Cardiac Magnetic Resonance Identifies Responders to Cardiac Resynchronization Therapy with an Assessment of Septal Scar and Left Ventricular Dyssynchrony
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Cardiac Magnetic Resonance (CMR)
Myocardial Scar
2.3. Indices of LV Dyssynchrony
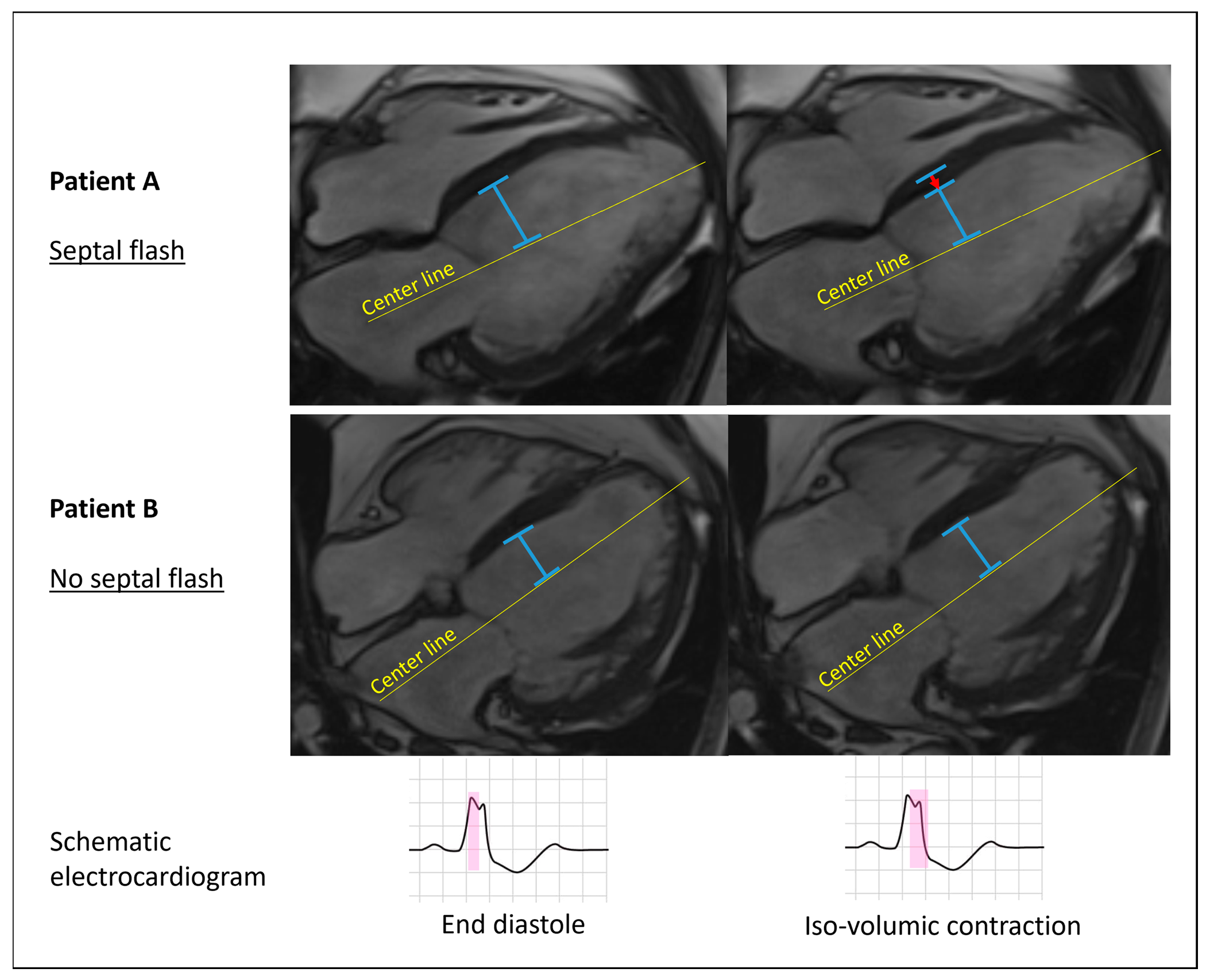
2.3.1. Septal Flash
2.3.2. Myocardial Work
2.4. Echocardiography
2.5. Device Implantation
2.6. Endpoints
2.7. Statistical Analyses
3. Results
3.1. Study Population
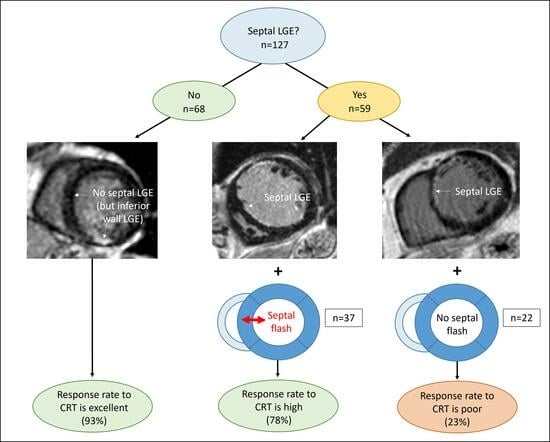
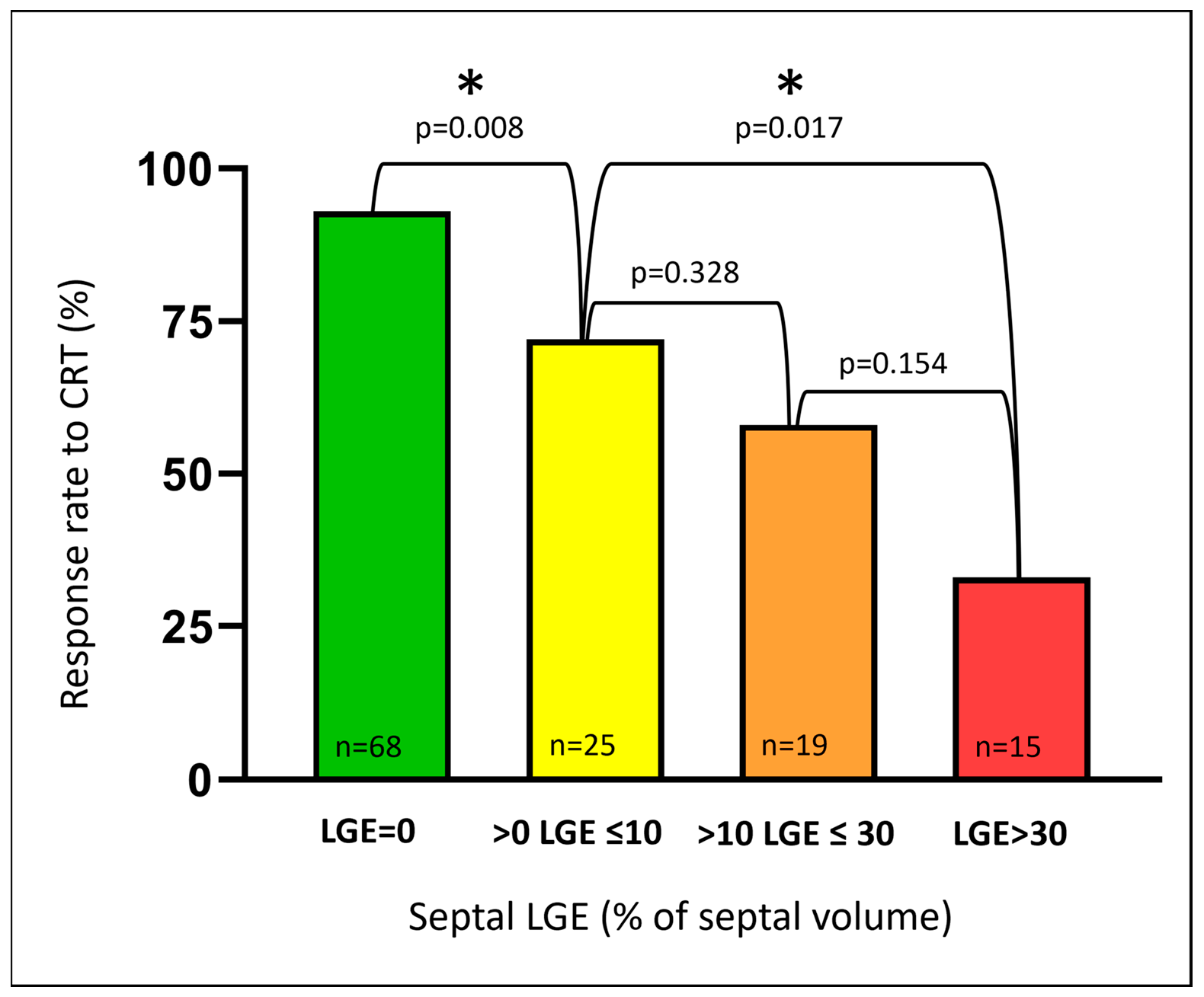
3.2. Septal Scar (LGE)
3.3. Septal Flash
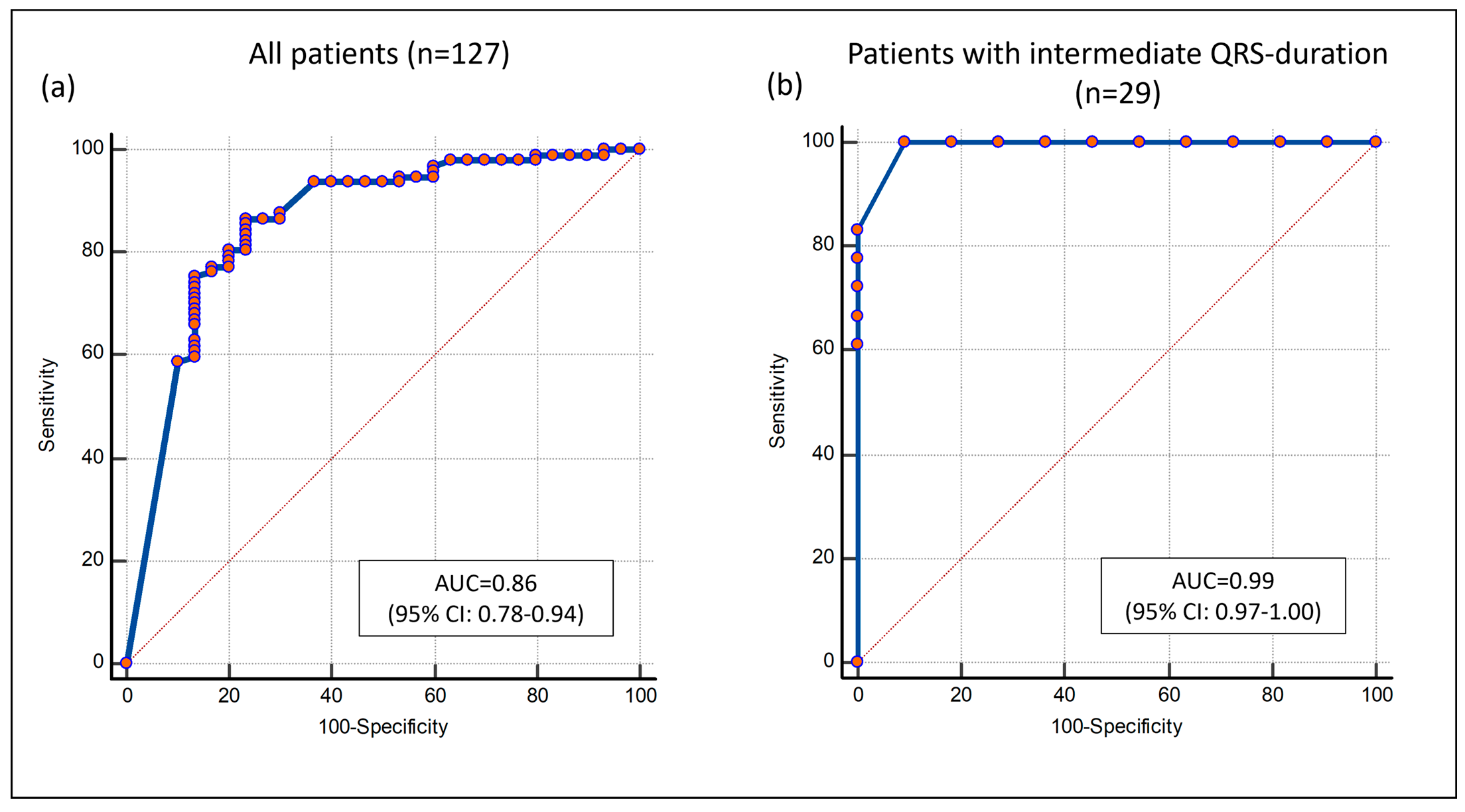
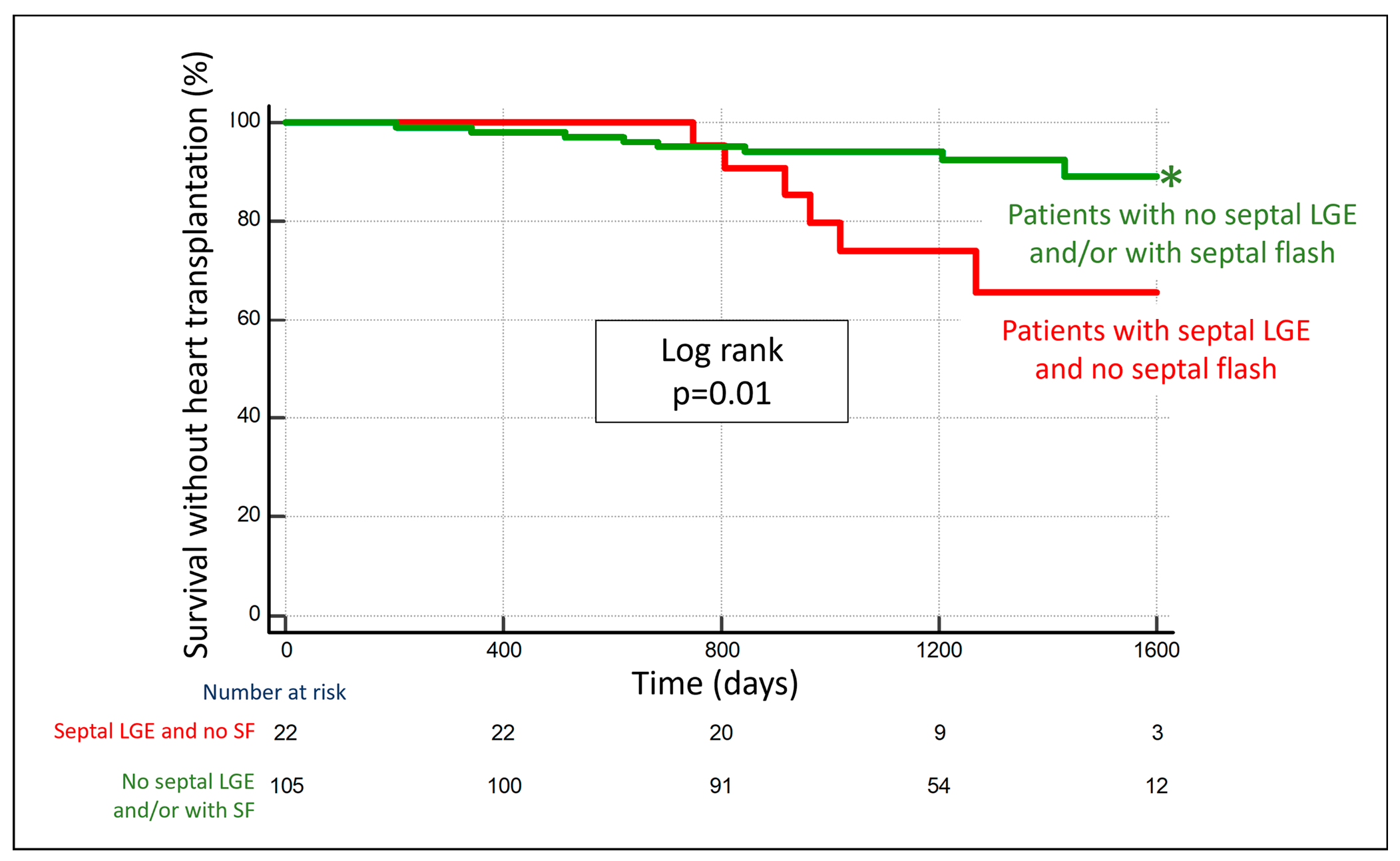
3.4. Combining Septal LGE and Septal Flash
3.5. Lateral-to-Septal Work Difference
3.6. Septal Flash with Echocardiography
4. Discussion
4.1. Septal Markers Define the CRT Response
4.2. LV Lateral-to-Septal Work Difference
4.3. Clinical Implications
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cleland, J.G.; Daubert, J.C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L.; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef]
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Packer, M.; Clavell, A.L.; Hayes, D.L.; et al. Cardiac Resynchronization in Chronic Heart Failure. N. Engl. J. Med. 2002, 346, 1845–1853. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA). Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Mollema, S.A.; Bleeker, G.B.; van der Wall, E.E.; Schalij, M.J.; Bax, J.J. Usefulness of QRS duration to predict response to cardiac resynchronization therapy in patients with end-stage heart failure. Am. J. Cardiol. 2007, 100, 1665–1670. [Google Scholar] [CrossRef]
