How Imaging Techniques Improve Ventricular Arrhythmia Ablation: A Multimodality-Based Approach
Abstract
:1. Introduction
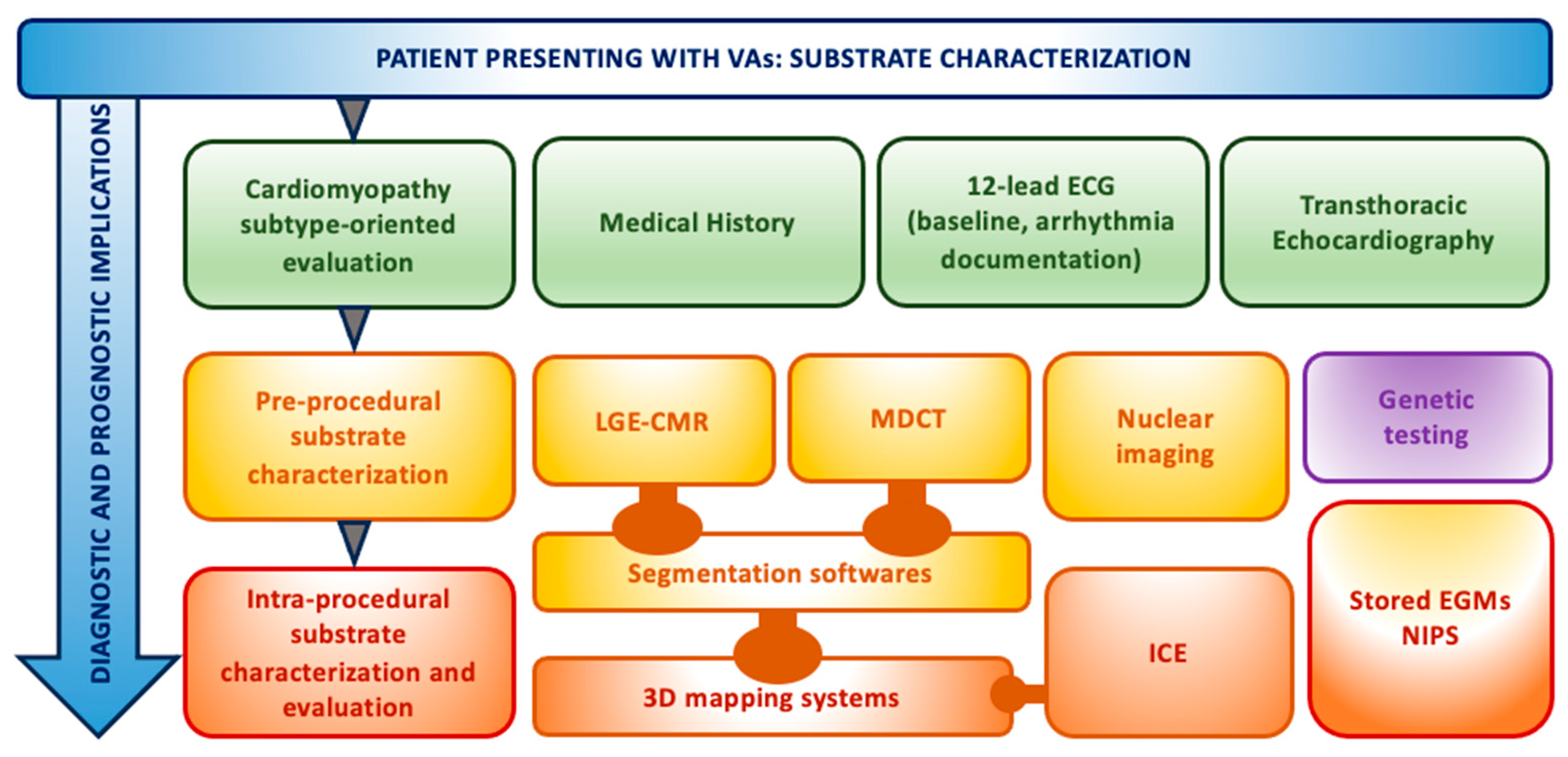
2. Pre-Procedural Assessment
2.1. Transthoracic Echocardiography
2.2. Cardiac Magnetic Resonance
2.3. Cardiac Computed Tomography
2.4. Segmentation Software
2.5. Nuclear Imaging
3. Intra-Procedural Assessment
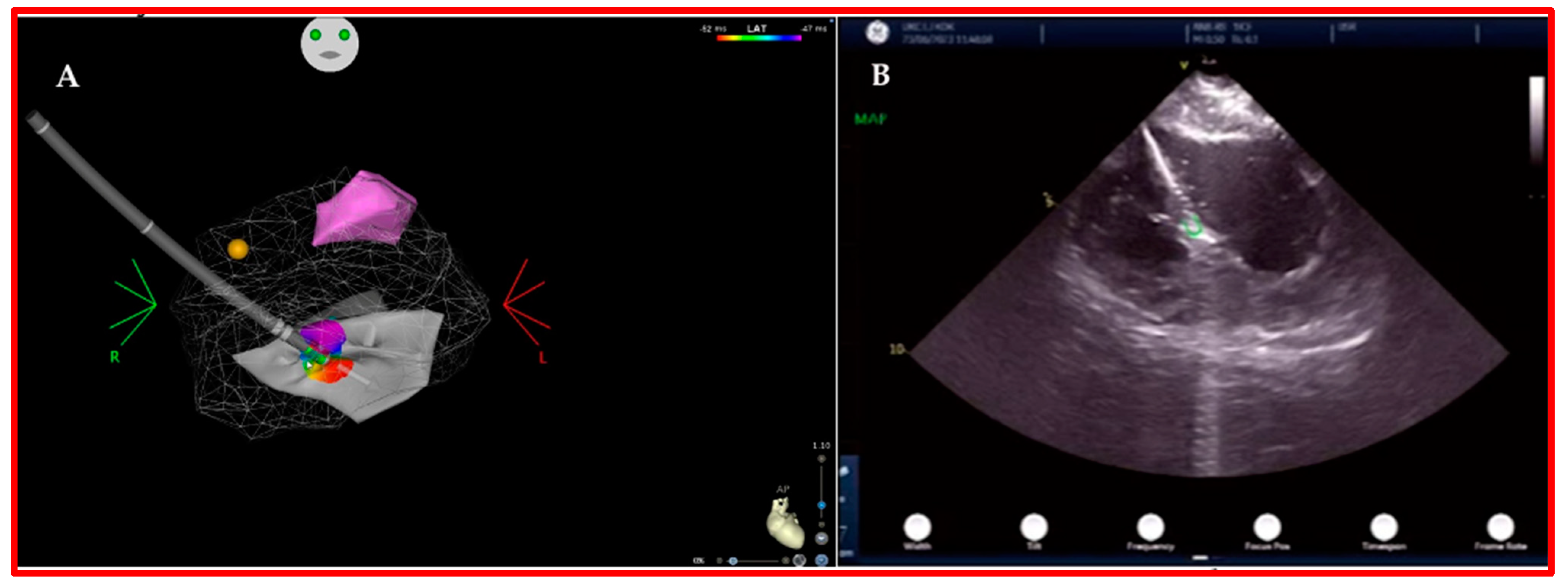
3.1. Intracardiac Echography
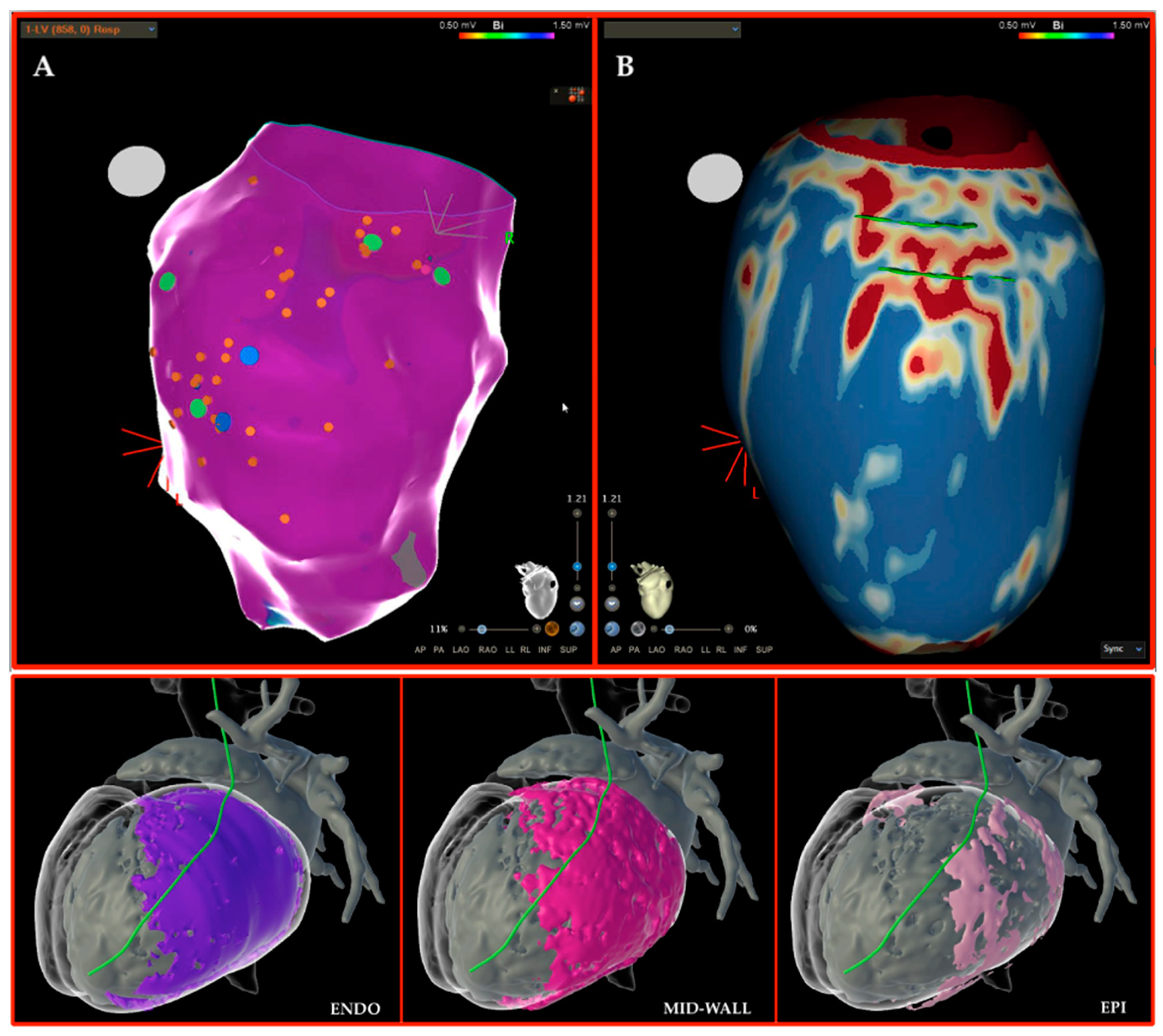
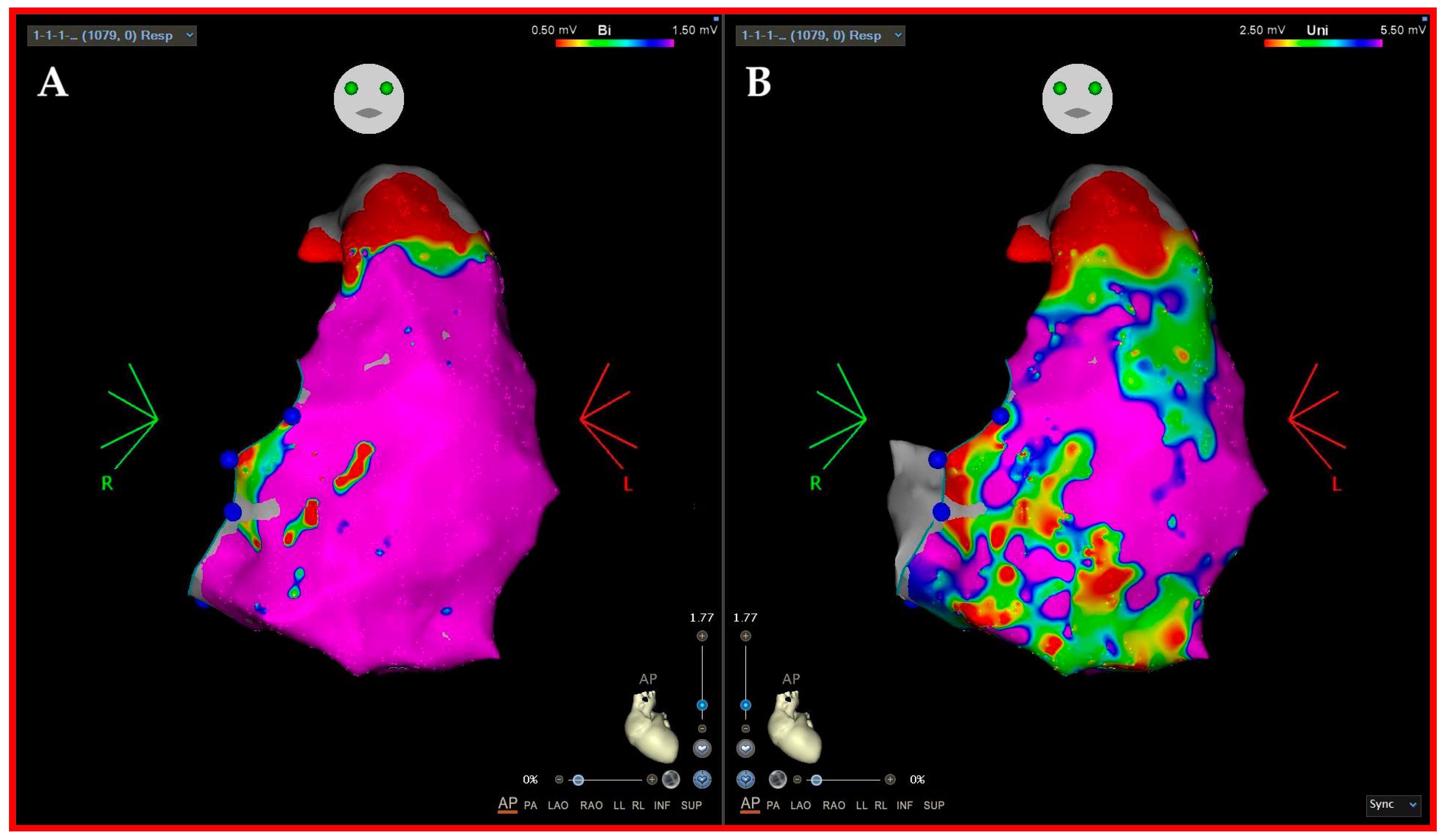
3.2. Three-Dimensional (3D)-Mapping Systems
4. Our Experience and Workflow
- A detailed substrate high-density mapping in sinus or paced rhythm (voltage, LAVA, LPs, DeEP mapping) performed by a multipolar-mapping catheter;
- Induction of VT;
- Pace mapping to find the site of interest according to the clinical or induced VT if it is not re-inducible or not hemodynamically tolerated;
- In case of hemodynamically tolerated VT, we perform activation mapping to define the reentry circuit with areas of slow conduction coupled with entrainment mapping;
- Ablation of LAVA/LP/DeEP at the site of interest in non-inducible or untolerated VTs or the slow conducting critical isthmus in tolerated VTs;
- Complete substrate modification (elimination of all LAVA/LP/DeEP);
- Re-mapping the ablated areas with a multipolar high-density mapping catheter;
- Testing for final VT non-inducibility with programmed ventricular stimulation with up to four extrastimuli from two different sites with one of those close to the ablated low-voltage area. Isoproterenol administration during programmed ventricular stimulation depends on patient characteristics and operator’s preference.
5. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-Dimensional |
| 4D | Four-Dimensional |
| ARVC | Arrhythmogenic Right Ventricular Cardiomyopathy |
| BrS | Brugada Syndrome |
| CIED | Cardiac Implantable Electronic Device |
| CMR | Cardiac Magnetic Resonance |
| CPVT | Catecholaminergic Polymorphic Ventricular Tachycardia |
| CT | Computed Tomography |
| DCM | Dilated Cardiomyopathy |
| DeEP | Delayed Evoked Potential |
| EAM | Electro-Anatomical Mapping |
| ECG | Electrocardiogram |
| ECGi | Electrocardiographic Imaging |
| ECMO | Extra-Corporeal Membrane Oxygenation |
| EGM | Intracavitary Electrogram |
| ERS | Early Repolarization Syndrome |
| HCM | Hypertrophic Cardiomyopathy |
| ICD | Implantable Cardioverter Defibrillator |
| ICE | Intracardiac Echography |
| ICM | Ischemic Cardiomyopathy |
| IHD | Ischemic Heart Disease |
| ILAM | Isochronal Latest Activation Mapping |
| LAVA | Local Abnormal Ventricular Activity |
| LGE-CMR | Late Gadolinium Enhancement Cardiac Magnetic Resonance |
| LP | Late Potential |
| LQTS | Long QT Syndrome |
| LV | Left Ventricle |
| LVOT | Left Ventricular Outflow Tract |
| MAD | Mitral Annular Disjunction |
| MDCT | Multi-Detector Computed Tomography |
| NDLVC | Non-Dilated Left Ventricular Cardiomyopathy |
| NICM | Non-Ischemic Cardiomyopathy |
| NIHD | Non-Ischemic Heart Disease |
| NIPS | Non-Invasive Programmed Stimulation |
| NSVT | Non-Sustained Ventricular Tachycardia |
| PCCT | Photon-Counting Computed Tomography |
| PET | Positron Emission Tomography |
| PFA | Pulsed-Field Ablation |
| PM | Papillary Muscle |
| PVC | Premature Ventricular Complex |
| RCM | Restrictive Cardiomyopathy |
| RF | Radiofrequency |
| RV | Right Ventricle |
| RVOT | Right Ventricular Outflow Tract |
| SCD | Sudden Cardiac Death |
| SHD | Structural Heart Disease |
| SPECT | Single-Photon Emission Computed Tomography |
| SQTS | Short QT Syndrome |
| TEE | Transesophageal Echocardiography |
| TTE | Transthoracic Echocardiography |
| VA | Ventricular Arrhythmia |
| VT | Ventricular Tachycardia |
References
- Della Bella, P.; Baratto, F.; Vergara, P.; Bertocchi, P.; Santamaria, M.; Notarstefano, P.; Calò, L.; Orsida, D.; Tomasi, L.; Piacenti, M.; et al. Does Timing of Ventricular Tachycardia Ablation Affect Prognosis in Patients with an Implantable Cardioverter Defibrillator? Results From the Multicenter Randomized PARTITA Trial. Circulation 2022, 145, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Tung, R.; Xue, Y.; Chen, M.; Jiang, C.; Shatz, D.Y.; Besser, S.A.; Hu, H.; Chung, F.-P.; Nakahara, S.; Kim, Y.-H.; et al. First-Line Catheter Ablation of Monomorphic Ventricular Tachycardia in Cardiomyopathy Concurrent with Defibrillator Implantation: The PAUSE-SCD Randomized Trial. Circulation 2022, 145, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Daubert, J.P.; Anstrom, K.J.; Daoud, E.G.; Gonzalez, M.; Saba, S.; Jackson, K.P.; Reece, T.; Gu, J.; Pokorney, S.D.; et al. Catheter ablation for ventricular tachycardia in patients with an implantable cardioverter defibrillator (CALYPSO) pilot trial. J. Cardiovasc. Electrophysiol. 2015, 26, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Cronin, E.M.; Bogun, F.M.; Maury, P.; Peichl, P.; Chen, M.; Namboodiri, N.; Aguinaga, L.; Leite, L.R.; Al-Khatib, S.M.; Anter, E.; et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias: Executive summary. EP Eur. 2020, 22, 450–495. [Google Scholar]
- Sapp, J.L.; Wells, G.A.; Parkash, R.; Stevenson, W.G.; Blier, L.; Sarrazin, J.-F.; Thibault, B.; Rivard, L.; Gula, L.; Leong-Sit, P.; et al. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N. Engl. J. Med. 2016, 375, 111–121. [Google Scholar] [CrossRef]
- Muser, D.; Tritto, M.; Mariani, M.V.; Di Monaco, A.; Compagnucci, P.; Accogli, M.; De Ponti, R.; Guarracini, F. Diagnosis and Treatment of Idiopathic Premature Ventricular Contractions: A Stepwise Approach Based on the Site of Origin. Diagnostics 2021, 11, 1840. [Google Scholar] [CrossRef]
- Muser, D.; Santangeli, P. Epicardial Ablation of Idiopathic Ventricular Tachycardia. Card. Electrophysiol. Clin. 2020, 12, 295–312. [Google Scholar] [CrossRef]
- Sadek, M.M.; Muser, D.; Santangeli, P.; Marchlinski, F.E. Epicardial Ablation in Nonischemic Ventricular Tachyardia. Card. Electrophysiol. Clin. 2020, 12, 321–328. [Google Scholar] [CrossRef]
- Robles, A.G.; Palamà, Z.; Nesti, M.; Tunzi, R.M.; Delise, P.; Cavarretta, E.; Penco, M.; Romano, S.; Sciarra, L. Sport Related Sudden Death: The Importance of Primary and Secondary Prevention. J. Clin. Med. 2022, 11, 4683. [Google Scholar] [CrossRef]
- Rudy, Y. Noninvasive electrocardiographic imaging of arrhythmogenic substrates in humans. Circ. Res. 2013, 112, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Sapp, J.L.; Dawoud, F.; Clements, J.C.; Horácek, B.M. Inverse solution mapping of epicardial potentials: Quantitative comparison with epicardial contact mapping. Circ. Arrhythm. Electrophysiol. 2012, 5, 1001–1009. [Google Scholar] [CrossRef]
- Varma, N.; Strom, M.; Chung, M.K. Noninvasive voltage and activation mapping of ARVD/C using ECG imaging. JACC Cardiovasc. Imaging 2013, 6, 1346–1347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cuculich, P.S.; Zhang, J.; Desouza, K.A.; Vijayakumar, R.; Chen, J.; Faddis, M.N.; Lindsay, B.D.; Smith, T.W.; Rudy, Y. Noninvasive electroanatomic mapping of human ventricular arrhythmias with electrocardiographic imaging. Sci. Transl. Med. 2011, 3, 98ra84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cooper, D.H.; Desouza, K.A.; Cuculich, P.S.; Woodard, P.K.; Smith, T.W.; Rudy, Y. Electrophysiologic Scar Substrate in Relation to VT: Noninvasive High-Resolution Mapping and Risk Assessment with ECGI. Pacing Clin. Electrophysiol. PACE 2016, 39, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Gigli, L.; Della Bella, P. Catheter ablation of ventricular tachycardia in nonischemic cardiomyopathy. J. Arrhythmia 2018, 34, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, A.; Hartung, W.; Geller, C.; Klein, H. Clinical relevance of stored electrograms for implantable cardioverter-defibrillator (ICD) troubleshooting and understanding of mechanisms for ventricular tachyarrhythmias. Am. J. Cardiol. 1996, 78, 33–41. [Google Scholar] [CrossRef]
