Detection of GSTM1-null Genotype in Women Undergoing IVF Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Population and Participant Characteristics
2.2. Ethical Approval
2.3. DNA Extraction and GSTM1 Genotyping
2.4. Hormone Assays
2.5. Controlled Ovarian Stimulation (COS)
2.6. Embryological Processes and Pregnancy
2.7. Statistical Analysis
3. Results
3.1. Detection of the Presence/Absence of GSTM1-null Genotype (Deletion) in Infertile Women
3.2. Hormonal and IVF Parameters
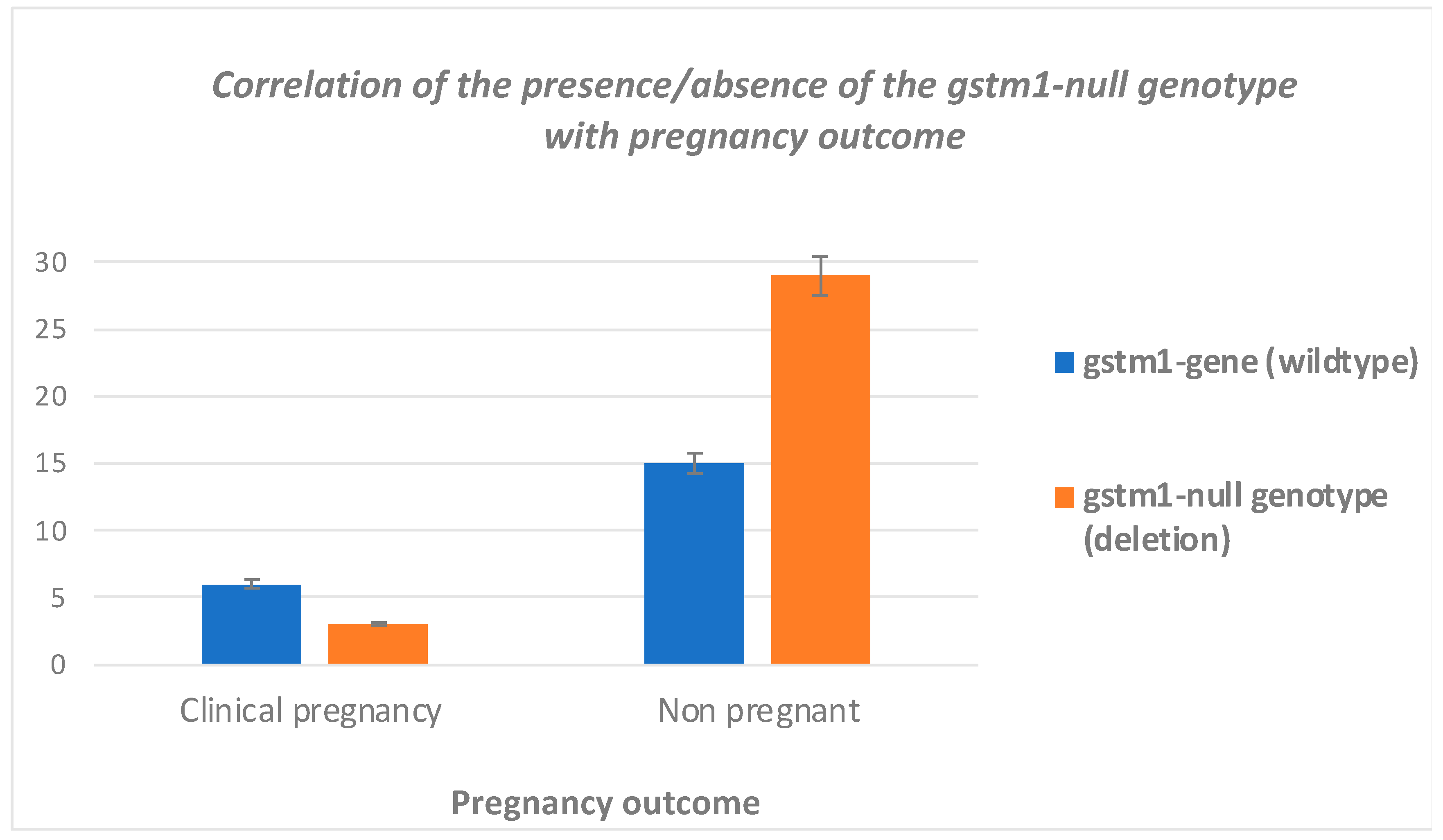
3.3. Clinical Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farquhar, C.M.; Bhattacharya, S.; Repping, S.; Mastenbroek, S.; Kamath, M.S.; Marjoribanks, J.; Boivin, J. Female subfertility. Nat. Rev. Dis. Primers 2019, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthopoulos, I.; Mavrogianni, D.; Drakaki, E.; Potiris, A.; Zikopoulos, A.; Zachariou, A.; Domali, E.; Drakakis, P.; Stavros, S. Detection of zeb1 Gene in Granulosa Cells in Women Undergoing IVF Treatment. J. Clin. Med. 2023, 12, 5652. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.P.P.; Scholten, J.T.M.; Mzyk, A.; Reyes-San-Martin, C.; Llumbet, A.E.; Hamoh, T.; Arts, E.; Schirhagl, R.; Cantineau, A.E.P. Male subfertility and oxidative stress. Redox Biol. 2021, 46, 102071. [Google Scholar] [CrossRef] [PubMed]
- Casteleiro Alves, M.M.; Almeida, M.; Oliani, A.H.; Breitenfeld, L.; Ramalhinho, A.C. CYP19A1 TC/CC Polymorphism, along with Deletion of GSTM1 and GSTT1 Genes, Strongly Influences Female Infertility Risk. Antioxidants 2023, 12, 940. [Google Scholar] [CrossRef]
- Panagopoulos, P.; Mavrogianni, D.; Christodoulaki, C.; Drakaki, E.; Chrelias, G.; Panagiotopoulos, D.; Potiris, A.; Drakakis, P.; Stavros, S. Effects of endocrine disrupting compounds on female fertility. Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 88, 102347. [Google Scholar] [CrossRef] [PubMed]
- Sanocka, D.; Kurpisz, M. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2004, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sharma, R. Oxidative stress and its implications in female infertility—A clinician’s perspective. Reprod. Biomed. Online 2005, 11, 641–650. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Ruder, E.H.; Hartman, T.J.; Goldman, M.B. Impact of oxidative stress on female fertility. Curr. Opin. Obstet. Gynecol. 2009, 21, 219–222. [Google Scholar] [CrossRef]