- Vernooy, K.; Cornelussen, R.N.M.; Verbeek, X.A.A.M.; Vanagt, W.Y.R.; van Hunnik, A.; Kuiper, M.; Arts, T.; Crijns, H.J.G.M.; Prinzen, F.W. Cardiac resynchronization therapy cures dyssynchronopathy in canine left bundle-branch block hearts. Eur. Heart J. 2007, 28, 2148–2155. [Google Scholar] [CrossRef]
- Vernooy, K.; Verbeek, X.A.; Peschar, M.; Crijns, H.J.; Arts, T.; Cornelussen, R.N.; Prinzen, F.W. Left bundle branch block induces ventricular remodelling and functional septal hypoperfusion. Eur. Heart J. 2005, 26, 91–98. [Google Scholar] [CrossRef]
- Parsai, C.; Bijnens, B.; Sutherland, G.R.; Baltabaeva, A.; Claus, P.; Marciniak, M.; Paul, V.; Scheffer, M.; Donal, E.; Derumeaux, G.; et al. Toward understanding response to cardiac resynchronization therapy: Left ventricular dyssynchrony is only one of multiple mechanisms. Eur. Heart J. 2009, 30, 940–949. [Google Scholar] [CrossRef]
- Stankovic, I.; Prinz, C.; Ciarka, A.; Daraban, A.M.; Kotrc, M.; Aarones, M.; Szulik, M.; Winter, S.; Belmans, A.; Neskovic, A.N.; et al. Relationship of visually assessed apical rocking and septal flash to response and long-term survival following cardiac resynchronization therapy (PREDICT-CRT). Eur. Heart J. Cardiovasc. Imaging 2016, 17, 262–269. [Google Scholar] [CrossRef]
- Maass, A.H.; Vernooy, K.; Wijers, S.C.; van ‘t Sant, J.; Cramer, M.J.; Meine, M.; Allaart, C.P.; De Lange, F.J.; Prinzen, F.W.; Gerritse, B.; et al. Refining success of cardiac resynchronization therapy using a simple score predicting the amount of reverse ventricular remodelling: Results from the Markers and Response to CRT (MARC) study. Europace 2018, 20, e1–e10. [Google Scholar] [CrossRef]
