involvement was defined as encompassing at least two spinal motion segments (i.e., ≥3 vertebrae),
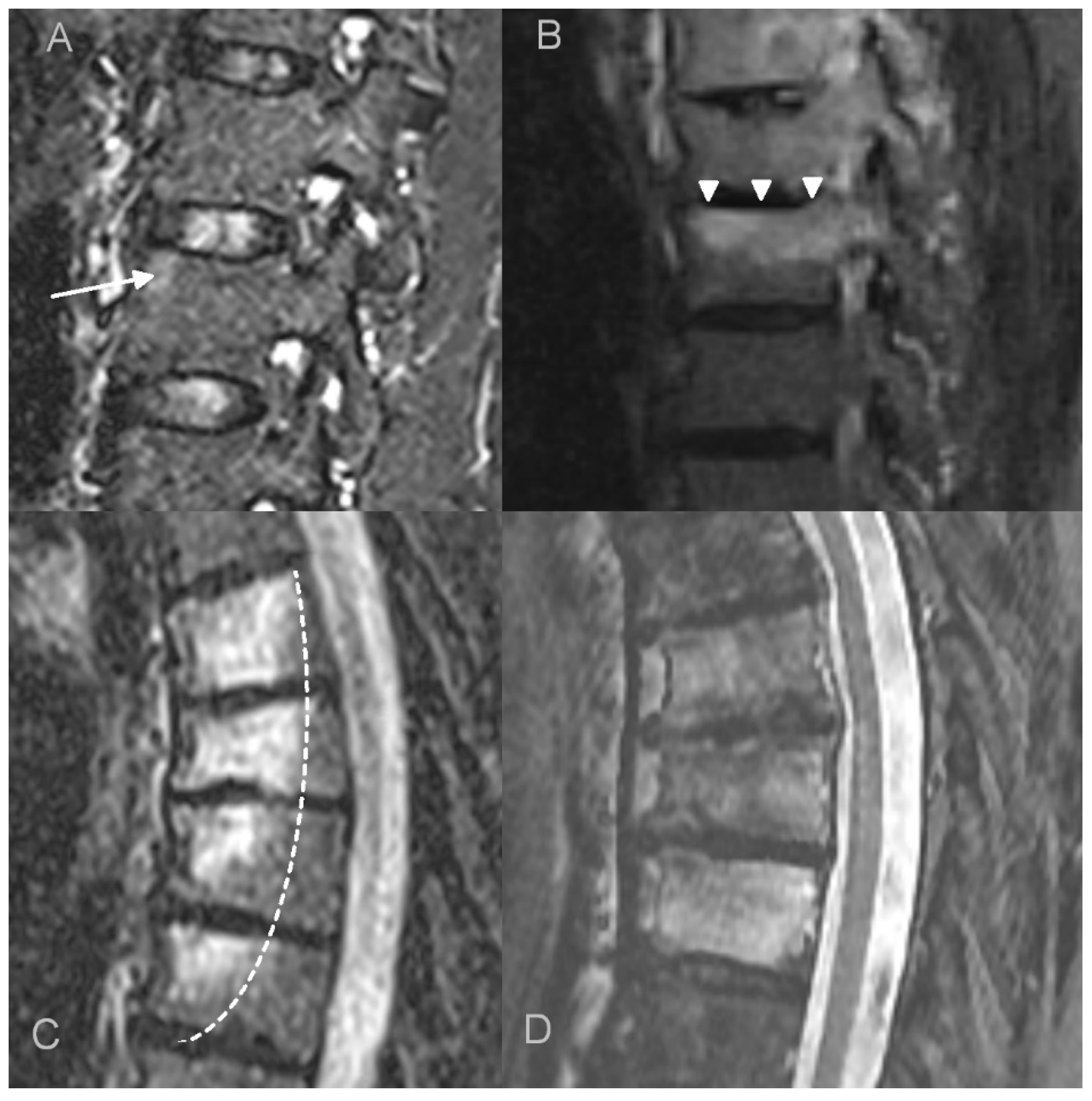
- bone marrow edema (BME) presence and pattern (Figure 1):
- vertebral body corner inflammatory lesions (CIL)—active Romanus lesion defined as small foci of BME at the vertebral corners,
- propagating BME (larger lesions extending along the endplates),
- diffuse solid or patchy BME involving the most of/whole vertebral body,
- the features of intervertebral disk disease:
- e.