- Llavanera, M.; Mateo-Otero, Y.; Bonet, S.; Barranco, I.; Fernandez-Fuertes, B.; Yeste, M. The triple role of glutathione S-transferases in mammalian male fertility. Cell. Mol. Life Sci. 2020, 77, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Kubiszeski, E.H.; de Medeiros, S.F.; da Silva Seidel, J.A.; Barbosa, J.S.; Galera, M.F.; Galera, B.B. Glutathione S-transferase M1 and T1 gene polymorphisms in Brazilian women with endometriosis. J. Assist. Reprod. Genet. 2015, 32, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Li, Y.; Wu, J.L.; Zhao, J.; Tian, Y.J.; Kang, S. Genetic Variation of Glutathione S-Transferase M1 Is Associated with Patients with Ovarian Endometriosis and Endometriosis-Related Primary Infertility. Public Health Genom. 2021, 24, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.M.P.; Marqui, A.B.T.; Bacala, B.T.; Balarin, M.A.S.; Resende, E.; Lima, M.F.P.; Gomes, M.K.O.; Cintra, M.T.R. Polymorphisms of the GSTT1 and GSTM1 genes in polycystic ovary syndrome. Rev. Assoc. Med. Bras. 2020, 66, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.M.C.; Almeida, M.; Oliani, A.H.; Breitenfeld, L.; Ramalhinho, A.C. Women with polycystic ovary syndrome and other causes of infertility have a higher prevalence of GSTT1 deletion. Reprod. Biomed. Online 2020, 41, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Baranov, V.S.; Ivaschenko, T.; Bakay, B.; Aseev, M.; Belotserkovskaya, R.; Baranova, H.; Malet, P.; Perriot, J.; Mouraire, P.; Baskakov, V.N.; et al. Proportion of the GSTM1 0/0 genotype in some Slavic populations and its correlation with cystic fibrosis and some multifactorial diseases. Hum. Genet. 1996, 97, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.B.; Pan, X.M.; Li, L.J.; Liang, W.B.; Bai, P.; Rao, L.; Su, X.W.; Wang, T.; Zhou, B.; Wei, Y.G.; et al. Null genotypes of GSTM1 and GSTT1 contribute to risk of cervical neoplasia: An evidence-based meta-analysis. PLoS ONE 2011, 6, e20157. [Google Scholar] [CrossRef]
- Baxter, S.W.; Thomas, E.J.; Campbell, I.G. GSTM1 null polymorphism and susceptibility to endometriosis and ovarian cancer. Carcinogenesis 2001, 22, 63–65. [Google Scholar] [CrossRef][Green Version]