- Bleeker, G.B.; Kaandorp, T.A.; Lamb, H.J.; Boersma, E.; Steendijk, P.; de Roos, A.; van der Wall, E.E.; Schalij, M.J.; Bax, J.J. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation 2006, 113, 969–976. [Google Scholar] [CrossRef]
- Chalil, S.; Stegemann, B.; Muhyaldeen, S.A.; Khadjooi, K.; Foley, P.W.; Smith, R.E.; Leyva, F. Effect of posterolateral left ventricular scar on mortality and morbidity following cardiac resynchronization therapy. Pacing Clin. Electrophysiol. 2007, 30, 1201–1209. [Google Scholar] [CrossRef]
- White, J.A.; Yee, R.; Yuan, X.; Krahn, A.; Skanes, A.; Parker, M.; Klein, G.; Drangova, M. Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J. Am. Coll. Cardiol. 2006, 48, 1953–1960. [Google Scholar] [CrossRef]
- Aalen, J.M.; Donal, E.; Larsen, C.K.; Duchenne, J.; Lederlin, M.; Cvijic, M.; Hubert, A.; Voros, G.; Leclercq, C.; Bogaert, J.; et al. Imaging predictors of response to cardiac resynchronization therapy: Left ventricular work asymmetry by echocardiography and septal viability by cardiac magnetic resonance. Eur. Heart J. 2020, 41, 3813–3823. [Google Scholar] [CrossRef]
- Larsen, C.K.; Galli, E.; Duchenne, J.; Aalen, J.M.; Stokke, C.; Fjeld, J.G.; Degtiarova, G.; Claus, P.; Gheysens, O.; Saberniak, J.; et al. Scar imaging in the dyssynchronous left ventricle: Accuracy of myocardial metabolism by positron emission tomography and function by echocardiographic strain. Int. J. Cardiol. 2023, 372, 122–129. [Google Scholar] [CrossRef]
- Heiberg, E.; Sjögren, J.; Ugander, M.; Carlsson, M.; Engblom, H.; Arheden, H. Design and validation of Segment--freely available software for cardiovascular image analysis. BMC Med. Imaging 2010, 10, 1. [Google Scholar] [CrossRef]
