Scheimpflug Corneal Densitometry Patterns at the Graft–Host Interface in DMEK and DSAEK: A 12-Month Longitudinal Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Surgical Procedures
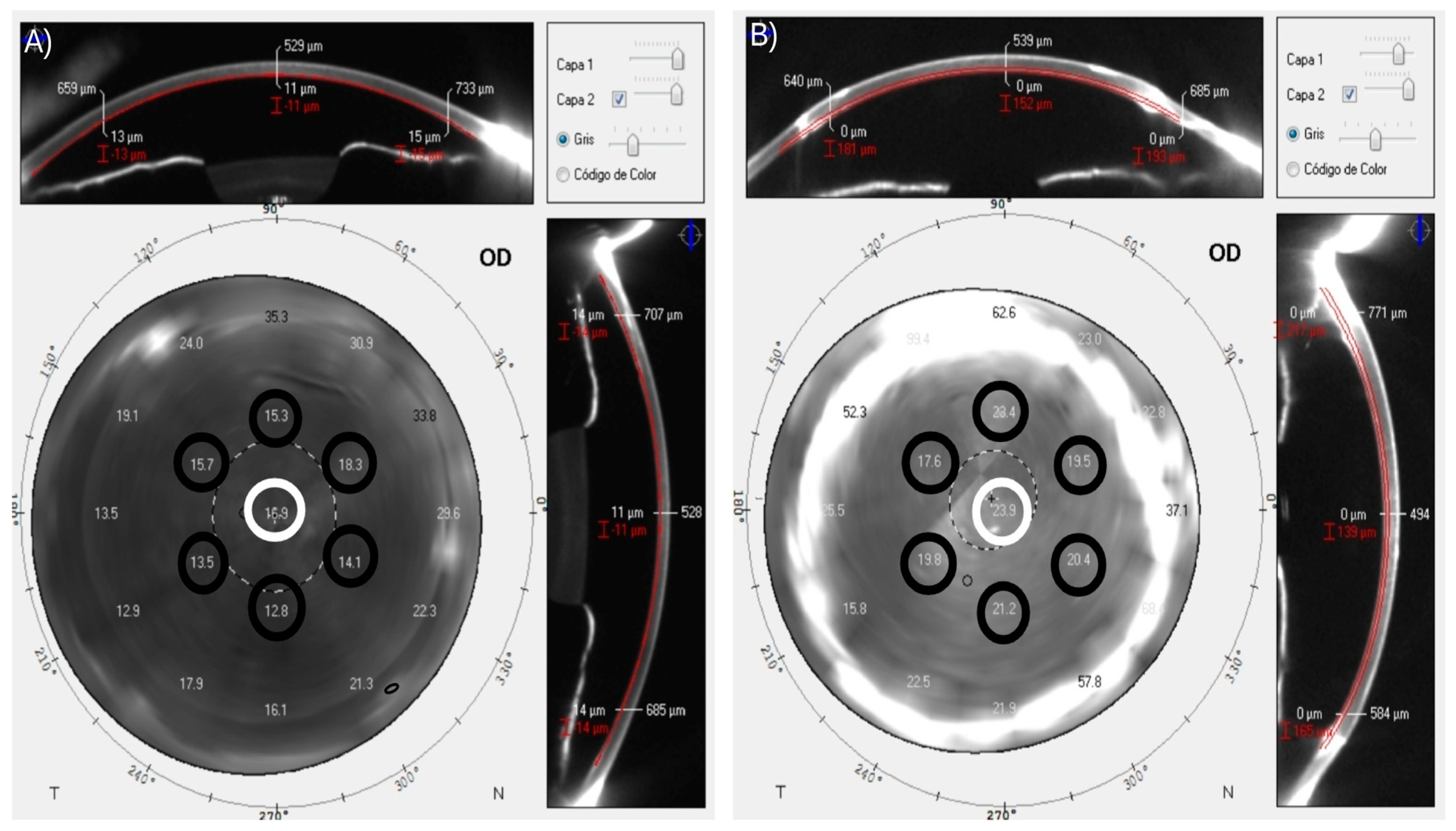
2.3. Measurement
2.4. Statistical Analysis
3. Results
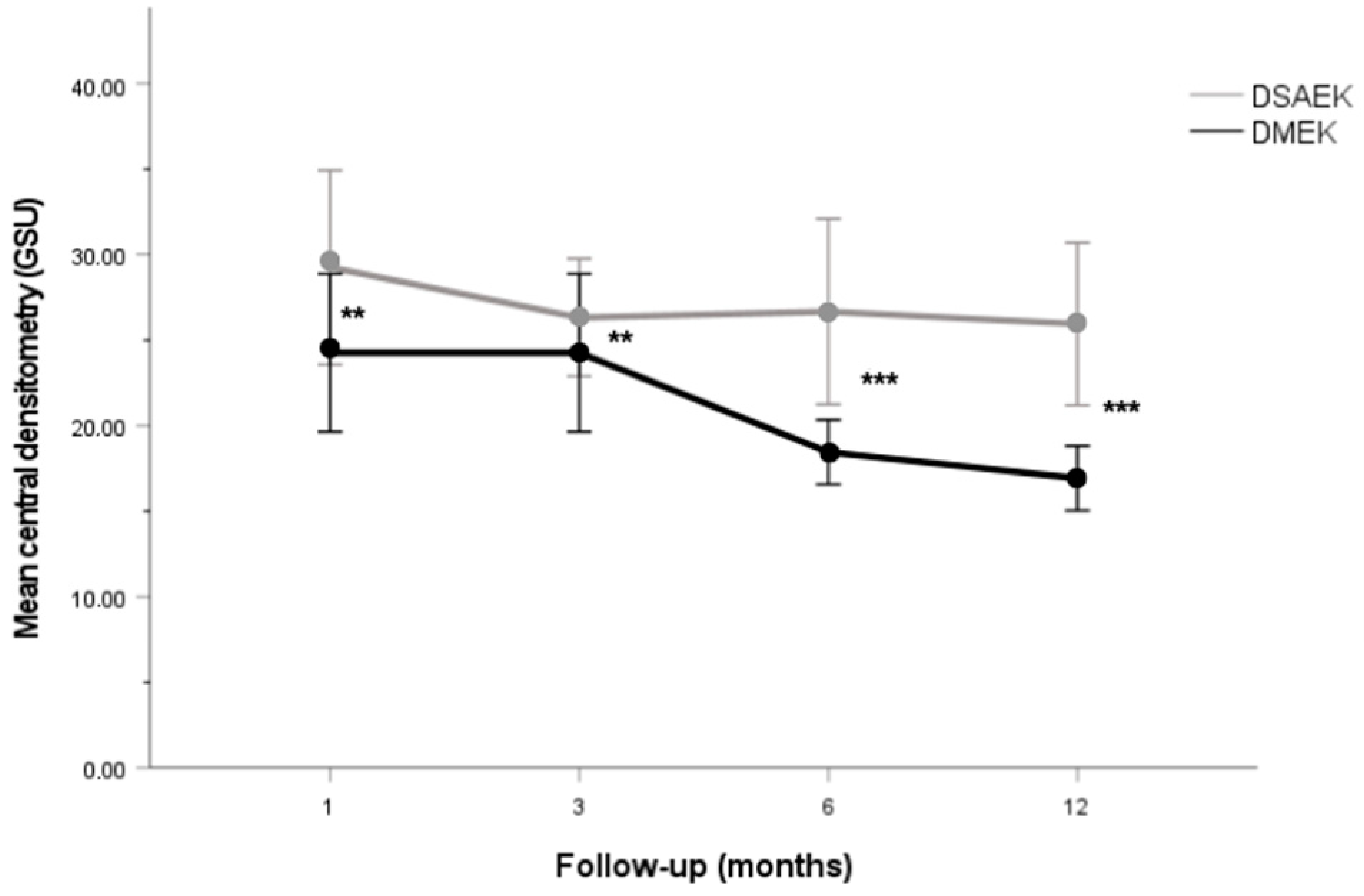
Corneal Densitometry Patterns
4. Discussion
Strengths and Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef]
- Price, M.O.; Feng, M.T.; Price, F.W. Endothelial Keratoplasty Update 2020. Cornea 2021, 40, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.E.; Guerra, P.S.; Sousa, D.C.; Gonçalves, A.I.; Quintas, A.M.; Rodrigues, W. DMEK versus DSAEK for Fuchs’ Endothelial Dystrophy: A Meta-Analysis. Eur. J. Ophthalmol. 2019, 29, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Dapena, I.; Ham, L.; Melles, G.R.J. Endothelial Keratoplasty: DSEK/DSAEK or DMEK—The Thinner the Better? Curr. Opin. Ophthalmol. 2009, 20, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Viberg, A.; Samolov, B.; Byström, B. Descemet Stripping Automated Endothelial Keratoplasty versus Descemet Membrane Endothelial Keratoplasty for Fuchs Endothelial Corneal Dystrophy: A National Registry-Based Comparison. Ophthalmology 2023, in press. [Google Scholar] [CrossRef]
- Wu, J.; Wu, T.; Li, J.; Wang, L.; Huang, Y. DSAEK or DMEK for Failed Penetrating Keratoplasty: A Systematic Review and Single-Arm Meta-Analysis. Int. Ophthalmol. 2021, 41, 2315–2328. [Google Scholar] [CrossRef]
- Maier, A.K.B.; Milek, J.; Joussen, A.M.; Dietrich-Ntoukas, T.; Lichtner, G. Systematic Review and Meta-Analysis: Outcomes After Descemet Membrane Endothelial Keratoplasty Versus Ultrathin Descemet Stripping Automated Endothelial Keratoplasty. Am. J. Ophthalmol. 2023, 245, 222–232. [Google Scholar] [CrossRef]
- Hurley, D.J.; Murtagh, P.; Guerin, M. Ultrathin Descemet Stripping Automated Endothelial Keratoplasty (UT-DSAEK) versus Descemet Membrane Endothelial Keratoplasty (DMEK)-a Systematic Review and Meta-Analysis. Eye 2023, 37, 3026–3032. [Google Scholar] [CrossRef]