- Yoshida, K.; Liu, T.-Y.; Scott, C.; Hero, A.; Yokokawa, M.; Gupta, S.; Good, E.; Morady, F.; Bogun, F. The value of defibrillator electrograms for recognition of clinical ventricular tachycardias and for pace mapping of post-infarction ventricular tachycardia. J. Am. Coll. Cardiol. 2010, 56, 969–979. [Google Scholar] [CrossRef]
- Kodali, S.; Shirai, Y.; Muser, D.; Callans, D.J.; Marchlinski, F.E.; Santangeli, P. Ventricular tachycardia in nonischemic cardiomyopathy: Anteroseptal vs. inferolateral origin based on ICD ventricular electrogram timing. J. Cardiovasc. Electrophysiol. 2019, 30, 2334–2343. [Google Scholar] [CrossRef]
- Mansencal, N.; Abi Nasr, I.; Pillière, R.; Farcot, J.C.; Joseph, T.; Lacombe, P.; Dubourg, O. Usefulness of contrast echocardiography for assessment of left ventricular thrombus after acute myocardial infarction. Am. J. Cardiol. 2007, 99, 1667–1670. [Google Scholar] [CrossRef]
- Thanigaraj, S.; Schechtman, K.B.; Pérez, J.E. Improved echocardiographic delineation of left ventricular thrombus with the use of intravenous second-generation contrast image enhancement. J. Am. Soc. Echocardiogr. 1999, 12, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Ederhy, S.; Mansencal, N.; Réant, P.; Piriou, N.; Barone-Rochette, G. Role of multimodality imaging in the diagnosis and management of cardiomyopathies. Arch. Cardiovasc. Dis. 2019, 112, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, S.; Leo, I.; Bianco, F.; Borrelli, N.; Beltrami, M.; Garofalo, M.; Giulia Milano, E.; Bisaccia, G.; Iellamo, F.; Paolo Bassareo, P.; et al. The Role of Multimodality Imaging in Pediatric Cardiomyopathies. J. Clin. Med. 2023, 12, 4866. [Google Scholar] [CrossRef] [PubMed]
- Muser, D.; Santangeli, P.; Castro, S.A.; Pathak, R.K.; Liang, J.J.; Hayashi, T.; Magnani, S.; Garcia, F.C.; Hutchinson, M.D.; Frankel, D.S.; et al. Long-Term Outcome after Catheter Ablation of Ventricular Tachycardia in Patients with Nonischemic Dilated Cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2016, 9, e004328. [Google Scholar] [CrossRef] [PubMed]
- Vaseghi, M.; Hu, T.Y.; Tung, R.; Vergara, P.; Frankel, D.S.; Di Biase, L.; Tedrow, U.B.; Gornbein, J.A.; Yu, R.; Mathuria, N.; et al. Outcomes of Catheter Ablation of Ventricular Tachycardia Based on Etiology in Nonischemic Heart Disease: An International Ventricular Tachycardia Ablation Center Collaborative Study. JACC Clin. Electrophysiol. 2018, 4, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, L.; Robles, A.G.; Costantino, R.; Forte, V.; Zingaro, M.; Rosa, I.; Guaricci, A.I.; Romano, S.; Sciarra, L.; Bartolomucci, F.; et al. How many clues make an evidence? An unusual case of aborted cardiac arrest due to mitral valve prolapse. Future Cardiol. 2023, 19, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Sabbag, A.; Essayagh, B.; Barrera, J.D.R.; Basso, C.; Berni, A.; Cosyns, B.; Deharo, J.-C.; Deneke, T.; Di Biase, L.; Enriquez-Sarano, M.; et al. EHRA expert consensus statement on arrhythmic mitral valve prolapse and mitral annular disjunction complex in collaboration with the ESC Council on valvular heart disease and the European Association of Cardiovascular Imaging endorsed cby the Heart Rhythm Society, by the Asia Pacific Heart Rhythm Society, and by the Latin American Heart Rhythm Society. Europace 2022, 24, 1981–2003. [Google Scholar] [CrossRef]
- Muser, D.; Liang, J.J.; Castro, S.A.; Hayashi, T.; Enriquez, A.; Troutman, G.S.; McNaughton, N.W.; Supple, G.; Birati, E.Y.; Schaller, R.; et al. Outcomes with prophylactic use of percutaneous left ventricular assist devices in high-risk patients undergoing catheter ablation of scar-related ventricular tachycardia: A propensity-score matched analysis. Heart Rhythm. 2018, 15, 1500–1506. [Google Scholar] [CrossRef]
- Zeppenfeld, K. Ventricular Tachycardia Ablation in Nonischemic Cardiomyopathy. JACC Clin. Electrophysiol. 2018, 4, 1123–1140. [Google Scholar] [CrossRef]
- Hasdemir, C.; Yuksel, A.; Camli, D.; Kartal, Y.; Simsek, E.; Musayev, O.; Isayev, E.; Aydin, M.; Can, L.H. Late gadolinium enhancement CMR in patients with tachycardia-induced cardiomyopathy caused by idiopathic ventricular arrhythmias. Pacing Clin. Electrophysiol. PACE 2012, 35, 465–470. [Google Scholar] [CrossRef]