- Engblom, H.; Tufvesson, J.; Jablonowski, R.; Carlsson, M.; Aletras, A.H.; Hoffmann, P.; Jacquier, A.; Kober, F.; Metzler, B.; Erlinge, D.; et al. A new automatic algorithm for quantification of myocardial infarction imaged by late gadolinium enhancement cardiovascular magnetic resonance: Experimental validation and comparison to expert delineations in multi-center, multi-vendor patient data. J. Cardiovasc. Magn. Reson. 2016, 18, 27. [Google Scholar] [CrossRef]
- Larsen, C.K.; Aalen, J.M.; Stokke, C.; Fjeld, J.G.; Kongsgaard, E.; Duchenne, J.; Degtiarova, G.; Gheysens, O.; Voigt, J.U.; Smiseth, O.A.; et al. Regional myocardial work by cardiac magnetic resonance and non-invasive left ventricular pressure: A feasibility study in left bundle branch block. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 143–153. [Google Scholar] [CrossRef]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef]
- Gold, M.R.; Daubert, C.; Abraham, W.T.; Ghio, S.; St John Sutton, M.; Hudnall, J.H.; Cerkvenik, J.; Linde, C. The effect of reverse remodeling on long-term survival in mildly symptomatic patients with heart failure receiving cardiac resynchronization therapy: Results of the REVERSE study. Heart Rhythm. 2015, 12, 524–530. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef]
- Hunold, P.; Schlosser, T.; Vogt, F.M.; Eggebrecht, H.; Schmermund, A.; Bruder, O.; Schüler, W.O.; Barkhausen, J. Myocardial late enhancement in contrast-enhanced cardiac MRI: Distinction between infarction scar and non-infarction-related disease. AJR Am. J. Roentgenol. 