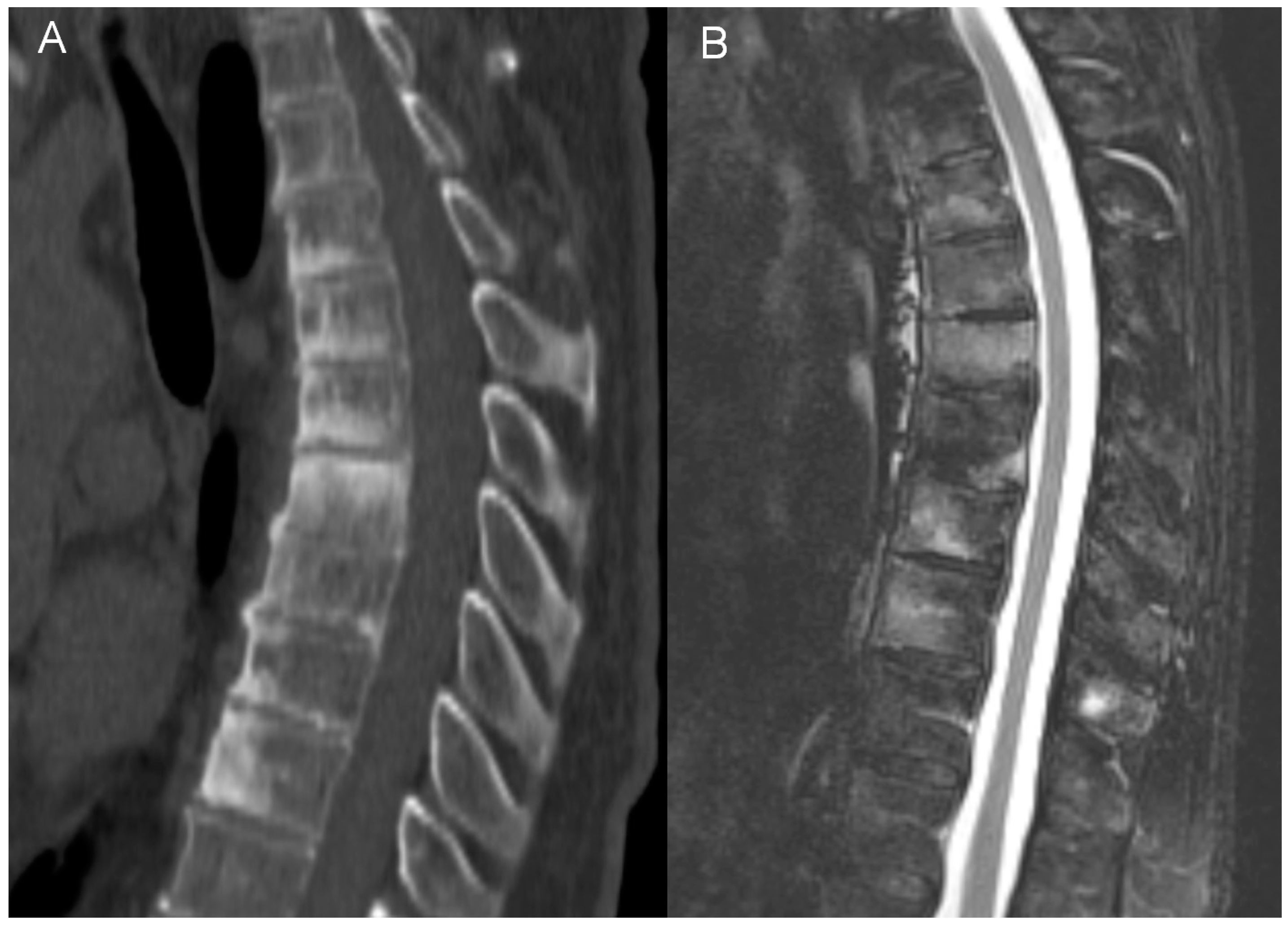
- general features of disc disease on plain films, computed tomography (CT) and magnetic resonance imaging (MRI): disc height reduction, endplate erosions, subchondral sclerosis, and intervertebral fusion,
- f.
- active aseptic spondylodiscitis (inflammatory Anderson lesion) on MRI defined as high disk signal on T2 with or without disc enhancement,
- g.
- unstable Anderson lesion defined as a pseudoarthrosis in an ankylotic spinal region,
- paraspinal soft-tissue abnormalities:
- h.
- the thickness of paravertebral soft tissue inflammation measured on both sagittal and axial T2 fat saturated or T1 fat saturated contrast enhanced images and the thickness of intracanal epidural inflammation measured on sagittal images (Figure 2),
- i.
