Bicuspid Morphology and Rapid Deployment Valve Replacement: Is This Still a Contraindication?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
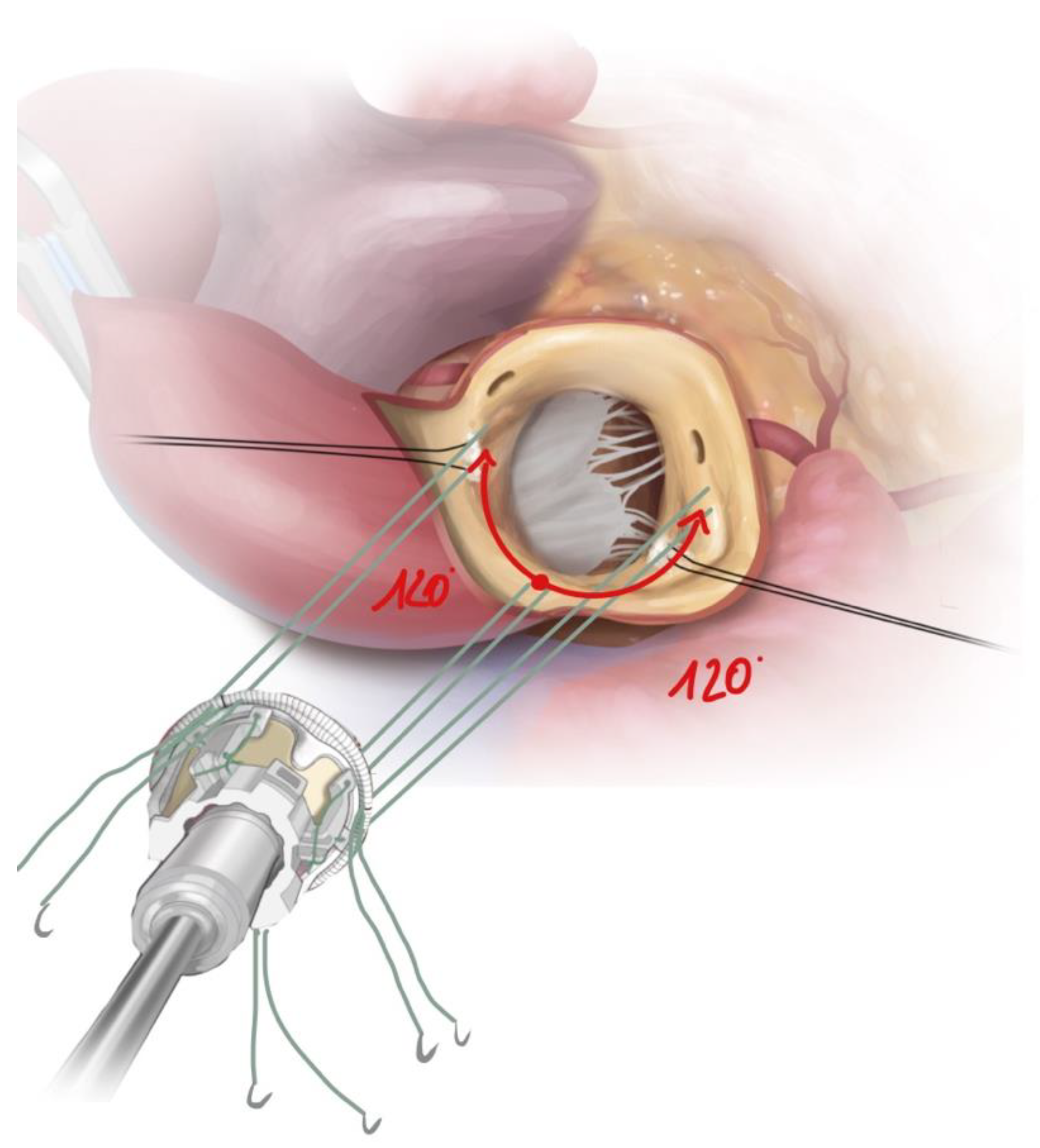
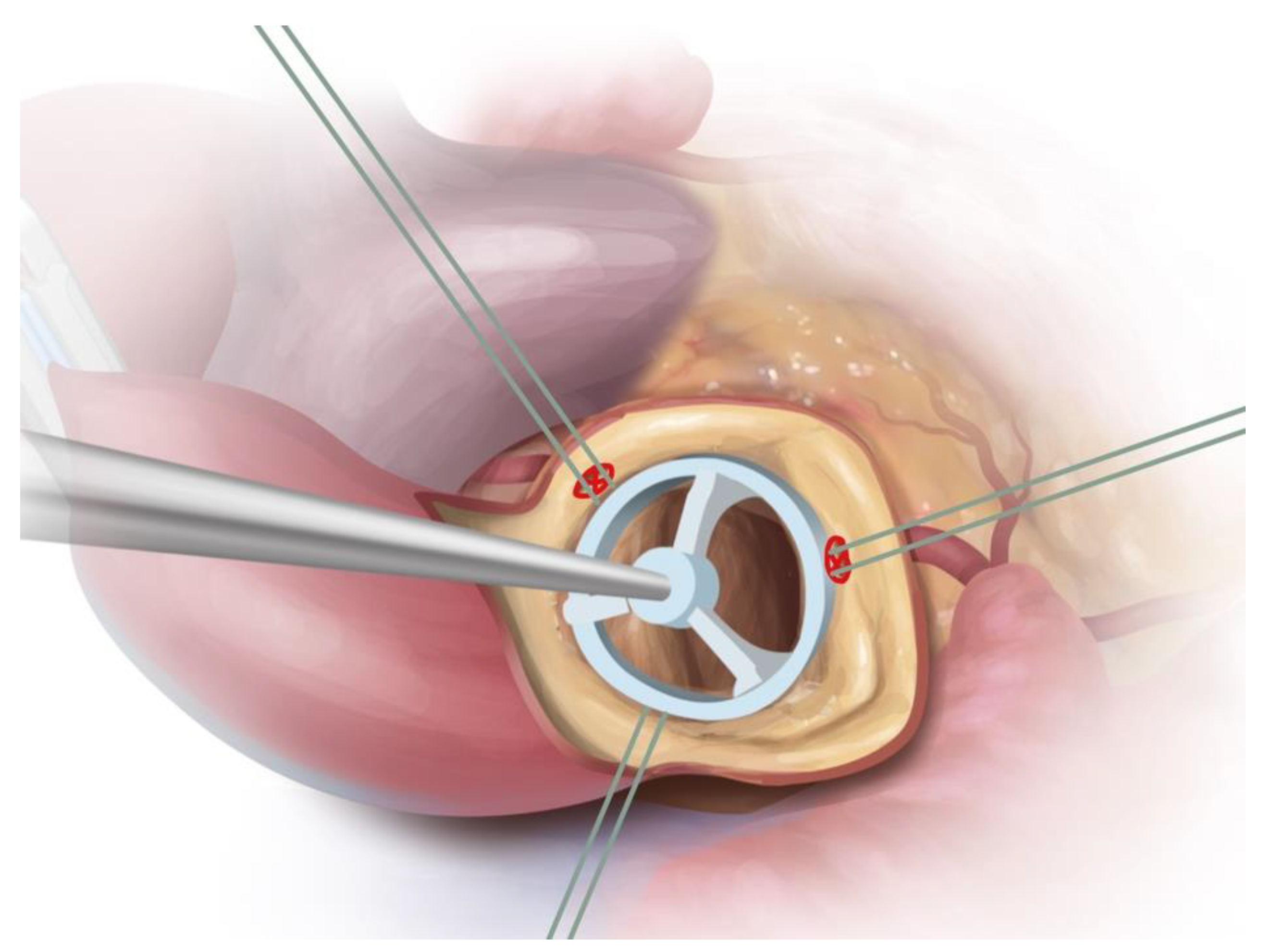
2.3. Surgical Approach
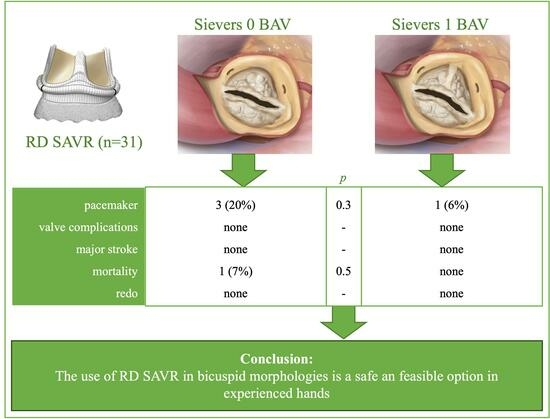
3. Results
3.1. Patient Characteristics
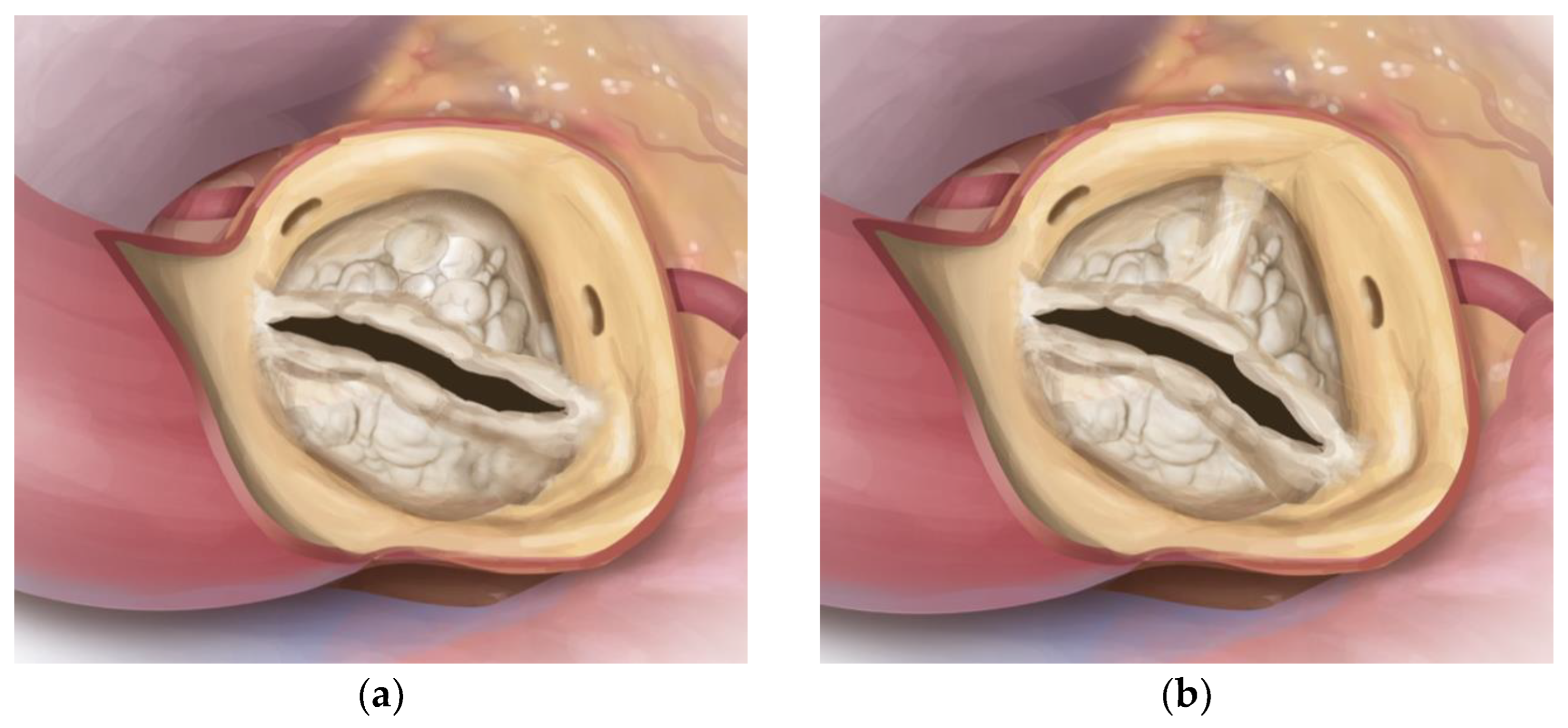
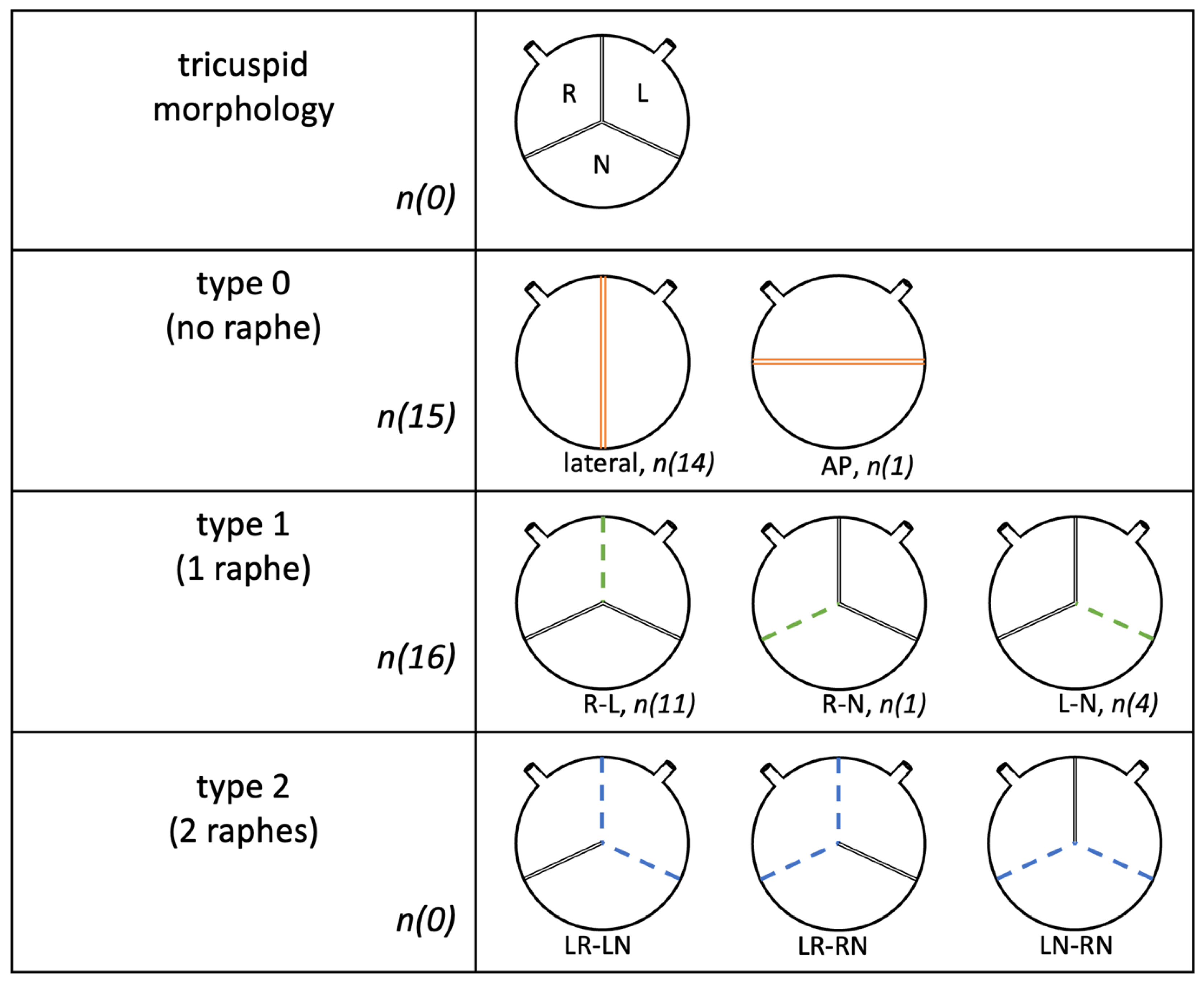
3.2. Valve Specific Data
3.3. Intraoperative Data
3.4. Postoperative Data
3.5. Follow-Up
4. Discussion
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vahanian, A.; Iung, B. The new ESC/EACTS Guidelines on the management of valvular heart disease. Arch. Cardiovasc. Dis. 2012, 105, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Sievers, H.-H.; Schmidtke, C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J. Thorac. Cardiovasc. Surg. 2007, 133, 1226–1233. [Google Scholar] [CrossRef]
- Roberts, W.C.; Ko, J.M. Frequency by Decades of Unicuspid, Bicuspid, and Tricuspid Aortic Valves in Adults Having Isolated Aortic Valve Replacement for Aortic Stenosis, with or without Associated Aortic Regurgitation. Circulation 2005, 111, 920–925. [Google Scholar] [CrossRef]
- Michelena, H.I.; Prakash, S.K.; Della Corte, A.; Bissell, M.M.; Anavekar, N.; Mathieu, P.; Bossé, Y.; Limongelli, G.; Bossone, E.; Benson, D.; et al. Bicuspid Aortic Valve: Identifying Knowledge Gaps and Rising to the Challenge From the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation 2014, 129, 2691–2704. [Google Scholar] [CrossRef] [PubMed]
- Kiyota, Y.; Della Corte, A.; Vieira, V.M.; Habchi, K.; Huang, C.-C.; Della Ratta, E.E.; Sundt, T.M.; Shekar, P.; Muehlschlegel, J.D.; Body, S.C. Risk and outcomes of aortic valve endocarditis among patients with bicuspid and tricuspid aortic valves. Open Heart 2017, 4, e000545. [Google Scholar] [CrossRef] [PubMed]