- Andreu, D.; Penela, D.; Acosta, J.; Fernández-Armenta, J.; Perea, R.J.; Soto-Iglesias, D.; de Caralt, T.M.; Ortiz-Perez, J.T.; Prat-González, S.; Borràs, R.; et al. Cardiac magnetic resonance-aided scar dechanneling: Influence on acute and long-term outcomes. Heart Rhythm. 2017, 14, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Andreu, D.; Ortiz-Pérez, J.T.; Boussy, T.; Fernández-Armenta, J.; de Caralt, T.M.; Perea, R.J.; Prat-González, S.; Mont, L.; Brugada, J.; Berruezo, A. Usefulness of contrast-enhanced cardiac magnetic resonance in identifying the ventricular arrhythmia substrate and the approach needed for ablation. Eur. Heart J. 2014, 35, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Siontis, K.C.; Kim, H.M.; Dabbagh, G.S.; Latchamsetty, R.; Stojanovska, J.; Jongnarangsin, K.; Morady, F.; Bogun, F.M. Association of preprocedural cardiac magnetic resonance imaging with outcomes of ventricular tachycardia ablation in patients with idiopathic dilated cardiomyopathy. Heart Rhythm. 2017, 14, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Soto-Iglesias, D.; Acosta, J.; Penela, D.; Fernández-Armenta, J.; Cabrera, M.; Martínez, M.; Vassanelli, F.; Alcaine, A.; Linhart, M.; Jáuregui, B.; et al. Image-based criteria to identify the presence of epicardial arrhythmogenic substrate in patients with transmural myocardial infarction. Heart Rhythm. 2018, 15, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Zghaib, T.; Ipek, E.G.; Hansford, R.; Ashikaga, H.; Berger, R.D.; Marine, J.E.; Spragg, D.D.; Tandri, H.; Zimmerman, S.L.; Halperin, H.; et al. Standard Ablation Versus Magnetic Resonance Imaging-Guided Ablation in the Treatment of Ventricular Tachycardia. Circ. Arrhythm. Electrophysiol. 2018, 11, e005973. [Google Scholar] [CrossRef]
- Srichai, M.B.; Junor, C.; Rodriguez, L.L.; Stillman, A.E.; Grimm, R.A.; Lieber, M.L.; Weaver, J.A.; Smedira, N.G.; WhiteClinical, R.D. imaging, and pathological characteristics of left ventricular thrombus: A comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am. Heart J. 2006, 152, 75–84. [Google Scholar] [CrossRef]
- Weinsaft, J.W.; Kim, R.J.; Ross, M.; Krauser, D.; Manoushagian, S.; LaBounty, T.M.; Cham, M.D.; Min, J.K.; Healy, K.; Wang, Y.; et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc. Imaging 2009, 2, 969–979. [Google Scholar] [CrossRef]
- Weinsaft, J.W.; Kim, H.W.; Shah, D.J.; Klem, I.; Crowley, A.L.; Brosnan, R.; James, O.G.; Patel, M.R.; Heitner, J.; Parker, M.; et al. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J. Am. Coll. Cardiol. 2008, 52, 148–157. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Casella, M.; Bergonti, M.; Narducci, M.L.; Persampieri, S.; Gasperetti, A.; Conte, E.; Catto, V.; Carbucicchio, C.; Guerra, F.; Pontone, G.; et al. Prior myocarditis and ventricular arrhythmias: The importance of scar pattern. Heart Rhythm. 2021, 18, 589–596. [Google Scholar] [CrossRef]
- Augusto, J.B.; Eiros, R.; Nakou, E.; Moura-Ferreira, S.; Treibel, T.A.; Captur, G.; Akhtar, M.M.; Protonotarios, A.; Gossios, T.D.; Savvatis, K.; et al. Dilated cardiomyopathy and arrhythmogenic left ventricular cardiomyopathy: A comprehensive genotype-imaging phenotype study. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Schelbert, E.B.; Messroghli, D.R. State of the Art: Clinical Applications of Cardiac T1 Mapping. Radiology 2016, 278, 658–676. [Google Scholar] [CrossRef] [PubMed]
- Roca-Luque, I.; Van Breukelen, A.; Alarcon, F.; Garre, P.; Tolosana, J.M.; Borras, R.; Sanchez, P.; Zaraket, F.; Doltra, A.; Ortiz-Perez, J.T.; et al. Ventricular scar channel entrances identified by new wideband cardiac magnetic resonance sequence to guide ventricular tachycardia ablation in patients with cardiac defibrillators. Europace 2020, 22, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, Y.; Cochet, H.; Jadidi, A.; Sacher, F.; Shah, A.; Derval, N.; Scherr, D.; Pascale, P.; Roten, L.; Denis, A.; et al. Regional myocardial wall thinning at multidetector computed tomography correlates to arrhythmogenic substrate in postinfarction ventricular tachycardia: Assessment of structural and electrical substrate. Circ. Arrhythm. Electrophysiol. 2013, 6, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Palmisano, A.; Antunes, S.; Maccabelli, G.; Colantoni, C.; Rancoita, P.M.V.; Baratto, F.; Di Serio, C.; Rizzo, G.; De Cobelli, F.; et al. Cardiac CT with Delayed Enhancement in the Characterization of Ventricular Tachycardia Structural Substrate: Relationship between CT-Segmented Scar and Electro-Anatomic Mapping. JACC Cardiovasc. Imaging 2016, 9, 822–832. [Google Scholar] [CrossRef]
- Tian, J.; Jeudy, J.; Smith, M.F.; Jimenez, A.; Yin, X.; Bruce, P.A.; Lei, P.; Turgeman, A.; Abbo, A.; Shekhar, R.; et al. Three-dimensional contrast-enhanced multidetector CT for anatomic, dynamic, and perfusion characterization of abnormal myocardium to guide ventricular tachycardia ablations. Circ. Arrhythm. Electrophysiol. 2010, 3, 496–504. [Google Scholar] [CrossRef]
- Ghannam, M.; Cochet, H.; Jais, P.; Sermesant, M.; Patel, S.; Siontis, K.C.; Morady, F.; Bogun, F. Correlation between computer tomography-derived scar topography and critical ablation sites in postinfarction ventricular tachycardia. J. Cardiovasc. Electrophysiol. 2018, 29, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Sacher, F.; Mahida, S.; Berte, B.; Lim, H.S.; Komatsu, Y.; Amraoui, S.; Denis, A.; Derval, N.; Laurent, F.; et al. Image Integration to Guide Catheter Ablation in Scar-Related Ventricular Tachycardia. J. Cardiovasc. Electrophysiol. 2016, 27, 699–708. [Google Scholar] [CrossRef]
- Cochet, H.; Komatsu, Y.; Sacher, F.; Jadidi, A.S.; Scherr, D.; Riffaud, M.; Derval, N.; Shah, A.; Roten, L.; Pascale, P.; et al. Integration of merged delayed-enhanced magnetic resonance imaging and multidetector computed tomography for the guidance of ventricular tachycardia ablation: A pilot study. J. Cardiovasc. Electrophysiol. 2013, 24, 419–426. [Google Scholar] [CrossRef]
- Desjardins, B.; Morady, F.; Bogun, F. Effect of epicardial fat on electroanatomical mapping and epicardial catheter ablation. J. Am. Coll. Cardiol. 2010, 56, 1320–1327. [Google Scholar] [CrossRef]