- presence of fluid collections/abscesses,
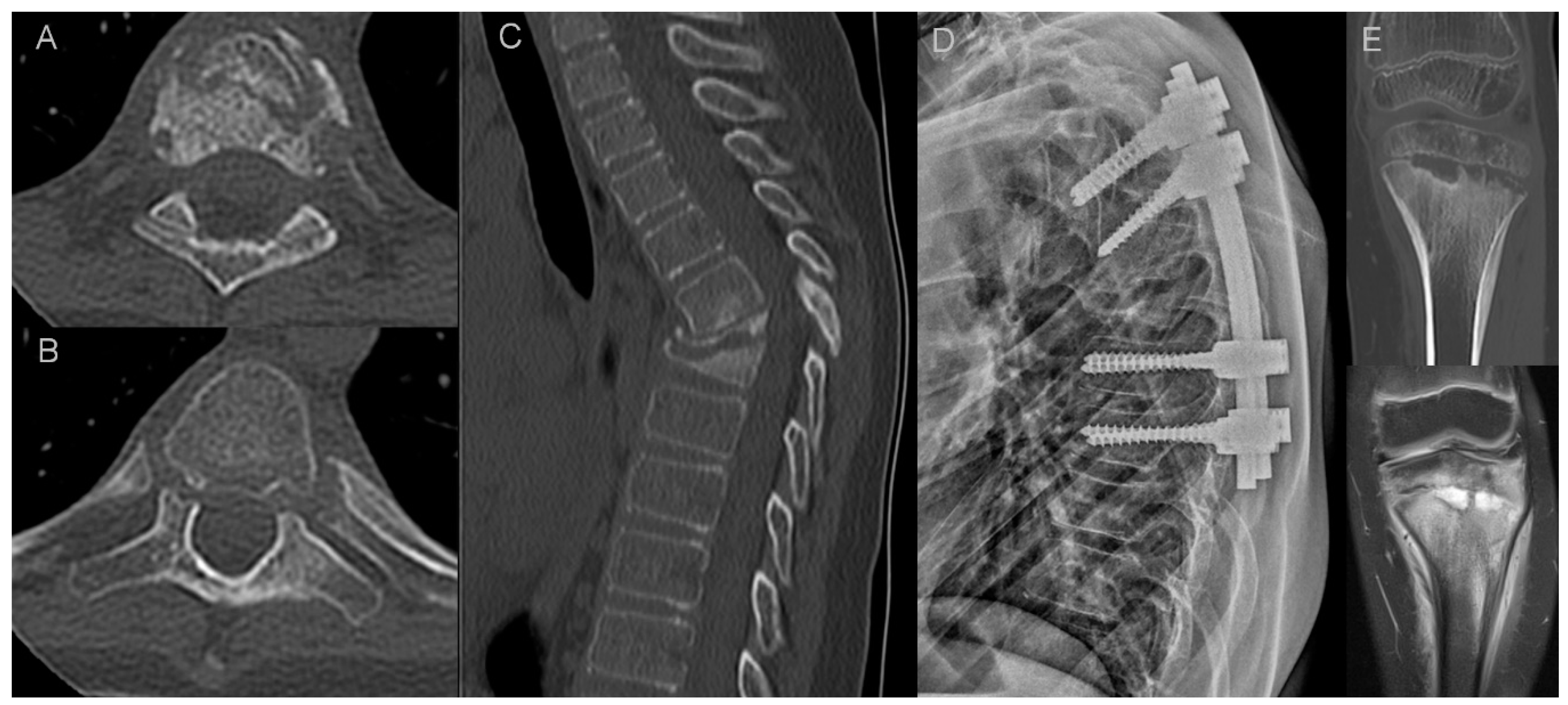
- osteodestructive lesions and secondary vertebral deformities with pathologic fractures defined as >20% vertebral collapse with no history of trauma; smaller deformities were classified either as wedge, biconcave or crush,
- osteosclerotic lesions defined as smaller foci of sclerosis adjacent to vertebral body walls or diffuse sclerosis (ivory vertebra),
- paravertebral ossification: presence of osteophytes (marginal or non-marginal) and syndesmophytes, and number of fused segments,
- facet joint arthropathy,
- costovertebral joint arthropathy.
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATW | anterior thoracic wall |
| AS | ankylosing spondylitis |
| BME | bone marrow edema |
| CNO | chronic non-bacterial osteomyelitis |
| CRMO | chronic recurrent multifocal osteomyelitis |
| CT | computed tomography |
| DISH | diffuse idiopathic skeletal hyperostosis |
| 18F-FDG PET/CT | F-18 fluorodeoxyglucose-positron emission tomography/computed tomography |
| LCH | Langerhans cell histiocytosis |
| MRI | magnetic resonance imaging |
| SAPHO | synovitis, acne, pustulosis, hyperostosis, osteitis |
| SPECT/CT | single-photon emission computed tomography |
| TB | tuberculosis |
| WBBS | whole-body bone scintigraphy |
References
- Buch, K.; Thuesen, A.C.B.; Brøns, C.; Schwarz, P. Chronic Non-bacterial Osteomyelitis: A Review. Calcif. Tissue Int. 2019, 104, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Valkema, P.A.; Luymes, C.H.; Witteveen, J.E.; le Cessie, S.; Appelman-Dijkstra, N.M.; Hogendoorn, P.C.W.; Hamdy, N.A.T. High prevalence of autoimmune disease in the rare inflammatory bone disorder sternocostoclavicular hyperostosis: Survey of a Dutch cohort. Orphanet J. Rare Dis. 2017, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, J.-M.; Corvec, S.; Hayem, G. SAPHO, autophagy, IL-1, FoxO1, and Propionibacterium (Cutibacterium) acnes. Jt. Bone Spine 2018, 85, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Magrey, M.; Khan, M.A. New insights into synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome. Curr. Rheumatol. Rep. 2009, 11, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, H.; Tang, H.; Zhang, Z.; Zou, L.; Yu, H.; Sun, L.; Li, X.; Tang, X.; Lu, M. Clinical characteristics and outcomes of chronic nonbacterial osteomyelitis in children: A multicenter case series. Pediatr. Rheumatol. Online J. 2022, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Van Doornum, S.; Barraclough, D.; McColl, G.; Wicks, I. SAPHO: Rare or just not recognized? Semin. Arthritis Rheum. 2000, 30, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ziegeler, K.; Eshed, I.; Diekhoff, T.; Hermann, K.G. Imaging of Joints and Bones in Autoinflammation. J. Clin. Med. 2020, 9, 4074. [Google Scholar] [CrossRef] [PubMed]
- Beretta-Piccoli, B.C.; Sauvain, M.J.; Gal, I.; Schibler, A.; Saurenmann, T.; Kressebuch, H.; Bianchetti, M.G. Synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome in childhood: A report of ten cases and review of the literature. Eur. J. Pediatr. 2000, 159, 594–601. [Google Scholar] [CrossRef]
- Walsh, P.; Manners, P.J.; Vercoe, J.; Burgner, D.; Murray, K.J. Chronic recurrent multifocal osteomyelitis in children: Nine years’ experience at a statewide tertiary paediatric rheumatology referral centre. Rheumatology 2015, 54, 1688–1691. [Google Scholar] [CrossRef]
- Jansson, A.F.; Grote, V.; ESPED Study Group. Nonbacterial osteitis in children: Data of a German Incidence Surveillance Study. Acta Paediatr. 2011, 100, 1150–1157. [Google Scholar] [CrossRef]