2005, 184, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Sohal, M.; Amraoui, S.; Chen, Z.; Sammut, E.; Jackson, T.; Wright, M.; O’Neill, M.; Gill, J.; Carr-White, G.; Rinaldi, C.A.; et al. Combined identification of septal flash and absence of myocardial scar by cardiac magnetic resonance imaging improves prediction of response to cardiac resynchronization therapy. J. Interv. Card. Electrophysiol. 2014, 40, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Zweerink, A.; Friedman, D.J.; Klem, I.; van de Ven, P.M.; Vink, C.; Biesbroek, P.S.; Hansen, S.M.; Kim, R.J.; van Rossum, A.C.; Atwater, B.D.; et al. Segment Length in Cine Strain Analysis Predicts Cardiac Resynchronization Therapy Outcome Beyond Current Guidelines. Circ. Cardiovasc. Imaging 2021, 14, e012350. [Google Scholar] [CrossRef]
- Chung, E.S.; Leon, A.R.; Tavazzi, L.; Sun, J.P.; Nihoyannopoulos, P.; Merlino, J.; Abraham, W.T.; Ghio, S.; Leclercq, C.; Bax, J.J.; et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008, 117, 2608–2616. [Google Scholar] [CrossRef] [PubMed]
- Kass, D.A. An epidemic of dyssynchrony: But what does it mean? J. Am. Coll. Cardiol. 2008, 51, 12–17. [Google Scholar] [CrossRef]
- Tayal, B.; Sogaard, P.; Risum, N. Why Dyssynchrony Matters in Heart Failure? Card. Electrophysiol. Clin. 2019, 11, 39–47. [Google Scholar] [CrossRef]
- Lumens, J.; Leenders, G.E.; Cramer, M.J.; De Boeck, B.W.; Doevendans, P.A.; Prinzen, F.W.; Delhaas, T. Mechanistic evaluation of echocardiographic dyssynchrony indices: Patient data combined with multiscale computer simulations. Circ. Cardiovasc. Imaging 2012, 5, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, C.; Redfield, M.M.; Powell, B.D.; Lin, G.M.; Herges, R.M.; Hodge, D.O.; Olson, L.J.; Hayes, D.L.; Espinosa, R.E.; Rea, R.F.; et al. Dyssynchrony indices to predict response to cardiac resynchronization therapy: A comprehensive prospective single-center study. Circ. Heart Fail. 2010, 3, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.C.; Sauer, W.H.; Duque, M.; Koplan, B.A.; Braunstein, E.D.; Marín, J.E.; Aristizabal, J.; Niño, C.D.; Bastidas, O.; Martinez, J.M.; et al. Left Bundle Branch Area Pacing versus Biventricular Pacing as Initial Strategy for Cardiac Resynchronization. JACC Clin. Electrophysiol. 2023, 9, 1568–1581. [Google Scholar] [CrossRef]
- Mao, Y.; Duchenne, J.; Yang, Y.; Garweg, C.; Yang, Y.; Sheng, X.; Zhang, J.; Ye, Y.; Wang, M.; Paton, M.F.; et al. Left bundle branch pacing better preserves ventricular mechanical synchrony than right ventricular pacing A two-center study. Eur. Heart J.-Cardiovasc. Imaging 2023, jead296, Online ahead of print. [Google Scholar] [CrossRef]
| All Patients (n = 136) | Responders (n = 103) | Non-Responders (n = 33) | p-Value | |
|---|---|---|---|---|
| Age (years) | 66 ± 10 | 67 ± 9 | 64 ± 11 | 0.071 |
| Male sex—no. (%) | 92 (68) | 65 (63) | 27 (82) | 0.046 |
| NYHA functional class—no. (%) | ||||
| I | 6 (4) | 6 (6) | 0 (0) | |
| II | 85 (63) | 65 (63) | 20 (61) | |
| III | 44 (32) | 32 (31) | 12 (36) | |
| IV | 1 (1) | 0 (0) | 1 (3) | |
| Medications—no. (%) | ||||
| ACE-inhibitor/ARB | 131 (96) | 100 (97) | 31 (94) | 0.403 |
| Beta blocker | 124 (91) | 92 (89) | 32 (97) | 0.178 |
| Aldosterone antagonist | 56 (41) | 42 (41) | 14 (42) | 0.797 |
