Complex Relationship of Left Ventricular Rotational Mechanics and Deformation Represented by Strain Parameters in Healthy Adults—Detailed Analysis from the Three-Dimensional Speckle-Tracking Echocardiographic MAGYAR-Healthy Study
Abstract
1. Introduction
2. Subjects and Methods
2.1. Subject Population
2.2. Two-Dimensional Doppler Echocardiography
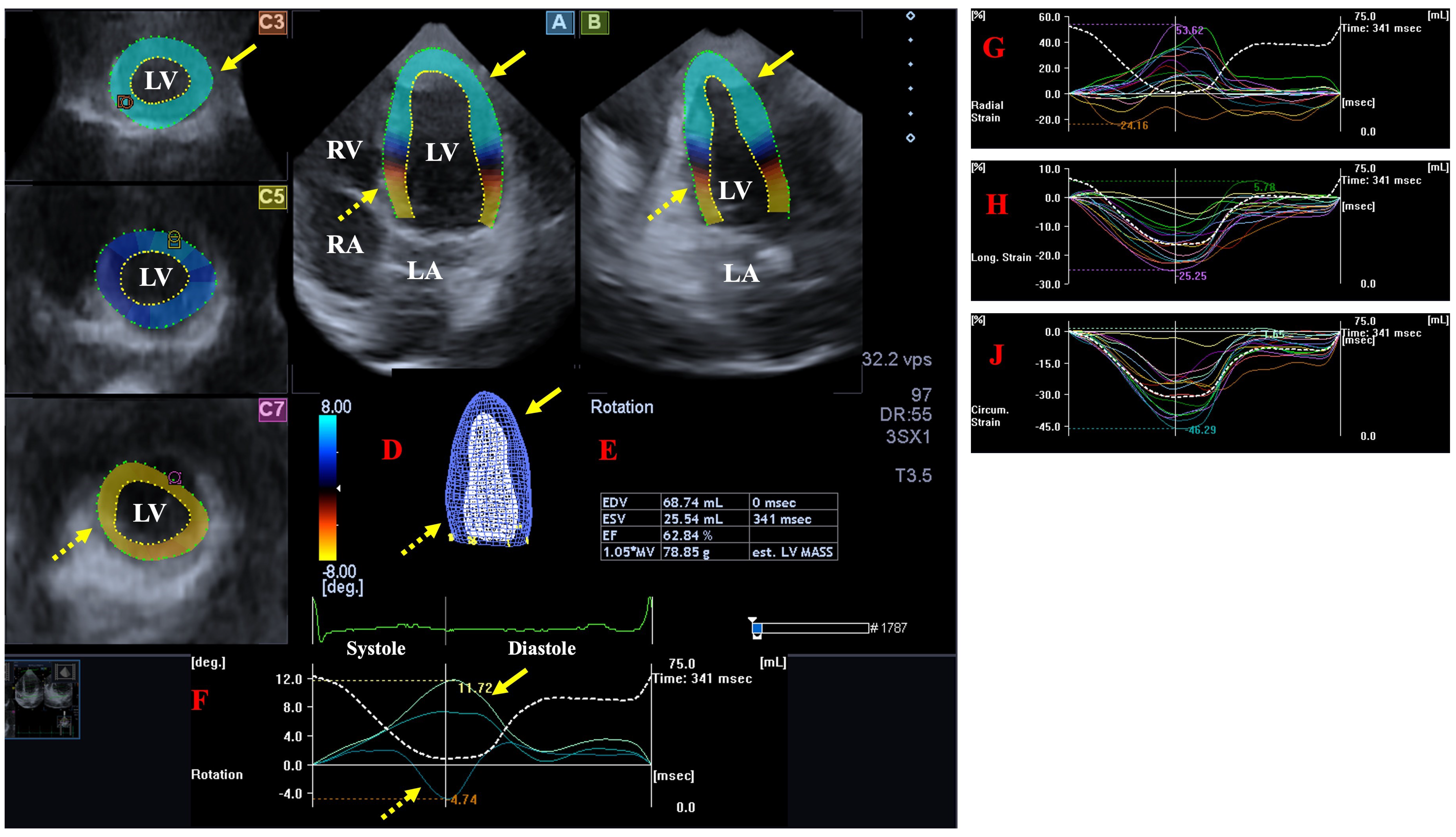
2.3. Three-Dimensional Speckle-Tracking Echocardiography
- The following LV rotational parameters were provided: apical and basal LV rotations, LV twist and time-to-peak LV twist [25].