- Michelena, H.I.; Della Corte, A.; Evangelista, A.; Maleszewski, J.J.; Edwards, W.D.; Roman, M.J.; Devereux, R.B.; Fernández, B.; Asch, F.M.; Barker, A.J.; et al. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional and research purposes. Eur. J. Cardio-Thorac. Surg. 2021, 60, 448–476. [Google Scholar] [CrossRef] [PubMed]
- Gersak, B.; Fischlein, T.; Folliguet, T.A.; Meuris, B.; Teoh, K.H.; Moten, S.C.; Solinas, M.; Miceli, A.; Oberwalder, P.J.; Rambaldini, M.; et al. Sutureless, rapid deployment valves and stented bioprosthesis in aortic valve replacement: Recommendations of an International Expert Consensus Panel. Eur. J. Cardio-Thorac. Surg. 2016, 49, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Berretta, P.; Di Eusanio, M. Aortic valve replacement with sutureless and rapid deployment aortic valve prostheses. J. Geriatr. Cardiol. JGC 2016, 13, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Kocher, A.A.; Laufer, G.; Haverich, A.; Shrestha, M.; Walther, T.; Misfeld, M.; Kempfert, J.; Gillam, L.; Schmitz, C.; Wahlers, T.C.; et al. One-year outcomes of the Surgical Treatment of Aortic Stenosis with a Next Generation Surgical Aortic Valve (TRITON) trial: A prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J. Thorac. Cardiovasc. Surg. 2013, 145, 110–115, discussion 115–116. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Fortin, W.; Mazine, A.; Bouchard, D.; Carrier, M.; El Hamamsy, I.; Lamarche, Y.; Demers, P. Sutureless aortic valve replacement in patients who have bicuspid aortic valve. J. Thorac. Cardiovasc. Surg. 2015, 150, 851–857. [Google Scholar] [CrossRef][Green Version]