- Stuart, A.J.; Romano, V.; Virgili, G.; Shortt, A.J. Descemet’s Membrane Endothelial Keratoplasty (DMEK) versus Descemet’s Stripping Automated Endothelial Keratoplasty (DSAEK) for Corneal Endothelial Failure. Cochrane Database Syst. Rev. 2016, 25, CD012097. [Google Scholar] [CrossRef]
- Woo, J.H.; Ang, M.; Htoon, H.M.; Tan, D. Descemet Membrane Endothelial Keratoplasty Versus Descemet Stripping Automated Endothelial Keratoplasty and Penetrating Keratoplasty. Am. J. Ophthalmol. 2019, 207, 288–303. [Google Scholar] [CrossRef]
- Dunbar, G.E.; Titus, M.; Stein, J.D.; Meijome, T.E.; Mian, S.I.; Woodward, M.A. Patient-Reported Outcomes After Corneal Transplantation. Cornea 2021, 40, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Dunker, S.L.; Dickman, M.M.; Wisse, R.P.L.; Nobacht, S.; Wijdh, R.H.J.; Bartels, M.C.; Tang, N.E.M.L.; van den Biggelaar, F.J.H.M.; Kruit, P.J.; Winkens, B.; et al. Quality of Vision and Vision-Related Quality of Life after Descemet Membrane Endothelial Keratoplasty: A Randomized Clinical Trial. Acta Ophthalmol. 2021, 99, e1127–e1134. [Google Scholar] [CrossRef]
- Torras-Sanvicens, J.; Blanco-Domínguez, I.; Sánchez-González, J.M.; Rachwani-Anil, R.; Spencer, J.F.; Sabater-Cruz, N.; Peraza-Nieves, J.; Rocha-De-lossada, C. Visual Quality and Subjective Satisfaction in Ultrathin Descemet Stripping Automated Endothelial Keratoplasty (UT-DSAEK) versus Descemet Membrane Endothelial Keratoplasty (DMEK): A Fellow-Eye Comparison. J. Clin. Med. 2021, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sela, T.C.; Iflah, M.; Muhsen, K.; Zahavi, A. Descemet Membrane Endothelial Keratoplasty Compared with Ultrathin Descemet Stripping Automated Endothelial Keratoplasty: A Meta-Analysis. BMJ Open Ophthalmol. 2023, 8, e001397. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, K.; Droutsas, K.; Hou, J.; Sangsari, S.; Liarakos, V.S.; Melles, G.R.J. Optical Quality of the Cornea after Descemet Membrane Endothelial Keratoplasty. Am. J. Ophthalmol. 2014, 158, 71–79. [Google Scholar] [CrossRef]