- The following global LV strains, featuring the whole LV were measured [26]:
- ○
- LV radial strain (LV-gRS)—for characterizing thinning/thickening of the myocardial tissue.
- ○
- LV longitudinal strain (LV-gLS)—for characterizing lengthening/shortening of the myocardial tissue.
- ○
- LV circumferential strain (LV-gCS)—for characterizing widening/narrowing of the myocardial tissue.
2.4. Statistical Analysis
3. Results
3.1. Clinical Data
3.2. Two-Dimensional Doppler Echocardiography
3.3. Three-Dimensional Speckle-Tracking Echocardiography
3.4. Classification of Subjects
3.5. Increasing LV Apical and Basal Rotations and LV Strains
3.6. Increasing LV Strains and LV Apical and Basal Rotations
3.7. Feasibility of 3DSTE-Derived LV Quantifications
4. Discussion
5. Limitations
- Although healthy individuals were included in the study and there were no known factors that could have influenced the findings, it cannot be ruled out 100% that they had some kind of subclinical latent disease. Further laboratory and imaging tests could have ruled them out.
- An important technical problem that still exists today is 3DSTE image quality, which is worse than that of 2D echocardiography due to technical reasons (lower spatial and temporal resolution) [13,14,15,16,17]. During 3DSTE analysis, ECG gating and data acquisition within four–six heart cycles are required to achieve optimal image quality. In addition, to create the 3D full volume, subvolumes are first recorded, which are later stitched together by the software. However, this makes an opportunity to create artifacts. In addition, respiratory movement and arrhythmias can make data acquisition and therefore imaging difficult.
- In the present study, only LV data were analyzed. However, 3DSTE-derived (left and right) atrial analysis could have been performed at the same time, as demonstrated in recent studies [34].
- Although there may be debate about which chamber the ventricular septum is part of, it was clearly considered part of the LV in the present investigation.
- Three-dimensional STE allows the determination of complex/multidimensional LV strains as well, such as 3D and area strains, which are formed from unidimensional LV strains. However, due to their complex nature, these parameters were not examined.
- The relationship between global LV strains and rotational parameters determined only by 3DSTE was studied. The presentation of other morphological abnormalities of LV, the measurement of the diameters or areas of LV in a particular selected plane or the analysis of segmental or regional strain and rotational parameters of the LV were not the aim of this study.