- Coti, I.; Werner, P.; Kaider, A.; Mach, M.; Kocher, A.; Laufer, G.; Andreas, M. Rapid-deployment aortic valve replacement for patients with bicuspid aortic valve: A single-centre experience. Eur. J. Cardio-Thorac. Surg. 2022, 62, ezac017. [Google Scholar] [CrossRef]
- Miceli, A.; Berretta, P.; Fiore, A.; Andreas, M.; Solinas, M.; Santarpino, G.; Kappert, U.; Misfeld, M.; Savini, C.; Albertini, A.; et al. Sutureless and rapid deployment implantation in bicuspid aortic valve: Results from the sutureless and rapid-deployment aortic valve replacement international registry. Ann. Cardiothorac. Surg. 2020, 9, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M. Replacement of bicuspid aortic valve with sutureless bioprosthesis: A word of caution here. J. Thorac. Cardiovasc. Surg. 2018, 156, e91. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.K.; Ramlawi, B.; Deeb, G.M.; Zahr, F.; Song, H.K.; Kleiman, N.S.; Chetcuti, S.J.; Michelena, H.I.; Mangi, A.A.; Skiles, J.A.; et al. Transcatheter Aortic Valve Replacement in Low-risk Patients With Bicuspid Aortic Valve Stenosis. JAMA Cardiol. 2020, 6, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Laufer, G.; Haverich, A.; Andreas, M.; Mohr, F.W.; Walther, T.; Shrestha, M.; Rahmanian, P.; Holzhey, D.; Roth, M.; Schmitz, C.; et al. Long-term outcomes of a rapid deployment aortic valve: Data up to 5 years. Eur. J. Cardio-Thorac. Surg. 2017, 52, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Barbeito, M.; Arribas, J.M.; Vazquez, A.; Carnero, M.; Sarralde, J.A.; Cal-Purriños, N.; Cánovas, S.J.; Maroto, L.; Gutiérrez, F.; Hornero, F.; et al. Risk Factors for Postoperative Pacemaker Implantation After Rapid Deployment Aortic Valve Replacement: Results from the RADAR Registry. Adv. Ther. 2021, 38, 1832–1842. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, A.; Salizzoni, S.; Filippini, C.; Tessari, C.; Bagozzi, L.; Messina, A.; Troise, G.; Tomba, M.D.; Rambaldini, M.; Dalén, M.; et al. Surgical aortic valve replacement with new-generation bioprostheses: Sutureless versus rapid-deployment. J. Thorac. Cardiovasc. Surg. 2020, 159, 432–442.e1. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, G.R.; Accola, K.D.; Grossi, E.A.; Woo, Y.J.; Mumtaz, M.A.; Sabik, J.F.; Slachman, F.N.; Patel, H.J.; Borger, M.A.; Garrett, H.E.; et al. TRANSFORM (Multicenter Experience With Rapid Deployment Edwards INTUITY Valve System for Aortic Valve Replacement) US clinical trial: Performance of a rapid deployment aortic valve. J. Thorac. Cardiovasc. Surg. 2016, 153, 241–251.e2. [Google Scholar] [CrossRef]
- Hayıroğlu, M.; Altay, S. The Role of Artificial Intelligence in Coronary Artery Disease and Atrial Fibrillation. Balk. Med. J. 2023, 40, 151–152. [Google Scholar] [CrossRef]
| Sievers Type 0 Collective (n = 15) | Sievers Type 1 Collective (n = 16) | p (Sievers Type 0 vs. Type 1 Collective) | |
|---|---|---|---|
| follow-up, m | 15.9 ± 9.1 | 14.5 ± 10.7 | 0.593 c |
| age, y | 64.5 ± 7.5 | 62 ± 8.0 | 0.700 a |
| gender, n (%) | 0.013 b | ||
| female | 2 (13) | 9 (56) | |
| mean New Euroscore II,% | 2.9 ± 4 | 2.1 ± 1.2 | 0.146 a |
| arterial hypertension, n (%) | 13 (87) | 8 (50) | 0.054 d |
| diabetes mellitus, n (%) | 3 (20) | 0 (0) | 0.101 d |
| smoking history, active, n (%) | 2 (13) | 2 (13) | 1.000 d |
| alcohol abuse, n (%) | 1 (7) | 0 (0) | 0.733 d |
| obesity (mean BMI), kg/m2 | 26.9 ± 3.8 | 29.5 ± 7.4 | 0.059 a |
| hyperlipoproteinemia, n (%) | 12 (80) | 8 (50) | 0.081 b |
| family history, n (%) | 4 (27) | 2 (13) | 0.394 d |
| peripheral artery disease (pAVK), n (%) | 1 (7) | 1 (6) | 1.000 d |
| cerebral artery disease (cAVK), n (%) | 2 (13) | 1 (6) | 0.600 d |