| Sinus rhythm—no. (%) | 129 (95) | 99 (96) | 30 (91) | 0.239 |
| Heart failure etiology—no. (%) | ||||
| Ischemic | 42 (31) | 23 (22) | 19 (58) | <0.001 |
| Non-ischemic | 94 (69) | 80 (78) | 14 (42) | <0.001 |
| QRS duration (milliseconds) | 164 ± 17 | 166 ± 16 | 158 ± 18 | 0.021 |
| Left bundle branch block (%) | 124 (91) | 98 (95) | 26 (79) | 0.004 |
| LV EDV indexed (ml/m2) | 145 ± 46 | 139 ± 46 | 164 ± 41 | 0.008 |
| LV ESV indexed (ml/m2) | 76 ± 32 | 73 ± 31 | 88 ± 32 | 0.580 |
| LV ejection fraction (%) | 27 ± 8 | 28 ± 8 | 23 ± 6 | 0.003 |
| Anterior LGE (%) | 0 (0–6.5) | 0 (0–0.1) | 12.2 (0.8–36.2) | <0.001 |
| Septal LGE (%) | 0 (0–12.2) | 0 (0–3.2) | 16.3 (1.7–39.6) | <0.001 |
| Inferior LGE (%) | 0 (0–9.8) | 0 (0–3.9) | 10.5 (0.4–30.1) | <0.001 |
| Lateral LGE (%) | 0 (0–5.5) | 0 (0–0) | 5.6 (0–23.1) | <0.001 |
| Septal flash—no. (%) | 104 (76) | 92 (89) | 12 (36) | <0.001 |
| Lateral-to-septal work difference (mmHg·%) | 1551 ± 1080 | 1710 ± 1085 | 1061 ± 917 | 0.003 |
| Number of Patients | Number with Septal Involvement (% of Total) | |
|---|---|---|
LGE present
| 64 37 20 7 | 59 (92%) 33 (89%) 19 (95%) 6 (86%) |
Location of infarcts (ischemic LGE)
| 25 14 12 | 25 (100%) 10 (71%) 11 (92%) |
Classification of non-ischemic LGE
| 12 7 12 | 11 (92%) 7 (100%) 11 (92%) |
| Multivariable Analysis | ||||
|---|---|---|---|---|
| Regression Variable | B | 95% CI | VIF | p-Value |
| QRS duration (ms) | −0.036 | −0.249 to 0.177 | 1.158 | 0.738 |
| Left bundle branch block (yes/no) | −9.18 | −21.31 to 2.94 | 1.110 | 0.136 |
| Septal LGE (%) | 0.521 | 0.311 to 0.731 | 1.153 | <0.001 |
| Septal flash (yes/no) | −18.39 | −27.16 to −9.61 | 1.325 | <0.001 |