- Liu, S.; Tang, M.; Cao, Y.; Li, C. Synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome: Review and update. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20912865. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, A.; Range, U.; Hahn, G.; Siepmann, T.; Berner, R.; Hedrich, C.M. Unexpectedly high incidences of chronic non-bacterial as compared to bacterial osteomyelitis in children. Rheumatol. Int. 2016, 36, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Jurik, A.G.; Klicman, R.F.; Simoni, P.; Robinson, P.; Teh, J.; Jurik, A.G. SAPHO and CRMO: The Value of Imaging. Semin. Musculoskelet. Radiol. 2018, 22, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Cassar-Pullicino, V.N.; Casale, R.; Magarelli, N.; Semprini, A.; Colosimo, C. The SAPHO syndrome revisited with an emphasis on spinal manifestations. Skelet. Radiol. 2015, 44, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, C.; Xu, W.; Wu, X.; Sun, X.; Zhang, W.; Jing, H.; Gu, Z.; Yuan, S.; Li, L.; et al. Spinal and sacroiliac involvement in SAPHO syndrome: A single center study of a cohort of 354 patients. Semin. Arthritis Rheum. 2019, 48, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Hospach, T.; Langendoerfer, M.; von Kalle, T.; Maier, J.; Dannecker, G.E. Spinal involvement in chronic recurrent multifocal osteomyelitis (CRMO) in childhood and effect of pamidronate. Eur. J. Pediatr. 2010, 169, 1105–1111. [Google Scholar] [CrossRef]
- Singh, A.K.; Ranjan, R.S. SAPHO syndrome: A radiological case report. Indian J. Radiol. Imaging 2020, 30, 84–88. [Google Scholar] [CrossRef]
- Biuden, S.; Maatallah, K.; Riahi, H.; Ferjani, H.; Kaffel, M.D.; Hamdi, W. SAPHO Syndrome Mimicking Infectious Spondylodiscitis and Bone Metastasis. Case Rep. Rheumatol. 2021, 2021, 5577257. [Google Scholar] [CrossRef]
- Roderick, M.R.; Shah, R.; Rogers, V.; Finn, A.; Ramanan, A.V. Chronic recurrent multifocal osteomyelitis (CRMO)—Advancing the diagnosis. Pediatr. Rheumatol. Online J. 2016, 14, 47. [Google Scholar] [CrossRef]
- Kahn, M.-F.; Khan, M.A. The SAPHO syndrome. Baillieres Clin. Rheumatol. 1994, 8, 333–362. [Google Scholar] [CrossRef]
- Peffers, G.; James, S.L.J.; Stirling, A.; Jobanputra, P. Thoracic spine osteitis: A distinct clinical entity, a variant of SAPHO or late-onset non-bacterial osteitis? Rheumatology 2012, 51, 191–193. [Google Scholar] [CrossRef][Green Version]
- McGauvran, A.M.; Kotsenas, A.L.; Diehn, F.E.; Wald, J.T.; Carr, C.M.; Morris, J.M. SAPHO Syndrome: Imaging Findings of Vertebral Involvement. Am. J. Neuroradiol. 2016, 37, 1567–1572. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, S.S.; Lee, Y.-J.; Lee, S.-Y.; Park, N.-H.; Choi, Y.-J.; Chung, E.-C.; Rho, M.-H. Clinical correlation of a new practical MRI method for assessing central lumbar spinal stenosis. Br. J. Radiol. 2013, 86, 20120180. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, J.W.; Koh, Y.H.; Hur, S.; Kim, S.J.; Chai, J.W.; Kang, H.S. New MRI grading system for the cervical canal stenosis. Am. J. Roentgenol. 2011, 197, W134–W140. [Google Scholar] [CrossRef]
- Anderson, S.E.; Heini, P.; Sauvain, M.J.; Stauffer, E.; Geiger, L.; Johnston, J.O.; Roggo, A.; Kalbermatten, D.; Steinbach, L.S. Imaging of chronic recurrent multifocal osteomyelitis of childhood first presenting with isolated primary spinal involvement. Skelet. Radiol. 2003, 32, 328–336. [Google Scholar] [CrossRef]
- Shah, A.; Rosenkranz, M.; Thapa, M. Review of spinal involvement in Chronic recurrent multifocal osteomyelitis (CRMO): What radiologists need to know about CRMO and its imitators. Clin. Imaging 2022, 81, 122–135. [Google Scholar] [CrossRef]