- Mavrogianni, D.L.P.; Liokari, M.-E.; Giannoulis, G.; Anagnostou, E.; Chatzipapas, C.; Protopapas, A.; Drakakis, P. ESR1, FSH-receptor, and GSTM1, Polymorphisms in Infertile Greek Women with Advanced Endometriosis—A Pilot Study. Gynecol. Obstet. Curr. Res. 2019, 1, GOCR-1-101. [Google Scholar]
- Suryanarayana, V.; Deenadayal, M.; Singh, L. Association of CYP1A1 gene polymorphism with recurrent pregnancy loss in the South Indian population. Hum. Reprod. 2004, 19, 2648–2652. [Google Scholar] [CrossRef]
- Mear, L.; Herr, M.; Fauconnier, A.; Pineau, C.; Vialard, F. Polymorphisms and endometriosis: A systematic review and meta-analyses. Hum. Reprod. Update 2020, 26, 73–102. [Google Scholar] [CrossRef] [PubMed]
- Potiris, A.; Voitse, A.; Mavrogianni, D.; Machairiotis, N.; Drakaki, E.; Papamentzelopoulou, M.; Karampitsakos, T.; Zikopoulos, A.; Evgeni, E.; Drakakis, P.; et al. Association of GSTM1 Polymorphism and Redox Potential with Idiopathic Male Infertility. J. Clin. Med. 2023, 12, 6775. [Google Scholar] [CrossRef] [PubMed]
- Christ, J.P.; Cedars, M.I. Current Guidelines for Diagnosing PCOS. Diagnostics 2023, 13, 1113. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.J.; Zhang, S.H.; Li, L. Comparing and evaluating six methods of extracting human genomic DNA from whole blood. J. Forensic Sci. 2009, 25, 109–111, 114. [Google Scholar]
- Loutradis, D.; Patsoula, E.; Minas, V.; Koussidis, G.A.; Antsaklis, A.; Michalas, S.; Makrigiannakis, A. FSH receptor gene polymorphisms have a role for different ovarian response to stimulation in patients entering IVF/ICSI-ET programs. J. Assist. Reprod. Genet. 2006, 23, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.C.; Wildt, D.E. Effect of the quality of the cumulus-oocyte complex in the domestic cat on the ability of oocytes to mature, fertilize and develop into blastocysts in vitro. J. Reprod. Fertil. 1997, 110, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Grondahl, C. Oocyte maturation. Basic and clinical aspects of in vitro maturation (IVM) with special emphasis of the role of FF-MAS. Dan. Med. Bull. 2008, 55, 1–16. [Google Scholar]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef]