- This study is a retrospective study, which limits the value of the conclusions that can be drawn.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bloechlinger, S.; Grander, W.; Bryner, J.; Dünser, M.W. Left ventricular rotation: A neglected aspect of the cardiac cycle. Intensive Care Med. 2011, 37, 156–163. [Google Scholar] [CrossRef]
- Omar, A.M.S.; Vallabhajosyula, S.; Sengupta, P.P. Left Ventricular Twist and Torsion. Research Observations and Clinical Applications. Circ. Cardiovasc. Imaging 2015, 8, e003029. [Google Scholar] [CrossRef]
- Nakatani, S. Left ventricular rotation and twist: Why should we learn? J. Cardiovasc. Ultrasound 2011, 19, 1–6. [Google Scholar] [CrossRef]
- Sengupta, P.P.; Tajik, A.J.; Chandrasekaran, K.; Khandheria, B.K. Twist mechanics of the left ventricle: Principles and application. JACC Cardiovasc. Imaging 2008, 1, 366–376. [Google Scholar] [CrossRef]
- Stohr, E.J.; Shave, R.E.; Baggish, A.L.; Weiner, R.B. Left ventricular twist mechanics in the context of normal physiology and cardiovascular disease: A review of studies using speckle tracking echocardiography. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H633–H644. [Google Scholar] [CrossRef]
- Dorobantu, D.M.; Wadey, C.A.; Amir, N.H.; Stuart, A.G.; Williams, C.A.; Pieles, G.E. The Role of Speckle Tracking Echocardiography in the Evaluation of Common Inherited Cardiomyopathies in Children and Adolescents: A Systematic Review. Diagnostics 2021, 11, 635. [Google Scholar] [CrossRef]
- Meyers, B.A.; Brindise, M.C.; Kutty, S.; Vlachos, P.P. A method for direct estimation of left ventricular global longitudinal strain rate from echocardiograms. Sci. Rep. 2022, 12, 4008. [Google Scholar] [CrossRef]
- Narang, A.; Addetia, K. An introduction to left ventricular strain. Curr. Opin. Cardiol. 2018, 33, 455–463. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef]
- Edvardsen, T.; Klaeboe, L.G. Imaging and heart failure: Myocardial strain. Curr. Opin. Cardiol. 2019, 34, 490–494. [Google Scholar] [CrossRef]
- Kocabay, G.; Muraru, D.; Peluso, D.; Cucchini, U.; Mihaila, S.; Padayattil-Jose, S.; Gentian, D.; Iliceto, S.; Vinereanu, D.; Badano, L.P. Normal left ventricular mechanics by two-dimensional speckle-tracking echocardiography. Reference values in healthy adults. Rev. Esp. Cardiol. (Engl. Ed.) 2014, 67, 651–658. [Google Scholar] [CrossRef]
- Parisi, V.; Losi, M.A.; Contaldi, C.; Chiacchio, E.; Pastore, F.; Scatteia, A.; Giamundo, A.; di Nardo, C.; Lombardi, R.; Betocchi, S. Speckle-tracking analysis based on 2D echocardiography does not reliably measure left ventricular torsion. Clin. Physiol. Funct. Imaging 2013, 33, 117–121. [Google Scholar] [CrossRef]
- Nabeshima, Y.; Seo, Y.; Takeuchi, M. A review of current trends in three-dimensional analysis of left ventricular myocardial strain. Cardiovasc. Ultrasound 2020, 18, 23. [Google Scholar] [CrossRef]
- Ammar, K.A.; Paterick, T.E.; Khandheria, B.K.; Jan, M.F.; Kramer, C.; Umland, M.M.; Tercius, A.J.; Baratta, L.; Tajik, A.J. Myocardial mechanics: Understanding and applying three-dimensional speckle tracking echocardiography in clinical practice. Echocardiography 2012, 97, 861–872. [Google Scholar] [CrossRef]