- Turnbull, A.M.J.; Tsatsos, M.; Hossain, P.N.; Anderson, D.F. Determinants of Visual Quality after Endothelial Keratoplasty. Surv. Ophthalmol. 2016, 61, 257–271. [Google Scholar] [CrossRef]
- Pickel, J.; Chamberlain, W.D.; Lin, C.C.; Austin, A.; Clover, J.; Lietman, T.M.; Rose-Nussbaumer, J. Predictors of Vision-Related Quality of Life After Endothelial Keratoplasty in the Descemet Endothelial Thickness Comparison Trials. Cornea 2021, 40, 449–452. [Google Scholar] [CrossRef]
- Patel, S.V.; Baratz, K.H.; Hodge, D.O.; Maguire, L.J.; McLaren, J.W. The Effect of Corneal Light Scatter on Vision after Descemet Stripping with Endothelial Keratoplasty. Arch. Ophthalmol. 2009, 127, 153–160. [Google Scholar] [CrossRef]
- Nemeth, G.; Hassan, J.; Modis, L.; Hassan, Z. Long-Term Changes in Backscattered Light Measurements in Keratoconus Corneas Treated with Collagen Cross-Linking. Curr. Eye Res. 2018, 43, 18–26. [Google Scholar] [CrossRef]
- Malhotra, C.; Jain, A.K.; Dwivedi, S.; Chakma, P.; Rohilla, V.; Sachdeva, K. Characteristics of Pre-Descemet Membrane Corneal Dystrophy by Three Different Imaging Modalities-In Vivo Confocal Microscopy, Anterior Segment Optical Coherence Tomography, and Scheimpflug Corneal Densitometry Analysis. Cornea 2015, 34, 829–832. [Google Scholar] [CrossRef]
- Pakbin, M.; Khabazkhoob, M.; Pakravan, M.; Fotouhi, A.; Jafarzadehpur, E.; Aghamirsalim, M.; Hashemi, H. Repeatability of Corneal Densitometry Measurements Using a Scheimpflug Camera in Healthy Normal Corneas. J. Curr. Ophthalmol. 2022, 34, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Dhubhghaill, S.N.; Rozema, J.J.; Jongenelen, S.; Ruiz Hidalgo, I.; Zakaria, N.; Tassignon, M.-J. Normative Values for Corneal Densitometry Analysis by Scheimpflug Optical Assessment. Investig. Ophthalmol. Vis. Sci. 2014, 55, 162–168. [Google Scholar] [CrossRef]