- Da Broi, M.G.; Navarro, P.A. Oxidative stress and oocyte quality: Ethiopathogenic mechanisms of minimal/mild endometriosis-related infertility. Cell Tissue Res. 2016, 364, 1–7. [Google Scholar] [CrossRef]
- Bodal, V.K.; Dhir, M.; Ahi, K.S.; Kaur, S.; Singh, M.; Bandhari, L. Role of Glutathione S-Transferase M1 and Glutathione S Transferase Theta 1 Gene Polymorphism, Histopathological, and Immunohistochemistry in Carcinoma Breast. Int. J. Appl. Basic Med. Res. 2021, 11, 243–247. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, A.; Singh, N.; Behera, D.; Sharma, S. Genetic polymorphisms in GSTM1, GSTT1 and GSTP1 genes and risk of lung cancer in a North Indian population. Cancer Epidemiol. 2015, 39, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Lao, X.; Peng, Q.; Lu, Y.; Li, S.; Qin, X.; Chen, Z.; Chen, J. Glutathione S-transferase gene GSTM1, gene-gene interaction, and gastric cancer susceptibility: Evidence from an updated meta-analysis. Cancer Cell Int. 2014, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Lavaris, A.; Gazouli, M.; Brouzas, D.; Moschos, M.M. Polymorphism Analysis of GSTM1 and OPA1 Genes in Greek Patients with Primary Open-angle Glaucoma. In Vivo 2016, 30, 473–477. [Google Scholar]
- Stamenkovic, M.; Lukic, V.; Suvakov, S.; Simic, T.; Sencanic, I.; Pljesa-Ercegovac, M.; Jaksic, V.; Babovic, S.; Matic, M.; Radosavljevic, A.; et al. GSTM1-null and GSTT1-active genotypes as risk determinants of primary open angle glaucoma among smokers. Int. J. Ophthalmol. 2018, 11, 1514–1520. [Google Scholar] [CrossRef]
- Yu, Y.; Weng, Y.; Guo, J.; Chen, G.; Yao, K. Association of glutathione S transferases polymorphisms with glaucoma: A meta-analysis. PLoS ONE 2013, 8, e54037. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.E.; Hong, Y.C.; Park, H.; Ha, M.; Koo, B.S.; Chang, N.; Roh, Y.M.; Kim, B.N.; Kim, Y.J.; Kim, B.M.; et al. Interaction between GSTM1/GSTT1 polymorphism and blood mercury on birth weight. Environ. Health Perspect. 2010, 118, 437–443. [Google Scholar] [CrossRef]
- Yohannes, Y.B.; Nakayama, S.M.M.; Yabe, J.; Toyomaki, H.; Kataba, A.; Nakata, H.; Muzandu, K.; Ikenaka, Y.; Choongo, K.; Ishizuka, M. Glutathione S-transferase gene polymorphisms in association with susceptibility to lead toxicity in lead- and cadmium-exposed children near an abandoned lead-zinc mining area in Kabwe, Zambia. Environ. Sci. Pollut. Res. Int. 2022, 29, 6622–6632. [Google Scholar] [CrossRef]