- Principi, N.; Esposito, S. Infectious Discitis and Spondylodiscitis in Children. Int. J. Mol. Sci. 2016, 17, 539. [Google Scholar] [CrossRef]
- Dagirmanjian, A.; Schils, J.; McHenry, M.; Modic, M.T.; Dagirmanjian, J.S.A.; Hanrahan, C.J.; Shah, L.M.; Sharif, H.S.; Smith, A.; Weinstein, M.; et al. MR imaging of vertebral osteomyelitis revisited. Am. J. Roentgenol. 1996, 167, 1539–1543. [Google Scholar] [CrossRef]
- Postini, A.M.; Andreacchio, A.; Boffano, M.; Pagano, M.; Del Prever, A.B.; Fagioli, F. Langerhans cell histiocytosis of bone in children: A long-term retrospective study. J. Pediatr. Orthop. B 2012, 21, 457–462. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Xi, Z.-J. Langerhans cell histiocytosis of bone in children: A clinicopathologic study of 108 cases. World J. Pediatr. 2010, 6, 255–259. [Google Scholar] [CrossRef]
- Baky, F.; Milbrandt, T.A.; Arndt, C.; Houdek, M.T.; Larson, A.N. Vertebra Plana in Children May Result from Etiologies Other Than Eosinophilic Granuloma. Clin. Orthop. Relat. Res. 2020, 478, 2367–2374. [Google Scholar] [CrossRef] [PubMed]
- Nachtigal, A.; Cardinal, E.; Bureau, N.J.; Sainte-Marie, L.G.; Milette, F. Vertebral involvement in SAPHO syndrome: MRI findings. Skelet. Radiol. 1999, 28, 163–168. [Google Scholar] [CrossRef]
- Toussirot, E.; Dupond, J.L.; Wendling, D. Spondylodiscitis in SAPHO syndrome. A series of eight cases. Ann. Rheum. Dis. 1997, 56, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Laredo, J.-D.; Vuillemin-Bodaghi, V.; Boutry, N.; Cotten, A.; Parlier-Cuau, C. SAPHO syndrome: MR appearance of vertebral involvement. Radiology 2007, 242, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Court, C.; Charlez, C.; Molina, V.; Clerc, D.; Miquel, A.; Nordin, J.Y. Isolated thoracic spine lesion: Is this the presentation of a SAPHO syndrome? A case report. Eur. Spine J. 2005, 14, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Colina, M.; Govoni, M.; Orzincolo, C.; Trotta, F. Clinical and radiologic evolution of synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome: A single center study of a cohort of 71 subjects. Arthritis Rheumatol. 2009, 61, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Garcia, A.; Sarria-Estrada, S.; Torrents-Odin, C.; Casas-Gomila, L.; Franquet, E. Imaging findings of Pott’s disease. Eur. Spine J. 2013, 22 (Suppl. S4), 567–578. [Google Scholar] [CrossRef]
- Moorthy, S.; Prabhu, N.K. Spectrum of MR imaging findings in spinal tuberculosis. AJR Am. J. Roentgenol. 2002, 179, 979–983. [Google Scholar] [CrossRef]
- Nakamura, J.-I.; Yamada, K.; Mitsugi, N.; Saito, T. A case of SAPHO syndrome with destructive spondylodiscitis suspicious of tuberculous spondylitis. Mod. Rheumatol. 2010, 20, 93–97. [Google Scholar] [CrossRef]
- Rana, A.; Krishnan, V.; Aggarwal, A. Unusual patterns of tuberculosis on cross-sectional imaging: A pictorial review. Egypt. J. Radiol. Nucl. Med. 2022, 53, 190. [Google Scholar] [CrossRef]
- Shetty, A.; Kanna, R.M.; Rajasekaran, S. TB spine—Current aspects on clinical presentation, diagnosis, and management options. Semin. Spine Surg. 2016, 28, 150–162. [Google Scholar] [CrossRef]
- Hermann, K.-G.A.; Althoff, C.E.; Schneider, U.; Zühlsdorf, S.; Lembcke, A.; Hamm, B.; Bollow, M. Spinal Changes in patients with spondyloarthritis: Comparison of MR imaging and radiographic appearances. Radiographics 2005, 25, 559–569. [Google Scholar] [CrossRef]
- Canella, C.; Schau, B.; Ribeiro, E.; Sbaffi, B.; Marchiori, E. MRI in Seronegative Spondyloarthritis: Imaging Features and Differential Diagnosis in the Spine and Sacroiliac Joints. Am. J. Roentgenol. 2013, 200, 149–157. [Google Scholar] [CrossRef]