| chronic lung disease, n (%) | 0 (0) | 1 (6) | 1.000 d |
| pulmonary embolism, n (%) | 0 (0) | 0 (0) | |
| rheumatic fever, n (%) | 0 (0) | 0 (0) | |
| congenital heart disease, n (%) | 0 (0) | 0 (0) | |
| TIA, n (%) | 0 (0) | 0 (0) | |
| stroke, n (%) | |||
| embolic | 0 (0) | 1 (6) | 1.000 d |
| hemorrhagic | 0 (0) | 0 (0) | |
| myocardial infarction, n (%) | 0 (0) | 0 (0) | |
| renal insufficiency dialysis, n (%) | 1 (7) | 0 (0) | 0.484 d |
| low cardiac output syndrome, n (%) | 0 (0) | 0 (0) | |
| mechanical ventilation, n (%) | 0 (0) | 0 (0) | |
| cancer, n (%) | 2 (13) | 1 (6) | 0.880 d |
| NYHA > II, n (%) | 12 (80) | 12 (75) | 1.000 d |
| angina pectoris, n (%) | 4 (27) | 2 (13) | 0.651 d |
| syncope, n (%) | 1 (7) | 1 (6) | 1.000 d |
| vertigo, n (%) | 3 (20) | 1 (6) | 0.333 d |
| CHA2DS2-VASc-Score | 2.4 ± 1.5 | 1.7 ± 1.0 | 0.200 a |
| antiplatelet, n (%) | |||
| ASS | 5 (33) | 3 (19) | 0.433 d |
| Clopidogrel | 3 (20) | 0 (0) | 0.101 d |
| ASS + Clopidogrel | 1 (7) | 0 (0) | 0.170 d |
| anticoagulation, n (%) | 0 (0) | 0 (0) |
| Sievers Type 0 Collective (n = 15) | Sievers Type 1 Collective (n = 16) | p (Sievers Type 0 vs. Type 1 Collective) | |
|---|---|---|---|
| aortic regurgitation, n (%) | 0 (0) | 1 (6) | 1.000 d |
| combined aortic stenosis and regurgitation, n (%) | 5 (33) | 4 (25) | 0.433 d |
| mean left ventricular ejection fraction, % | 56.0 ± 10.6 | 61.7 ± 7.0 | 0.124 a |
| mean gradient, mean mmHg | 40.8 ± 13.1 | 47.1 ± 14.5 | 0.766 a |
| maximal gradient, mean mmHg | 67.0 ± 17.5 | 79.6 ± 24.3 | 0.261 a |
| aortic orifice area, mean cm2 | 0.85 ± 0.29 | 0.80 ± 0.26 | 0.781 c |
| aortic aneurysm, n (%) | 4 (27) | 5 (31) | 0.546 d |
| endocarditis | 0 (0) | 1 (6) | 1.000 d |