- Xu, X.B.; Liu, S.R.; Ying, H.Q.; A, Z.C. Null genotype of GSTM1 and GSTT1 may contribute to susceptibility to male infertility with impaired spermatogenesis in Chinese population. Biomarkers 2013, 18, 151–154. [Google Scholar] [CrossRef]
- Li, X.; Pan, J.; Liu, Q.; Xiong, E.; Chen, Z.; Zhou, Z.; Su, Y.; Lu, G. Glutathione S-transferases gene polymorphisms and risk of male idiopathic infertility: A systematic review and meta-analysis. Mol. Biol. Rep. 2013, 40, 2431–2438. [Google Scholar] [CrossRef]
- Wu, W.; Lu, J.; Tang, Q.; Zhang, S.; Yuan, B.; Li, J.; Di, W.; Sun, H.; Lu, C.; Xia, Y.; et al. GSTM1 and GSTT1 null polymorphisms and male infertility risk: An updated meta-analysis encompassing 6934 subjects. Sci. Rep. 2013, 3, 2258. [Google Scholar] [CrossRef]
- de Oliveira, E.; de Aquino Castro, R.; Vieira Gomes, M.T.; Cotrim Guerreiro da Silva, I.D.; Baracat, E.C.; Rodrigues de Lima, G.; Ferreira Sartori, M.G.; Batista Castello Girao, M.J. Role of glutathione S-transferase (GSTM1) gene polymorphism in development of uterine fibroids. Fertil. Steril. 2009, 91, 1496–1498. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ramirez, O.C.; Perez-Morales, R.; Castro, C.; Flores-Diaz, A.; Soto-Cruz, K.E.; Astorga-Ramos, A.; Gonsebatt, M.E.; Casas, L.; Valdes-Flores, M.; Rubio, J. Polymorphisms of catechol estrogens metabolism pathway genes and breast cancer risk in Mexican women. Breast 2013, 22, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Reding, K.W.; Weiss, N.S.; Chen, C.; Li, C.I.; Carlson, C.S.; Wilkerson, H.W.; Farin, F.M.; Thummel, K.E.; Daling, J.R.; Malone, K.E. Genetic polymorphisms in the catechol estrogen metabolism pathway and breast cancer risk. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Saadat, M.; Bahaoddini, S.; Saadat, I. Alteration of serum sex hormonal profile in male gasoline filling station workers in respect to their polymorphism of glutathione S-transferase M1. Environ. Toxicol. Pharmacol. 2013, 35, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Saadat, M.; Saadat, I. Serum testosterone in females exposed to natural sour gas with respect to polymorphisms of XRCC1, GSTM1, and GSTT1. Mol. Biol. Rep. 2011, 38, 89–94. [Google Scholar] [CrossRef]