- Will, R.; Bhalla, A.; Palmer, R.; Ring, F.; Calin, A. Osteoporosis in early ankylosing spondylitis: A primary pathological event? Lancet 1989, 2, 1483–1485. [Google Scholar] [CrossRef]
- Baek, H.J.; Kang, S.W.; Lee, Y.J.; Shin, K.C.; Lee, E.B.; Yoo, C.D.; Song, Y.W. Osteopenia in men with mild and severe ankylosing spondylitis. Rheumatol. Int. 2005, 26, 30–34. [Google Scholar] [CrossRef]
- Østergaard, M.; Lambert, R.G. Imaging in ankylosing spondylitis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 301–311. [Google Scholar] [CrossRef]
- Jang, J.H.; Ward, M.M.; Rucker, A.N.; Reveille, J.D.; Davis, J.C.; Weisman, M.H.; Learch, T.J. Ankylosing spondylitis: Patterns of radiographic involvement—A re-examination of accepted principles in a cohort of 769 patients. Radiology 2011, 258, 192–198. [Google Scholar] [CrossRef]
- Sudoł-Szopińska, I.; Matuszewska, G.; Pracoń, G. Radiographic Atlas of Inflammatory Rheumatic Diseases; Medisfera: Otwock, Poland, 2022. [Google Scholar]
- Arad, U.; Elkayam, O.; Eshed, I. Magnetic resonance imaging in diffuse idiopathic skeletal hyperostosis: Similarities to axial spondyloarthritis. Clin. Rheumatol. 2017, 36, 559–1549. [Google Scholar] [CrossRef]
- Resnick, D.; Niwayama, G. Radiographic and Pathologic Features of Spinal Involvement in Diffuse Idiopathic Skeletal Hyperostosis (DISH). Radiology 1976, 119, 559–568. [Google Scholar] [CrossRef]
- Sugimoto, H.; Tamura, K.; Fujii, T. The SAPHO syndrome: Defining the radiologic spectrum of diseases comprising the syndrome. Eur. Radiol. 1998, 8, 800–806. [Google Scholar] [CrossRef]
- Rohekar, G.; Inman, R.D. Conundrums in nosology: Synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome and spondylarthritis. Arthritis Rheumatol. 2006, 55, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Earwaker, J.W.S.; Cotten, A. SAPHO: Syndrome or concept? Imaging findings. Skelet. Radiol. 2003, 32, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Depasquale, R.; Kumar, N.; Lalam, R.; Tins, B.; Tyrrell, P.; Singh, J.; Cassar-Pullicino, V. SAPHO: What radiologists should know. Clin. Radiol. 2012, 67, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Jans, L.; Jurik, A.G.; Sudoł-Szopińska, I.; Schueller-Weidekamm, C.; Eshed, I.; Rennie, W.J. Anterior Chest Wall in Axial Spondyloarthritis: Imaging, Interpretation, and Differential Diagnosis. Semin. Musculoskelet. Radiol. 2018, 22, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.J.; Shamsuddin, H. Sternoclavicular Septic Arthritis: Review of 180 cases. Medicine 2004, 83, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S. Sternoclavicular Tuberculosis. Med. J. Armed Forces India 2008, 64, 373–374. [Google Scholar] [CrossRef]
- Sun, X.; Li, C.; Cao, Y.; Shi, X.; Li, L.; Zhang, W.; Wu, X.; Wu, N.; Jing, H.; Zhang, W. F-18 FDG PET/CT in 26 patients with SAPHO syndrome: A new vision of clinical and bone scintigraphy correlation. J. Orthop. Surg. Res. 2018, 13, 120. [Google Scholar] [CrossRef]
- Freyschmidt, J.; Sternberg, A. The bullhead sign: Scintigraphic pattern of sternocostoclavicular hyperostosis and pustulotic arthroosteitis. Eur. Radiol. 1998, 8, 807–812. [Google Scholar] [CrossRef]
- Mettler, F.A., Jr.; Huda, W.; Yoshizumi, T.T.; Mahesh, M. Effective doses in radiology and diagnostic nuclear medicine: A catalog. Radiology 2008, 248, 254–263. [Google Scholar] [CrossRef]
- Andronikou, S.; Kraft, J.K.; Offiah, A.C.; Jones, J.; Douis, H.; Thyagarajan, M.; Barrera, C.A.; Zouvani, A.; Ramanan, A.V. Whole-body MRI in the diagnosis of paediatric CNO/CRMO. Rheumatology 2020, 59, 2671–2680. [Google Scholar] [CrossRef]