- Urbano-Moral, J.A.; Patel, A.R.; Maron, M.S.; Arias-Godinez, J.A.; Pandian, N.G. Three-dimensional speckle-tracking echocardiography: Methodological aspects and clinical potential. Echocardiography 2012, 29, 997–1010. [Google Scholar] [CrossRef]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef]
- Seo, Y.; Ishizu, T.; Atsumi, A.; Kawamura, R.; Aonuma, K. Three-dimensional speckle tracking echocardiography. Circ. J. 2014, 78, 1290–1301. [Google Scholar] [CrossRef]
- Seo, Y.; Ishizu, T.; Enomoto, Y.; Sugimori, H.; Yamamoto, M.; Machino, T.; Kawamura, R.; Aonuma, K. Validation of 3-dimensional speckle tracking imaging to quantify regional myocardial deformation. Circ. Cardiovasc. Imaging 2009, 2, 451–454. [Google Scholar] [CrossRef]
- Kleijn, S.A.; Brouwer, W.P.; Aly, M.F.A.; Rüssel, I.K.; de Roest, G.J.; Beek, A.M.; van Rossum, A.C.; Kamp, O. Comparison between three-dimensional speckle-tracking echocardiography and cardiac magnetic resonance imaging for quantification of left ventricular volumes and function. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 834–839. [Google Scholar] [CrossRef]
- Kleijn, S.A.; Aly, M.F.A.; Terwee, C.B.; van Rossum, A.C.; Kamp, O. Reliability of left ventricular volumes and function measurements using three-dimensional speckle tracking echocardiography. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 159–168. [Google Scholar] [CrossRef]
- Ashraf, M.; Myronenko, A.; Nguyen, T.; Inage, A.; Smith, W.; Lowe, R.I.; Thiele, K.; Gibbons-Kroeker, C.A.; Tyberg, J.V.; Smallhorn, J.F.; et al. Defining left ventricular apex-to-base twist mechanics computed from high-resolution 3D echocardiography: Validation against sonomicrometry. JACC Cardiovasc. Imaging 2010, 3, 227–234. [Google Scholar] [CrossRef]
- Zhou, Z.; Ashraf, M.; Hu, D.; Dai, X.; Xu, Y.; Kenny, B.; Cameron, B.; Nguyen, T.; Xiong, L.; Sahn, D.J. Three-dimensional speckle-tracking imaging for left ventricular rotation measurement: An in vitro validation study. J. Ultrasound Med. 2010, 29, 903–909. [Google Scholar] [CrossRef]
- Andrade, J.; Cortez, L.D.; Campos, O.; Arruda, A.L.; Pinheiro, J.; Vulcanis, L.; Shiratsuchi, T.S.; Kalil-Filho, R.; Cerri, G.S. Left ventricular twist: Comparison between two- and three-dimensional speckle-tracking echocardiography in healthy volunteers. Eur. J. Echocardiogr. 2011, 12, 76–79. [Google Scholar] [CrossRef][Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Ruzsa, Z.; Achim, A.; Ambrus, N.; Lengyel, C. Left ventricular rotational mechanics and left ventricular volumes: Is there a relationship in healthy adults? Three-dimensional speckle-tracking echocardiography-derived insights from the MAGYAR-Healthy Study. Quant. Imaging Med. Surg. 2023, 13, 6583–6589. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Ruzsa, Z.; Achim, A.; Ambrus, N.; Lengyel, C. Complexity of left ventricular strains in response to elevated volumes in healthy adults—Detailed analysis from the three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. Int. J. Cardiol. Heart Vasc. 2023, 47, 101236. [Google Scholar] [CrossRef]