- Polimanti, R.; Piacentini, S.; Lazzarin, N.; Vaquero, E.; Re, M.A.; Manfellotto, D.; Fuciarelli, M. Glutathione S-transferase genes and the risk of recurrent miscarriage in Italian women. Fertil. Steril. 2012, 98, 396–400. [Google Scholar] [CrossRef]
- Nair, R.R.; Khanna, A.; Singh, K. Association of GSTT1 and GSTM1 polymorphisms with early pregnancy loss in an Indian population and a meta-analysis. Reprod. Biomed. Online 2013, 26, 313–322. [Google Scholar] [CrossRef]
- Karimlo, F.K.; Mashayekhi, F.; Sorouri, Z.Z.; Bahador, M.H.; Salehi, Z. Association of GSTM1 and GSTT1 gene polymorphisms and in-vitro fertilisation outcome in a population in northern Iran. J. Obstet. Gynaecol. 2015, 35, 46–48. [Google Scholar] [CrossRef]
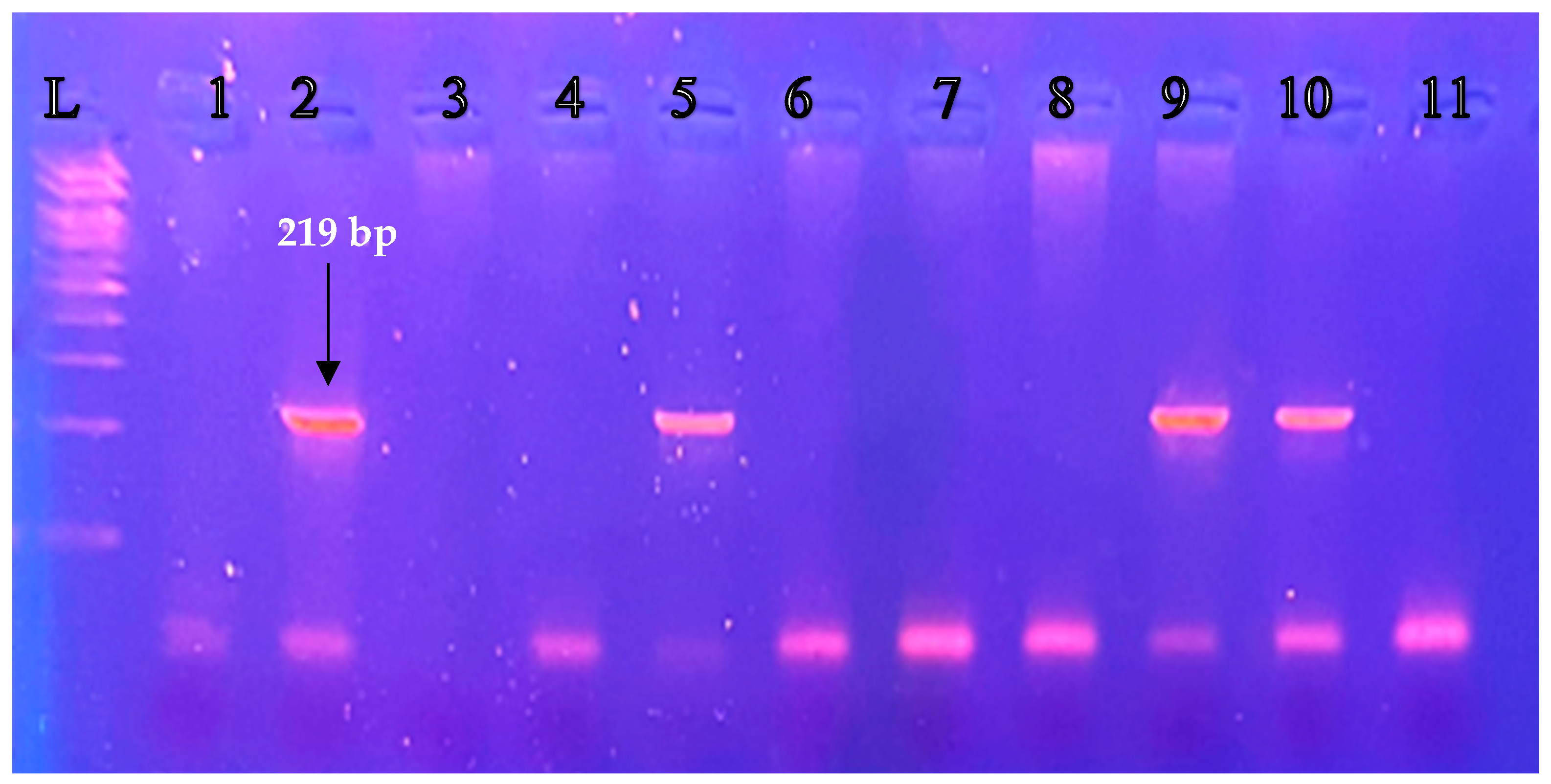
| Control Group | Infertility Group | |
|---|---|---|
| Sample size | 49 | 125 |
| Ethnicity (Caucasian) | 49 | 125 |
| Age (in years) | 37.10 ± 4.67 | 36.20 ± 5.24 |
| Weight (in kilograms) | 59.2 ± 6 | 60.9 ± 9 |
| BMI (kg/m2) | 23.7 ± 1.8 | 22.87 ± 3.22 |
| Infertility duration (in years) | n/a | 4.20 ± 2.5 |
| Group | Risk Estimate | |||||
|---|---|---|---|---|---|---|
| Control | Infertile | OR | 95% CI for OR | p-Value | ||
| GSTM1-null type | 9 (18.37%) | 49 (39.20%) | 2.865 | (1.278 6.424) | 0.0087 | |
| GSTM1-wild type | 40 (81.63%) | 76 (60.80%) | ||||
| Variables | GSTM1-null Genotype (Deletion) | N | Mean | SD | p-Value (5%) |
|---|---|---|---|---|---|
| Infertility Duration | Presence | 32 | 4.50 | 2.83 | 0.510 |
| Absence | 22 | 3.82 | 2.32 | ||
| Days of Stimulation | Presence | 32 | 9.78 | 1.26 | 0.300 |
| Absence | 20 | 9.30 | 1.26 | ||
| Number of Follicles | Presence | 32 | 6.13 | 3.09 | 0.017 * |
| Absence | 21 | 8.29 | 2.99 | ||
| Number of COC | Presence | 32 | 5.45 | 3.03 | 0.025 * |
| Absence | 22 | 7.36 | 3.23 | ||
| Number of excellent/good quality COC | Presence | 32 | 4.13 | 2.57 | 0.042 * |
| Absence | 21 | 5.43 | 2.29 | ||
| % Maturation Rate | Presence | 32 | 71% | 20% | 0.412 |
| Absence | 21 | 69% | 12% | ||
| Number of 2PN embryos | Presence | 32 | 4.13 | 2.21 | 0.013 * |
| Absence | 21 | 5.57 | 2.40 | ||