- Taddio, A.; Ferrara, G.; Insalaco, A.; Pardeo, M.; Gregori, M.; Finetti, M.; Pastore, S.; Tommasini, A.; Ventura, A.; Gattorno, M. Dealing with Chronic Non-Bacterial Osteomyelitis: A practical approach. Pediatr. Rheumatol. Online J. 2017, 15, 87. [Google Scholar] [CrossRef]
- Arnoldi, A.P.; Schlett, C.L.; Douis, H.; Geyer, L.L.; Voit, A.M.; Bleisteiner, F.; Jansson, A.F.; Weckbach, S. Whole-body MRI in patients with Non-bacterial Osteitis: Radiological findings and correlation with clinical data. Eur. Radiol. 2017, 27, 2391–2399. [Google Scholar] [CrossRef]
- Zhao, Y.; Sato, T.S.; Nielsen, S.M.; Beer, M.; Huang, M.; Iyer, R.S.; McGuire, M.; Ngo, A.-V.; Otjen, J.P.; Panwar, J.; et al. Development of a Scoring Tool for Chronic Nonbacterial Osteomyelitis Magnetic Resonance Imaging and Evaluation of its Interrater Reliability. J. Rheumatol. 2020, 47, 739–747. [Google Scholar] [CrossRef]
- Okuno, H.; Watanuki, M.; Kuwahara, Y.; Sekiguchi, A.; Mori, Y.; Hitachi, S.; Miura, K.; Ogura, K.; Watanabe, M.; Hosaka, M.; et al. Clinical features and radiological findings of 67 patients with SAPHO syndrome. Mod. Rheumatol. 2018, 28, 703–708. [Google Scholar] [CrossRef]
- Bron, J.L.; de Vries, M.K.; Snieders, M.N.; van der Horst-Bruinsma, I.E.; van Royen, B.J. Discovertebral (Andersson) lesions of the spine in ankylosing spondylitis revisited. Clin. Rheumatol. 2009, 28, 883–892. [Google Scholar] [CrossRef]
- Mario, G.; Ch, S.N.; Felice, D.; Laura, G.; Salvatore, C.; Lara, P.; Ligarotti, G.K.; Rossano, A.; Andrea, S. Enhancing contrast agents and radiotracers performance through hyaluronic acid-coating in neuroradiology and nuclear medicine. Hell. J. Nucl. Med. 2017, 20, 166–168. [Google Scholar]
- Placente, D.; Ruso, J.M.; Baldini, M.; Laiuppa, J.A.; Sieben, J.M.; Santillán, G.E.; Messina, P.V. Self-fluorescent antibiotic MoOx–hydroxyapatite: A nano-theranostic platform for bone infection therapies. Nanoscale 2019, 11, 17277–17292. [Google Scholar] [CrossRef] [PubMed]
- Hulsen, D.J.W.; Mitea, C.; Arts, J.J.; Loeffen, D.; Geurts, J. Diagnostic value of hybrid FDG-PET/MR imaging of chronic osteomyelitis. Eur. J. Hybrid Imaging 2022, 6, 15. [Google Scholar] [CrossRef]



| A. Bristol Criteria for the Diagnosis of CRMO | |
| The presence of typical clinical findings (bone pain ± localized swelling without significant local or systemic features of inflammation or infection) | |
| AND | |
| The presence of typical radiological findings (plain X-ray: showing combination of lytic areas, sclerosis and new bone formation, or preferably STIR MRI: showing bone marrow edema ± bone expansion, lytic areas, and periosteal reaction) | |
| AND EITHER | |
| >1 bone (or clavicle alone) without significantly raised CRP (<30 mg/L) | if unifocal disease (other than clavicle), or CRP >30 mg/L, with bone biopsy showing inflammatory changes (plasma cells, osteoclasts, fibrosis, or sclerosis) with no bacterial growth while not on antibiotic therapy |
| B. Kahn Critieria for the diagnosis of SAPHO syndrome (from Kahn; American College of Rheumatology 67th Annual Scientific Meeting, October 2003) | |
| Inclusion criteria | |
| Bone ± joint involvement associated with PPP and psoriasis vulgaris Bone ± joint involvement associated with severe acne Isolated sterile (a) hyperostosis/osteitis (adults) CRMO (children) Bone ± joint involvement associated with chronic bowel diseases | |
| Exclusion | |
| Infectious osteitis Tumoral conditions of bone Non-inflammatory condensing lesions of bone Exception: growth of propionibacterium acnes. | |