- Buckberg, G.D. Basic science review: The helix and the heart. J. Thorac. Cardiovasc. Surg. 2002, 124, 863–883. [Google Scholar] [CrossRef]
- Armour, J.A.; Randall, W.C. Structural basis for cardiac function. Am. J. Physiol. 1970, 218, 1517–1523. [Google Scholar] [CrossRef]
- Greenbaum, R.A.; Ho, S.Y.; Gibson, D.G.; Becker, A.E.; Anderson, R.H. Left ventricular fibre architecture in man. Br. Heart J. 1981, 45, 248–263. [Google Scholar] [CrossRef]
- Sengupta, P.P.; Korinek, J.; Belohlavek, M.; Narula, J.; Vannan, M.A.; Jahangir, A.; Khandheria, B.K. Left ventricular structure and function: Basic science for cardiac imaging. J. Am. Coll. Cardiol. 2006, 48, 1988–2001. [Google Scholar] [CrossRef]
- Jacob, R.; Dierberger, B.; Kissling, G. Functional significance of the Frank-Starling mechanism under physiological and pathophysiological conditions. Eur. Heart J. 1992, 13 (Suppl. E), 7–14. [Google Scholar] [CrossRef]
- Nemes, A.; Domsik, P.; Kalapos, A.; Forster, T. Is three-dimensional speckle-tracking echocardiography able to identify different patterns of left atrial dysfunction in selected disorders?: Short summary of the MAGYAR-Path Study. Int. J. Cardiol. 2016, 220, 535–537. [Google Scholar] [CrossRef]
- Phelan, D.; Collier, P.; Thavendiranathan, P.; Popović, Z.B.; Hanna, M.; Plana, J.C.; Marwick, T.H.; Thomas, J.D. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012, 98, 1442–1448. [Google Scholar] [CrossRef]
- Mohseni-Badalabadi, R.; Mirjalili, T.; Jalali, A.; Davarpasand, T.; Hosseinsabet, A. A systematic review and meta-analysis of the normal reference value of the longitudinal left atrial strain by three dimensional speckle tracking echocardiography. Sci. Rep. 2022, 12, 4395. [Google Scholar] [CrossRef]
- Kormányos, Á.; Kalapos, A.; Domsik, P.; Lengyel, C.; Forster, T.; Nemes, A. Normal values of left ventricular rotational parameters in healthy adults-Insights from the three-dimensional speckle tracking echocardiographic MAGYAR-Healthy Study. Echocardiography 2019, 36, 714–721. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Kalapos, A.; Domsik, P.; Gyenes, N.; Ambrus, N.; Lengyel, C. Normal reference values of left ventricular strain parameters in healthy adults: Real-life experience from the single-center three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. J. Clin. Ultrasound 2021, 49, 368–377. [Google Scholar] [CrossRef]
| Basal LV Rotation < 2.03° (n = 23) | 2.03° ≤ Basal LV Rotation ≤ 6.17° (n = 123) | 6.17° < Basal LV Rotation (n = 28) | Apical LV Rotation < 5.60° (n = 25) | 5.60° ≤ Apical LV Rotation ≤ 13.02° (n = 121) | 13.02° < Apical LV Rotation (n = 28) | |
|---|---|---|---|---|---|---|
| basal LV rotation (°) | −1.33 ± 0.62 | −3.75 ± 1.08 * | −7.78 ± 1.02 *† | −4.25 ± 2.54 | −4.14 ± 1.96 | −3.80 ± 2.02 |
| apical LV rotation (°) | 9.76 ± 4.31 | 9.39 ± 3.68 | 8.52 ± 3.68 | 3.63 ± 1.51 | 9.03 ± 1.90 § | 15.56 ± 1.86 §‡ |
| LV twist (°) | 11.08 ± 4.44 | 13.14 ± 3.60 * | 16.3 ± 3.97 *† | 7.88 ± 3.01 | 13.17 ± 2.43 § | 19.36 ± 2.5 §‡ |
| time-to-peak LV twist (ms) | 297.4 ± 103.8 | 361.3 ± 137.5 * | 342.4 ± 80.8 | 338.5 ± 125.7 | 353.3 ± 132.4 | 345.3 ± 104.6 |
| LV-gRS (%) | 24.2 ± 7.3 | 25.7 ± 9.5 | 23.9 ± 7.9 | 23.9 ± 7.6 | 25.0 ± 8.6 | 28.6 ± 12.2 ‡ |