- Alnawaiseh, M.; Rosentreter, A.; Prokosch, V.; Eveslage, M.; Eter, N.; Zumhagen, L. Changes in Corneal Densitometry in Patients with Fuchs Endothelial Dystrophy after Endothelial Keratoplasty. Curr. Eye Res. 2017, 42, 163–167. [Google Scholar] [CrossRef]
- Schaub, F.; Enders, P.; Bluhm, C.; Bachmann, B.O.; Cursiefen, C.; Heindl, L.M. Two-Year Course of Corneal Densitometry After Descemet Membrane Endothelial Keratoplasty. Am. J. Ophthalmol. 2017, 175, 60–67. [Google Scholar] [CrossRef]
- Droutsas, K.; Lazaridis, A.; Giallouros, E.; Kymionis, G.; Chatzistefanou, K.; Sekundo, W. Scheimpflug Densitometry After DMEK Versus DSAEK-Two-Year Outcomes. Cornea 2018, 37, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, K.E.; Chamberlain, W.; Rose-Nussbaumer, J.; Austin, A.; Stell, L.; Lin, C.C. Corneal Light Scatter After Ultrathin Descemet Stripping Automated Endothelial Keratoplasty Versus Descemet Membrane Endothelial Keratoplasty in Descemet Endothelial Thickness Comparison Trial: A Randomized Controlled Trial. Cornea 2020, 39, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Peraza-Nieves, J.; Sánchez-González, J.M.; Rocha-de-Lossada, C.; Rachwani-Anil, R.; Sánchez-Valera, M.; Borroni, D.; Torras-Sanvicens, J. Corneal Densitometry Patterns in Descemet Membrane Endothelial Keratoplasty and Descemet Stripping Automated Keratoplasty. Int. Ophthalmol. 2021, 1–9. [Google Scholar] [CrossRef]
- Busin, M.; Madi, S.; Santorum, P.; Scorcia, V.; Beltz, J. Ultrathin Descemet’s Stripping Automated Endothelial Keratoplasty with the Microkeratome Double-Pass Technique: Two-Year Outcomes. Ophthalmology 2013, 120, 1186–1194. [Google Scholar] [CrossRef]
- Dapena, I.; Moutsouris, K.; Droutsas, K.; Ham, L.; van Dijk, K.; Melles, G.R.J. Standardized “No-Touch” Technique for Descemet Membrane Endothelial Keratoplasty. Arch. Ophthalmol. 2011, 129, 88–94. [Google Scholar] [CrossRef]
- de Oliveira, R.C.; Wilson, S.E. Descemet’s Membrane Development, Structure, Function and Regeneration. Exp. Eye Res. 2020, 197, 108090. [Google Scholar] [CrossRef]