| Patient | Sex [M/F] | Age at the Initial Study [Years] | Imaging Studies Available | Biopsy | Spinal Region Involved | Other Sites of Involvement | Follow Up Duration | |||
|---|---|---|---|---|---|---|---|---|---|---|
| X-ray | CT | MRI | PET/Sc | |||||||
| 1. | F | 75 | 0 | 1 | 0 | 0 | - | Th | ATW | n/a |
| 2. | F | 65 | 11 | 1 | 6 | 2 | + (spine) | Th | ATW, SIJ | 5y 10mo 6d |
| 3. | M | 54 | 5 | 0 | 11 | 0 | - | Th, L-S | - | 5y 2mo 16d |
| 4. | F | 35 | 5 | 2 | 2 | 0 | + (spine) | Th | ATW | 3y 4mo 23d |
| 5. | F | 54 | 6 | 4 | 2 | 1 | - | Th | ATW | 15y 11mo 23d |
| 6. | F | 56 | 0 | 4 | 0 | 0 | - | Th | ATW, mandible | 3y 1mo 14d |
| 7. | M | 65 | 0 | 1 | 0 | 0 | - | Th | ATW | n/a |
| 8. | F | 68 | 0 | 1 | 0 | 0 | - | Th | ATW | n/a |
| 9. | F | 71 | 0 | 1 | 0 | 0 | - | Th | ATW | n/a |
| 10. | M | 62 | 3 | 1 | 1 | 1 | + (sternum) | C, Th | ATW | n/a |
| 11. | F | 59 | 6 | 2 | 5 | 1 | + (spine) | Th | ATW | 3y 4mo 14d |
| 12. | F | 63 | 3 | 2 | 1 | 2 | - | Th | ATW | 4mo 14d |
| 13. | F | 39 | 3 | 1 | 2 | 1 | - | L-S | - | 1mo 7d |
| 14. | M | 61 | 4 | 2 | 6 | 2 | - | Th, L-S | ATW | 1y 5mo 16d |
| 15. | F | 26 | 4 | 0 | 4 | 0 | - | Th | ATW, SIJ | n/a |
| 16. | M | 10 | 18 | 4 | 8 | 1 | + (spine) | Th | tibia | 8mo 14d |
| 17. | F | 11 | 7 | 1 | 6 * | 1 | + (spine) | Th, L-S | femur, fibulae, ATW | 1l 3m 21d |
| Pattern of BME | Patients (Number of Vertebrae per Patient) | ||
|---|---|---|---|
| All | Children | Adults | |
| corner lesion | 5 (3) | 1 (1) | 4 (3.5) |
| propagating | 6 (2) | 2 (1) | 4 (2.5) |
| semicircular | 3 (5.6) | 0 | 3 (5.6) |
| diffuse | 8 (2.5) | 2 (2) | 6 (2.6) |
| Osteosclerosis | BME | Facet Joints and Costovertebral Joints | Paraspinal Inflammation | Abscess | |
|---|---|---|---|---|---|
| CNO | Extensive | Extensive | + | + * | - |
| Infectious spondylitis | Variable | Extensive | + | + | + |
| AS | Limited to tiny corner lesions | Small to moderate | + | - | - |
| DISH | None | None to small | - | - | - |
| Malignancy | Possible | Rare | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byrdy-Daca, M.; Duczkowski, M.; Sudoł-Szopińska, I.; Żelewska, M.; Piłat, K.; Daca, F.; Nieciecki, M.; Sztwiertnia, P.; Walecki, J.; Cieszanowski, A.; et al. Spinal Involvement in Patients with Chronic Non-Bacterial Osteomyelitis (CNO): An Analysis of Distinctive Imaging Features. J. Clin. Med. 2023, 12, 7419. https://doi.org/10.3390/jcm12237419
Byrdy-Daca M, Duczkowski M, Sudoł-Szopińska I, Żelewska M, Piłat K, Daca F, Nieciecki M, Sztwiertnia P, Walecki J, Cieszanowski A, et al. Spinal Involvement in Patients with Chronic Non-Bacterial Osteomyelitis (CNO): An Analysis of Distinctive Imaging Features. Journal of Clinical Medicine. 2023; 12(23):7419. https://doi.org/10.3390/jcm12237419
Chicago/Turabian StyleByrdy-Daca, Marta, Marek Duczkowski, Iwona Sudoł-Szopińska, Marta Żelewska, Krzysztof Piłat, Filip Daca, Michał Nieciecki, Paweł Sztwiertnia, Jerzy Walecki, Andrzej Cieszanowski, and et al. 2023. "Spinal Involvement in Patients with Chronic Non-Bacterial Osteomyelitis (CNO): An Analysis of Distinctive Imaging Features" Journal of Clinical Medicine 12, no. 23: 7419. https://doi.org/10.3390/jcm12237419
APA StyleByrdy-Daca, M., Duczkowski, M., Sudoł-Szopińska, I., Żelewska, M., Piłat, K., Daca, F., Nieciecki, M., Sztwiertnia, P., Walecki, J., Cieszanowski, A., Świątkowski, J., Bereźniak, M., Sułkowska, K., Czubak, J., Gołębiowski, M., & Palczewski, P. (2023). Spinal Involvement in Patients with Chronic Non-Bacterial Osteomyelitis (CNO): An Analysis of Distinctive Imaging Features. Journal of Clinical Medicine, 12(23), 7419. https://doi.org/10.3390/jcm12237419