| LV-gCS (%) | −28.2 ± 3.1 | −28.2 ± 4.8 | −24.4 ± 5.6 *† | −25.2 ± 3.8 | −27.5 ± 4.4 § | −30.6 ± 6.5 §‡ |
| LV-gLS (%) | −15.9 ± 2.2 | −16.1 ± 2.5 | −16.0 ± 2.5 | −16.1 ± 1.4 | −15.9 ± 2.6 | −16.8 ± 2.5 |
| LV-gRS < 16.1% (n = 26) | 16.1% ≤ LV-gRS ≤ 34.70% (n = 123) | 34.70% < LV-gRS (n = 25) | LV-gCS < −22.6% (n = 18) | −22.6% ≤ LV-gCS ≤ −32.6% (n = 127) | −32.6% < LV-gCS (n = 29) | LV-gLS < −13.6% (n = 26) | −13.6% ≤ LV-gLS ≤ −18.6% (n = 122) | −18.6% < LV-gLS (n = 26) | |
|---|---|---|---|---|---|---|---|---|---|
| basal LV rotation (°) | −4.13 ± 1.88 | −4.12 ± 2.15 | 4.00 ± 1.83 | −5.93 ± 1.94 | −3.98 ± 2.05 § | −3.49 ± 1.59 §‡ | −3.95 ± 1.82 | −4.10 ± 2.15 | −4.24 ± 1.92 |
| apical LV rotation (°) | 8.12 ± 2.90 | 9.34 ± 3.93 | 10.38 ± 3.53 * | 8.77 ± 4.17 | 8.76 ± 3.41 | 12.05 ± 3.91 §‡ | 9.43 ± 2.54 | 8.88 ± 3.91 | 11.17 ± 3.68 #& |
| LV twist (°) | 12.24 ± 3.58 | 13.46 ± 4.24 | 14.37 ± 3.32 * | 14.70 ± 4.90 | 12.74 ± 3.78 § | 15.53 ± 3.80‡ | 13.38 ± 3.10 | 12.98 ± 4.26 | 15.41 ± 3.36 # |
| time-to-peak LV twist (ms) | 340.0 ± 171.1 | 348.5 ± 118.2 | 367.4 ± 115.7 | 318.1 ± 46.7 | 357.7 ± 135.0 | 334.6 ± 121.5 ‡ | 325.8 ± 145.1 | 361.4 ± 131.4 | 320.8 ± 69.1 |
| LV-gRS (%) | 12.8 ± 3.1 | 24.7 ± 4.6 * | 42.1 ± 6.5 *† | 20.6 ± 8.3 | 24.4 ± 8.1 § | 33.0 ± 10.5 §‡ | 26.6 ± 11.8 | 24.6 ± 8.4 | 27.9 ± 9.9 |
| LV-gCS (%) | −25.2 ± 4.3 | −27.5 ± 4.6 * | −30.7 ± 5.9 *† | −19.9 ± 2.3 | −26.9 ± 2.6 § | −35.9 ± 2.7 §‡ | −27.0 ± 4.4 | −27.2 ± 4.7 | −30.2 ± 5.7 #& |
| LV-gLS (%) | −15.3 ± 2.1 | −16.3 ± 2.4 * | −16.2 ± 3.1 | −15.7 ± 2.1 | −15.9 ± 2.3 | −17.1 ± 3.2 ‡ | −12.4 ± 1.5 | −16.1 ± 1.4 # | −19.9 ± 1.1 #& |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes, A.; Kormányos, Á.; Ambrus, N.; Lengyel, C. Complex Relationship of Left Ventricular Rotational Mechanics and Deformation Represented by Strain Parameters in Healthy Adults—Detailed Analysis from the Three-Dimensional Speckle-Tracking Echocardiographic MAGYAR-Healthy Study. J. Clin. Med. 2023, 12, 7389. https://doi.org/10.3390/jcm12237389
Nemes A, Kormányos Á, Ambrus N, Lengyel C. Complex Relationship of Left Ventricular Rotational Mechanics and Deformation Represented by Strain Parameters in Healthy Adults—Detailed Analysis from the Three-Dimensional Speckle-Tracking Echocardiographic MAGYAR-Healthy Study. Journal of Clinical Medicine. 2023; 12(23):7389. https://doi.org/10.3390/jcm12237389
Chicago/Turabian StyleNemes, Attila, Árpád Kormányos, Nóra Ambrus, and Csaba Lengyel. 2023. "Complex Relationship of Left Ventricular Rotational Mechanics and Deformation Represented by Strain Parameters in Healthy Adults—Detailed Analysis from the Three-Dimensional Speckle-Tracking Echocardiographic MAGYAR-Healthy Study" Journal of Clinical Medicine 12, no. 23: 7389. https://doi.org/10.3390/jcm12237389
APA StyleNemes, A., Kormányos, Á., Ambrus, N., & Lengyel, C. (2023). Complex Relationship of Left Ventricular Rotational Mechanics and Deformation Represented by Strain Parameters in Healthy Adults—Detailed Analysis from the Three-Dimensional Speckle-Tracking Echocardiographic MAGYAR-Healthy Study. Journal of Clinical Medicine, 12(23), 7389. https://doi.org/10.3390/jcm12237389