- Yamashita, S.; Sacher, F.; Mahida, S.; Berte, B.; Lim, H.S.; Komatsu, Y.; Amraoui, S.; Denis, A.; Derval, N.; Laurent, F.; et al. Role of high-resolution image integration to visualize left phrenic nerve and coronary arteries during epicardial ventricular tachycardia ablation. Circ. Arrhythm. Electrophysiol. 2015, 8, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Aghayev, A.; Murphy, D.J.; Keraliya, A.R.; Steigner, M.L. Recent developments in the use of computed tomography scanners in coronary artery imaging. Expert. Rev. Med. Devices 2016, 13, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Frijia, F.; Panetta, D.; Degiorgi, G.; De Gori, C.; Maffei, E.; Clemente, A.; Positano, V.; Cademartiri, F. Photon-Counting Computed Tomography (PCCT): Technical Background and Cardio-Vascular Applications. Diagnostics 2023, 13, 645. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, M.; Duchateau, J.; Sacher, F.; Martin, R.; Vlachos, K.; Kitamura, T.; Sermesant, M.; Cedilnik, N.; Cheniti, G.; Frontera, F.; et al. Are wall thickness channels defined by computed tomography predictive of isthmuses of postinfarction ventricular tachycardia? Heart Rhythm. 2019, 16, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Berte, B.; Cochet, H.; Dang, L.; Mahida, S.; Moccetti, F.; Hilfiker, G.; Bondietti, J.; Ruschitzka, F.; Jaïs, P.; Scharf, C.; et al. Image-guided ablation of scar-related ventricular tachycardia: Towards a shorter and more predictable procedure. J. Interv. Card. Electrophysiol. 2020, 59, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Soto-Iglesias, D.; Penela, D.; Jáuregui, B.; Acosta, J.; Fernández-Armenta, J.; Linhart, M.; Zucchelli, G.; Syrovnev, V.; Zaraket, F.; Terés, C.; et al. Cardiac Magnetic Resonance-Guided Ventricular Tachycardia Substrate Ablation. JACC Clin. Electrophysiol. 2020, 6, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Andreu, D.; Ortiz-Pérez, J.T.; Fernández-Armenta, J.; Guiu, E.; Acosta, J.; Prat-González, S.; De Caralt, T.M.; Perea, R.J.; Garrido, C.; Mont, L.; et al. 3D delayed-enhanced magnetic resonance sequences improve conducting channel delineation prior to ventricular tachycardia ablation. EP Eur. 2015, 17, 938–945. [Google Scholar] [CrossRef]
- Piers, S.R.; Zeppenfeld, K. Imaging-guided Ventricular Tachycardia Ablation. Arrhythmia Electrophysiol. Rev. 2013, 2, 128–134. [Google Scholar] [CrossRef]
- Matsunari, I.; Taki, J.; Nakajima, K.; Tonami, N.; Hisada, K. Myocardial viability assessment using nuclear imaging. Ann. Nucl. Med. 2003, 17, 169–179. [Google Scholar] [CrossRef]
- Tian, J.; Smith, M.F.; Ahmad, G.; Dilsizian, V.; Jimenez, A.; Dickfeld, T. Integration of 3-dimensional scar models from SPECT to guide ventricular tachycardia ablation. J. Nucl. Med. 2012, 53, 894–901. [Google Scholar] [CrossRef]
- Tung, R.; Bauer, B.; Schelbert, H.; Lynch, J.P.; Auerbach, M.; Gupta, P.; Schiepers, C.; Chan, S.; Ferris, J.; Barrio, M.; et al. Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: The potential role of occult inflammation in arrhythmogenesis. Heart Rhythm. 2015, 12, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Fallavollita, J.A.; Heavey, B.M.; Luisi, A.J.; Michalek, S.M.; Baldwa, S.; Mashtare, T.L.; Hutson, A.D.; Dekemp, R.A.; Haka, M.S.; Sajjad, M.; et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J. Am. Coll. Cardiol. 2014, 63, 141–149. [Google Scholar] [CrossRef]
- Kammerling, J.J.; Green, F.J.; Watanabe, A.M.; Inoue, H.; Barber, M.J.; Henry, D.P.; Zipes, D.P. Denervation supersensitivity of refractoriness in noninfarcted areas apical to transmural myocardial infarction. Circulation 1987, 76, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Abdulghani, M.; Smith, M.; Huang, R.; Asoglu, R.; Remo, B.F.; Turgeman, A.; Mesubi, O.; Sidhu, S.; Synowski, S.; et al. Three-dimensional 123I-meta-iodobenzylguanidine cardiac innervation maps to assess substrate and successful ablation sites for ventricular tachycardia: Feasibility study for a novel paradigm of innervation imaging. Circ. Arrhythm. Electrophysiol. 2015, 8, 583–591. [Google Scholar] [CrossRef]
- Simões, M.V.; Barthel, P.; Matsunari, I.; Nekolla, S.G.; Schömig, A.; Schwaiger, M.; Schmidt, G.; Bengel, F.M. Presence of sympathetically denervated but viable myocardium and its electrophysiologic correlates after early revascularised, acute myocardial infarction. Eur. Heart J. 2004, 25, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Hussey, P.; Wu, I.; Johnston, T. 2018 ACC/HRS/NASCI/SCAI/SCCT Expert Consensus Document on Optimal Use of Ionizing Radiation in Cardiovascular Imaging: Best Practices for Safety and Effectiveness-A Review for the Cardiac Anesthesiologist. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2902–2908. [Google Scholar] [CrossRef]
- Enriquez, A.; Saenz, L.C.; Rosso, R.; Silvestry, F.E.; Callans, D.; Marchlinski, F.E.; Garcia, F. Use of Intracardiac Echocardiography in Interventional Cardiology: Working with the Anatomy Rather Than Fighting It. Circulation 2018, 137, 2278–2294. [Google Scholar] [CrossRef]
- Demo, H.; Willoughby, C.; Jazayeri, M.A.; Razminia, M. Fluoroless Catheter Ablation of Cardiac Arrhythmias. Card. Electrophysiol. Clin. 2019, 11, 719–729. [Google Scholar] [CrossRef]
- Jan, M.; Žižek, D.; Kalinšek, T.P.; Kuhelj, D.; Trunk, P.; Kolar, T.; Kšela, J.; Rauber, M.; Yazici, M. Minimising radiation exposure in catheter ablation of ventricular arrhythmias. BMC Cardiovasc. Disord. 2021, 21, 306. [Google Scholar] [CrossRef]
- Lamberti, F.; Di Clemente, F.; Remoli, R.; Bellini, C.; De Santis, A.; Mercurio, M.; Dottori, S.; Gaspardone, A. Catheter ablation of idiopathic ventricular tachycardia without the use of fluoroscopy. Int. J. Cardiol. 2015, 190, 338–343. [Google Scholar] [CrossRef]