| Sievers Type 0 Collective (n = 15) | Sievers Type 1 Collective (n = 16) | p (Sievers Type 0 vs. Type 1 Collective) | |
|---|---|---|---|
| priority, n (%) | 1.000 d | ||
| urgent | 0 (0) | 1 (6) | |
| partial sternotomy, n (%) | 1 (7) | 3 (19) | 0.600 d |
| aortic prosthetic size, mean, mm, n (%) | 25.0 ± 1.9 | 25.1 ± 1.9 | 0.866 a |
| 19 | 0 (0) | 0 (0) | |
| 21 | 1 (7) | 1 (6) | |
| 23 | 3 (20) | 3 (19) | |
| 25 | 6 (40) | 6 (38) | |
| 27 | 5 (33) | 6 (38) | |
| concomitant procedures, n (%) | |||
| coronary artery bypass graft | 5 (33) | 1 (6) | 0.083 d |
| mitral valve | 1 (7) | 0 (0) | 0.484 d |
| tricuspid valve | 0 (0) | 0 (0) | |
| aortic surgery | 5 (33) | 9 (56) | 0.200 b |
| ventricular septal defect closure | 0 (0) | 0 (0) | |
| PFO closure | 0 (0) | 1 (6) | 1.000 d |
| left atrial appendage closure | 1 (7) | 0 (0) | 0484 d |
| intraoperative data, min | |||
| cardiopulmonary bypass time | 112.2 ± 40.3 | 127.2 ± 48.7 | 0.520 a |
| cross-clamp time | 74.9 ± 28.5 | 70.1 ± 21.4 | 0.547 a |
| Sievers Type 0 Collective (n = 15) | Sievers Type 1 Collective (n = 16) | p (Sievers Type 0 vs. Type 1 Collective) | |
|---|---|---|---|
| mean left-ventricular ejection fraction, % | 56.4 ± 9.5 | 61.7 ± 5.8 | 0.199 c |
| mean gradient, mean mmHg | 8.1 ± 3.3 | 8.4 ± 2.9 | 0.336 a |
| maximal gradient, mean mmHg | 13.9 ± 5.4 | 17.1 ± 6.7 | 0.375 a |
| mortality, n (%) | |||
| in-hospital | 1 (7) | 0 (0) | 0.484 d |
| during follow-up | 0 (0) | 0 (0) | |
| intraoperative complications, n (%) | |||
| paravalvular leak (aortic) | 0 (0) | 0 (0) | |
| valve deployment (aortic) | 0 (0) | 0 (0) | |
| extracorporeal membrane oxygenation or extracorporeal life support | 0 (0) | 0 (0) | |
| postoperative complications, n (%) | |||
| reintubation | 0 (0) | 0 (0) | |
| CPR, myocardial infarction | 1 (7) | 0 (0) | 0.484 d |