| Constant term | −8.513 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larsen, C.K.; Smiseth, O.A.; Duchenne, J.; Galli, E.; Aalen, J.M.; Lederlin, M.; Bogaert, J.; Kongsgaard, E.; Linde, C.; Penicka, M.; et al. Cardiac Magnetic Resonance Identifies Responders to Cardiac Resynchronization Therapy with an Assessment of Septal Scar and Left Ventricular Dyssynchrony. J. Clin. Med. 2023, 12, 7182. https://doi.org/10.3390/jcm12227182
Larsen CK, Smiseth OA, Duchenne J, Galli E, Aalen JM, Lederlin M, Bogaert J, Kongsgaard E, Linde C, Penicka M, et al. Cardiac Magnetic Resonance Identifies Responders to Cardiac Resynchronization Therapy with an Assessment of Septal Scar and Left Ventricular Dyssynchrony. Journal of Clinical Medicine. 2023; 12(22):7182. https://doi.org/10.3390/jcm12227182
Chicago/Turabian StyleLarsen, Camilla Kjellstad, Otto A. Smiseth, Jürgen Duchenne, Elena Galli, John Moene Aalen, Mathieu Lederlin, Jan Bogaert, Erik Kongsgaard, Cecilia Linde, Martin Penicka, and et al. 2023. "Cardiac Magnetic Resonance Identifies Responders to Cardiac Resynchronization Therapy with an Assessment of Septal Scar and Left Ventricular Dyssynchrony" Journal of Clinical Medicine 12, no. 22: 7182. https://doi.org/10.3390/jcm12227182
APA StyleLarsen, C. K., Smiseth, O. A., Duchenne, J., Galli, E., Aalen, J. M., Lederlin, M., Bogaert, J., Kongsgaard, E., Linde, C., Penicka, M., Donal, E., Voigt, J.-U., & Hopp, E. (2023). Cardiac Magnetic Resonance Identifies Responders to Cardiac Resynchronization Therapy with an Assessment of Septal Scar and Left Ventricular Dyssynchrony. Journal of Clinical Medicine, 12(22), 7182. https://doi.org/10.3390/jcm12227182