- Oloriz, T.; Silberbauer, J.; Maccabelli, G.; Mizuno, H.; Baratto, F.; Kirubakaran, S.; Vergara, P.; Bisceglia, C.; Santagostino, G.; Marzi, A.; et al. Catheter ablation of ventricular arrhythmia in nonischemic cardiomyopathy: Anteroseptal versus inferolateral scar sub-types. Circ. Arrhythm. Electrophysiol. 2014, 7, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, P.; Hutchinson, M.D.; Supple, G.E.; Callans, D.J.; Marchlinski, F.E.; Garcia, F.C. Right Atrial Approach for Ablation of Ventricular Arrhythmias Arising From the Left Posterior-Superior Process of the Left Ventricle. Circ. Arrhythm. Electrophysiol. 2016, 9, e004048. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Ren, J.-F.; Hutchinson, M.D.; Desjardins, B.; Tschabrunn, C.; Gerstenfeld, E.P.; Deo, R.; Dixit, S.; Garcia, F.C.; Cooper, J.M.; et al. Assessing epicardial substrate using intracardiac echocardiography during VT ablation. Circ. Arrhythm. Electrophysiol. 2011, 4, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Sciarra, L.; Palamà, Z.; Nesti, M.; Lanzillo, C.; Di Roma, M.; De Ruvo, E.; Robles, A.G.; Cavarretta, E.; Scarà, A.; De Luca, L.; et al. Contact-force monitoring increases accuracy of right ventricular voltage mapping avoiding ‘false scar’ detection in patients with no evidence of structural heart disease. Indian Pacing Electrophysiol. J. 2020, 20, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Hoogendoorn, J.C. Unipolar voltage mapping in right ventricular cardiomyopathy: Pitfalls, solutions and advantages. Europace 2023, 25, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Hawson, J.; Al-kaisey, A.; Anderson, R.D.; Watts, T.; Morton, J.; Kumar, S.; Kistler, P.; Kalman, J.; Lee, G. Substrate-based approaches in ventricular tachycardia ablation. Indian Pacing Electrophysiol. J. 2022, 22, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Guandalini, G.S.; Liang, J.J.; Marchlinski, F.E. Ventricular Tachycardia Ablation: Past, Present, and Future Perspectives. JACC Clin. Electrophysiol. 2019, 5, 1363–1383. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.F.; Miller, D.C.; Stinson, E.B.; Daily, P.O.; Fogarty, T.J.; Harrison, D.C. Surgical treatment of refractory life-threatening ventricular tachycardia. Am. J. Cardiol. 1973, 32, 909–912. [Google Scholar] [CrossRef]
- Josephson, M.E.; Harken, A.H.; Horowitz, L.N. Endocardial excision: A new surgical technique for the treatment of recurrent ventricular tachycardia. Circulation 1979, 60, 1430–1439. [Google Scholar] [CrossRef]
- Jaïs, P.; Maury, P.; Khairy, P.; Sacher, F.; Nault, I.; Komatsu, Y.; Hocini, M.; Forclaz, A.; Jadidi, A.S.; Weerasooryia, R.; et al. Elimination of Local Abnormal Ventricular Activities: A New End Point for Substrate Modification in Patients with Scar-Related Ventricular Tachycardia. Circulation 2012, 125, 2184–2196. [Google Scholar] [CrossRef]
- Vergara, P.; Trevisi, N.; Ricco, A.; Petracca, F.; Baratto, F.; Cireddu, M.; Bisceglia, C.; Maccabelli, G.; DELLA Bella, P. Late Potentials Abolition as an Additional Technique for Reduction of Arrhythmia Recurrence in Scar Related Ventricular Tachycardia Ablation. J. Cardiovasc. Electrophysiol. 2012, 23, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Briceño, D.F.; Romero, J.; Villablanca, P.A.; Londoño, A.; Diaz, J.C.; Maraj, I.; Batul, S.A.; Madan, N.; Patel, J.; Jagannath, A.; et al. Long-term outcomes of different ablation strategies for ventricular tachycardia in patients with structural heart disease: Systematic review and meta-analysis. Ep Eur. 2018, 20, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Porta-Sánchez, A.; Jackson, N.; Lukac, P.; Kristiansen, S.B.; Nielsen, J.M.; Gizurarson, S.; Massé, S.; Labos, C.; Viswanathan, K.; King, B.; et al. Multicenter Study of Ischemic Ventricular Tachycardia Ablation with Decrement-Evoked Potential (DEEP) Mapping with Extra Stimulus. JACC Clin. Electrophysiol. 2018, 4, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Yu, R.; Bradfield, J.S.; Vaseghi, M.; Buch, E.F.; Ajijola, O.; Macias, C.; Fujimura, O.; Mandapati, R.; Boyle, N.G.; et al. Relationship between sinus rhythm late activation zones and critical sites for scar-related ventricular tachycardia: Systematic analysis of isochronal late activation mapping. Circ. Arrhythm. Electrophysiol. 2015, 8, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, W.G.; Khan, H.; Sager, P.; Saxon, L.A.; Middlekauff, H.R.; Natterson, P.D.; Wiener, I. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation 1993, 88 Pt 1, 1647–1670. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.D.; Gerstenfeld, E.P.; Desjardins, B.; Bala, R.; Riley, M.P.; Garcia, F.C.; Dixit, S.; Lin, D.; Tzou, W.S.; Cooper, J.M.; et al. Endocardial unipolar voltage mapping to detect epicardial ventricular tachycardia substrate in patients with nonischemic left ventricular cardiomyopathy. Circ. Arrhythm. Electrophysiol. 2011, 4, 49–55. [Google Scholar] [CrossRef]
- Gökoğlan, Y.; Mohanty, S.; Gianni, C.; Santangeli, P.; Trivedi, C.; Güneş, M.F.; Bai, R.; Al-Ahmad, A.; Gallinghouse, G.J.; Horton, R.; et al. Scar Homogenization versus Limited-Substrate Ablation in Patients with Nonischemic Cardiomyopathy and Ventricular Tachycardia. J. Am. Coll. Cardiol. 2016, 68, 1990–1998. [Google Scholar] [CrossRef]
- Di Biase, L.; Burkhardt, J.D.; Lakkireddy, D.; Carbucicchio, C.; Mohanty, S.; Mohanty, P.; Trivedi, C.; Santangeli, P.; Bai, R.; Forleo, G.; et al. Ablation of Stable VTs versus Substrate Ablation in Ischemic Cardiomyopathy: The VISTA Randomized Multicenter Trial. J. Am. Coll. Cardiol. 2015, 66, 2872–2882. [Google Scholar] [CrossRef]