| renal failure, permanent | 1 (7) | 0 (0) | 0.484 d |
| major stroke or TIA | 0 (0) | 0 (0) | |
| delirium | 1 (7) | 2 (13) | 1.000 d |
| rethoracotomy (tamponade) | 2 (13) | 2 (13) | 1.000 d |
| new pacemaker implantation | 3 (20) | 1 (6) | 0.333 d |
| paravalvular leak | 0 (0) | 0 (0) | |
| dislocation of prothesis | 0 (0) | 0 (0) | |
| redo surgery | 0 (0) | 0 (0) | |
| postoperative infection | 9 (60) | 8 (50) | 0.576 b |
| endocarditis, sepsis or systemic inflammatory response syndrome | 0 (0) | 0 (0) | |
| pneumonia | 8 (53) | 6 (38) | 0.376 b |
| urogenital | 0 (0) | 1 (6) | 0.504 b |
| skin | 0 (0) | 1 (6) | 1.000 d |
| hospital and intensive care unit stay | |||
| ventilation time, h | 13.3 ± 12.2 | 12.7 ± 3.9 | 0.097 a |
| intensive care unit stay, d | 4.6 ± 5.8 | 3.6 ± 2.2 | 0.322 a |
| hospital stay, d | 12.1 ± 4.7 | 14.8 ± 4.3 | 0.548 a |
| follow-up complications 3-months, n (%) | 0 (0) | 0 (0) |
| BAV (n = 31) | TAV (n = 79) | p (BAV vs TAV) | |
|---|---|---|---|
| mean New Euroscore II,% | 2.40 ± 2.73 | 2.02 ± 1.69 | 0.146 a |
| aortic stenosis, n (%) | 25 (80.1) | 51 (64.6) | 0.444 b |
| aortic regurgitation, n (%) | 0 (0) | 5 (6.3) | 0.473 b |
| combined aortic stenosis and regurgitation, n (%) | 9 (29) | 23 (29.1) | 0.709 b |
| mean preoperative gradient, mean mmHg | 44.79 ± 13.67 | 40.24 ± 16.32 | 0.086 c |
| aortic orifice area, mean cm2 | 0.80 ± 0.27 | 0.84 ± 0.27 | 0.506 a |
| concomitant procedures, n (%) | |||
| coronary artery bypass graft | 5 (14.3) | 48 (60.8) | <0.001 b |
| mitral valve | 3 (8.6) | 8 (10.1) | 0.795 b |
| tricuspid valve | 0 (0) | 0 (0) | |
| aortic surgery | 13 (37.1) | 0 (0) | <0.001 b |
| aortic prosthetic size, mean, mm, n (%) | 25.1 ± 2 | 24.11 ± 1.49 | 0.003 a,c |