| Number of previous IVF cycles | Presence | 32 | 1.79 | 1.05 | 0.484 |
| Absence | 22 | 1.50 | 0.74 | ||
| Total FSH administered (IU) | Presence | 32 | 3060.94 | 1170.42 | 0.920 |
| Absence | 21 | 2984.52 | 893.19 | ||
| E2 (pg/mL) | Presence | 32 | 1460 | 808 | 0.000 * |
| Absence | 20 | 2512 | 1007 |
| Variables | GSTM1-Null Genotype (Deletion) | N | Mean | SD | p-Value (5%) |
|---|---|---|---|---|---|
| FSH (mIU/L) | Presence | 32 | 10 | 14 | 0.729 |
| Absence | 21 | 9 | 5 | ||
| LH (mIU/L) | Presence | 32 | 6.08 | 6.97 | 0.126 |
| Absence | 22 | 6.60 | 3.64 | ||
| PRL (ng/mL) | Presence | 28 | 12.97 | 6.61 | 0.194 |
| Absence | 17 | 15.36 | 7.04 | ||
| AMH (pg/L) | Presence | 32 | 9.56 | 8.06 | 0.232 |
| Absence | 22 | 13.95 | 11.69 | ||
| BMI (kg/m2) | Presence | 32 | 23.25 | 3.10 | 0.519 |
| Absence | 22 | 22.79 | 3.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysanthopoulos, I.; Petsavas, A.; Mavrogianni, D.; Potiris, A.; Machairiotis, N.; Drakaki, E.; Vrachnis, D.; Machairoudias, P.; Karampitsakos, T.; Perros, P.; et al. Detection of GSTM1-null Genotype in Women Undergoing IVF Treatment. J. Clin. Med. 2023, 12, 7269. https://doi.org/10.3390/jcm12237269
Chrysanthopoulos I, Petsavas A, Mavrogianni D, Potiris A, Machairiotis N, Drakaki E, Vrachnis D, Machairoudias P, Karampitsakos T, Perros P, et al. Detection of GSTM1-null Genotype in Women Undergoing IVF Treatment. Journal of Clinical Medicine. 2023; 12(23):7269. https://doi.org/10.3390/jcm12237269
Chicago/Turabian StyleChrysanthopoulos, Ioannis, Angelos Petsavas, Despoina Mavrogianni, Anastasios Potiris, Nikolaos Machairiotis, Eirini Drakaki, Dionysios Vrachnis, Pavlos Machairoudias, Theodoros Karampitsakos, Paraskevas Perros, and et al. 2023. "Detection of GSTM1-null Genotype in Women Undergoing IVF Treatment" Journal of Clinical Medicine 12, no. 23: 7269. https://doi.org/10.3390/jcm12237269
APA StyleChrysanthopoulos, I., Petsavas, A., Mavrogianni, D., Potiris, A., Machairiotis, N., Drakaki, E., Vrachnis, D., Machairoudias, P., Karampitsakos, T., Perros, P., Koratzanis, C., Drakakis, P., & Stavros, S. (2023). Detection of GSTM1-null Genotype in Women Undergoing IVF Treatment. Journal of Clinical Medicine, 12(23), 7269. https://doi.org/10.3390/jcm12237269