- Josephson, M.E.; Anter, E. Substrate Mapping for Ventricular Tachycardia: Assumptions and Misconceptions. JACC Clin. Electrophysiol. 2015, 1, 341–352. [Google Scholar] [CrossRef]
- Yavin, H.D.; Sroubek, J.; Yarnitsky, J.; Bubar, Z.P.; Higuchi, K.; Zilberman, I.; Basu, S.; Anter, E. Direction-aware mapping algorithms have minimal impact on bipolar voltage maps created using high-resolution multielectrode catheters. J. Cardiovasc. Electrophysiol. 2022, 33, 73–80. [Google Scholar] [CrossRef]
- Leshem, E.; Tschabrunn, C.M.; Jang, J.; Whitaker, J.; Zilberman, I.; Beeckler, C.; Govari, A.; Kautzner, J.; Peichl, P.; Nezafat, N.; et al. High-Resolution Mapping of Ventricular Scar: Evaluation of a Novel Integrated Multielectrode Mapping and Ablation Catheter. JACC Clin. Electrophysiol. 2017, 3, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Glashan, C.A.; Beukers, H.K.C.; Tofig, B.J.; Tao, Q.; Blom, S.; Mertens, B.; Kristiansen, S.B.; Zeppenfeld, Z. Mini-, Micro-, and Conventional Electrodes: An in Vivo Electrophysiology and Ex Vivo Histology Head-to-Head Comparison. JACC Clin. Electrophysiol. 2021, 7, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Žižek, D.; Antolič, B.; Prolič Kalinšek, T.; Štublar, J.; Kajdič, N.; Jelenc, M.; Jan, M. Intracardiac echocardiography-guided transseptal puncture for fluoroless catheter ablation of left-sided tachycardias. J. Interv. Card. Electrophysiol. 2021, 61, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Nazarian, S.; Kantsevoy, S.V.; Zviman, M.M.; Matsen, F.A.; Calkins, H.; Berger, R.D.; Halperin, H.R. Feasibility of endoscopic guidance for nonsurgical transthoracic atrial and ventricular epicardial ablation. Heart Rhythm. 2008, 5, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Kautzner, J.; Peichl, P. 3D and 4D echo—Applications in EP laboratory procedures. J. Interv. Card. Electrophysiol. 2008, 22, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Cauti, F.M.; Niscola, M.; Calore, F.; Fanti, V.; Polselli, M.; Di Pastena, A.; Iaia, L.; Bianchi, S. A novel Ventricular map of Electrograms DUration as a Method to identify areas of slow conduction for ventricular tachycardia ablation: The VEDUM pilot study. Heart Rhythm. 2021, 18, 1253–1260. [Google Scholar] [CrossRef]
- Rossi, P.; Cauti, F.M.; Niscola, M.; Magnocavallo, M.; Polselli, M.; Capone, S.; Della Rocca, D.G.; Rodriguez-Garrido, J.; Piccirillo, G.; Anguera, I.; et al. Ventricular Electrograms Duration Map to Detect Ventricular Arrhythmia Substrate: The VEDUM Project Study. Circ. Arrhythm. Electrophysiol. 2023, 16, e011729. [Google Scholar] [CrossRef]
- Monaci, S.; Qian, S.; Gillette, K.; Puyol-Antón, E.; Mukherjee, R.; Elliott, M.K.; Whitaker, J.; Rajani, R.; O’Neill, M.; Rinaldi, C.A.; et al. Non-invasive localization of post-infarct ventricular tachycardia exit sites to guide ablation planning: A computational deep learning platform utilizing the 12-lead electrocardiogram and intracardiac electrograms from implanted devices. Europace 2023, 25, 469–477. [Google Scholar] [CrossRef]
| Pseudo-delta wave ≥34 ms in the precordial leads |
| Intrinsicoid deflection to R-wave peak in V2 ≥85 msec |
| Shortest RS duration ≥121 ms in any precordial lead |
| Maximum deflection index (MDI) ≥55 msec |
| Q wave in D1 |
| Characteristics suggesting an antero-septal scar: |
| AV-block |
| Left bundle branch block |
| Wide QRS |
| Characteristics suggesting an inferolateral scar: |
| Low QRS voltages in limb leads |
| No Q waves in inferior leads |
| QRS fragmentation in lateral leads |
| S/R ratio ≥0.25 in V6 |
| r in V1 and s in V6 ≥0.15 mV |
| CMR | MDCT | ICE | |
|---|---|---|---|
| Radiation exposure | No | Yes | No |
| Scan duration | several minutes | few seconds | variable/real time |
| Planning benefits | pre-procedural | pre-procedural | intra-procedural |
| Fibrosis identification | ++ | +/- | +/- |
| Calcium identification | - | ++ | +/- |
| Thrombus identification | ++ | + | ++ |
| Coronary visualization/depiction | +/- | ++ | ostia and proximal tracts only |
| Fat identification | + | ++ | - |
| CIED generator artifacts | +++ | + | - |
| CIED leads artifacts | + | +++ | + |
| Integration with 3D-mapping systems | Yes | Yes | SOUNDSTAR/CARTOSOUND® only |
| Segmentation software elaboration | Yes | Yes | No |
| Intracavitary structures visualization | Yes | Yes | Yes, real time |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robles, A.G.; Jan, M.; Prolič Kalinšek, T.; Antolič, B.; Rauber, M.; Klemen, L.; Šinkovec, M.; Romano, S.; Sciarra, L.; Pernat, A. How Imaging Techniques Improve Ventricular Arrhythmia Ablation: A Multimodality-Based Approach. J. Clin. Med. 2023, 12, 7420. https://doi.org/10.3390/jcm12237420
Robles AG, Jan M, Prolič Kalinšek T, Antolič B, Rauber M, Klemen L, Šinkovec M, Romano S, Sciarra L, Pernat A. How Imaging Techniques Improve Ventricular Arrhythmia Ablation: A Multimodality-Based Approach. Journal of Clinical Medicine. 2023; 12(23):7420. https://doi.org/10.3390/jcm12237420
Chicago/Turabian StyleRobles, Antonio Gianluca, Matevž Jan, Tine Prolič Kalinšek, Bor Antolič, Martin Rauber, Luka Klemen, Matjaž Šinkovec, Silvio Romano, Luigi Sciarra, and Andrej Pernat. 2023. "How Imaging Techniques Improve Ventricular Arrhythmia Ablation: A Multimodality-Based Approach" Journal of Clinical Medicine 12, no. 23: 7420. https://doi.org/10.3390/jcm12237420
APA StyleRobles, A. G., Jan, M., Prolič Kalinšek, T., Antolič, B., Rauber, M., Klemen, L., Šinkovec, M., Romano, S., Sciarra, L., & Pernat, A. (2023). How Imaging Techniques Improve Ventricular Arrhythmia Ablation: A Multimodality-Based Approach. Journal of Clinical Medicine, 12(23), 7420. https://doi.org/10.3390/jcm12237420