| cardiopulmonary bypass time | 119.7 ± 44.5 | 111 ± 38.0 | 0.250 a |
| cross-clamp time | 72.5 ± 24.95 | 76.9 ± 26.2 | 0.661 a |
| mean postoperative gradient, mean mmHg | 8.3 ± 3.1 | 8.85 ± 5.56 | 0.804 c |
| mortality, n (%) | |||
| in-hospital | 1 (3.2) | 0 (0) | |
| during follow-up | 0 (0) | 0 (0) | |
| valvular complications, n (%) | 0 (0) | 0 (0) | |
| new pacemaker implantation | 3 (8.6) | 3 (3.8) | 0.362 b |
| redo surgery | 0 (0) | 0 (0) | |
| major stroke | 0 (0) | 0 (0) | |
| intensive care unit stay, d | 3.8 ± 4.1 | 3.6 ± 2.5 | 0.382 c |
| follow-up complications 3-months, n (%) | 0 (0) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von der Linden, J.; Herrmann, F.; Belyaev, S.; Juchem, G.; Peterss, S.; Hagl, C.; Dashkevich, A. Bicuspid Morphology and Rapid Deployment Valve Replacement: Is This Still a Contraindication? J. Clin. Med. 2023, 12, 7390. https://doi.org/10.3390/jcm12237390
von der Linden J, Herrmann F, Belyaev S, Juchem G, Peterss S, Hagl C, Dashkevich A. Bicuspid Morphology and Rapid Deployment Valve Replacement: Is This Still a Contraindication? Journal of Clinical Medicine. 2023; 12(23):7390. https://doi.org/10.3390/jcm12237390
Chicago/Turabian Stylevon der Linden, Julia, Florian Herrmann, Sergey Belyaev, Gerd Juchem, Sven Peterss, Christian Hagl, and Alexey Dashkevich. 2023. "Bicuspid Morphology and Rapid Deployment Valve Replacement: Is This Still a Contraindication?" Journal of Clinical Medicine 12, no. 23: 7390. https://doi.org/10.3390/jcm12237390
APA Stylevon der Linden, J., Herrmann, F., Belyaev, S., Juchem, G., Peterss, S., Hagl, C., & Dashkevich, A. (2023). Bicuspid Morphology and Rapid Deployment Valve Replacement: Is This Still a Contraindication? Journal of Clinical Medicine, 12(23), 7390. https://doi.org/10.3390/jcm12237390