- Tekin, K.; Sekeroglu, M.A.; Kiziltoprak, H.; Yilmazbas, P. Corneal Densitometry in Healthy Corneas and Its Correlation with Endothelial Morphometry. Cornea 2017, 36, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Satue, M.; Idoipe, M.; Gavin, A.; Romero-Sanz, M.; Liarakos, V.S.; Mateo, A.; Garcia-Martin, E.; Blasco-Martinez, A.; Sanchez-Perez, A. Early Changes in Visual Quality and Corneal Structure after DMEK: Does DMEK Approach Optical Quality of a Healthy Cornea? J. Ophthalmol. 2018, 2018, 2012560. [Google Scholar] [CrossRef]
- Rose, L.; Kelliher, C.; Jun, A.S. Endothelial Keratoplasty: Historical Perspectives, Current Techniques, Future Directions. Can. J. Ophthalmol. 2009, 44, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.Y.; Chiang, C.C. Endothelial Keratoplasty. Ophthalmology 2011, 118, 219. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.M.; Knupp, C. Corneal Structure and Transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Dirisamer, M.; Parker, J.; Naveiras, M.; Liarakos, V.S.; Ham, L.; Van Dijk, K.; Melles, G.R.J. Identifying Causes for Poor Visual Outcome after DSEK/DSAEK Following Secondary DMEK in the Same Eye. Acta Ophthalmol. 2013, 91, 131–139. [Google Scholar] [CrossRef]
- Hillenaar, T.; Sicam, V.A.D.P.; Vermeer, K.A.; Braaf, B.; Remeijer, L.; Cals, R.H.H.; de Boer, J.F. Wide-Range Calibration of Corneal Backscatter Analysis by in Vivo Confocal Microscopy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2136–2146. [Google Scholar] [CrossRef]
- McLaren, J.W.; Wacker, K.; Kane, K.M.; Patel, S.V. Measuring Corneal Haze by Using Scheimpflug Photography and Confocal Microscopy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 227–235. [Google Scholar] [CrossRef]
- Sung, M.S.; Yoon, K.C. Evaluation of Graft-Host Interface after Penetrating Keratoplasty Using Anterior Segment Optical Coherence Tomography. Jpn. J. Ophthalmol. 2014, 58, 282–289. [Google Scholar] [CrossRef]
- Agha, B.; Dawson, D.G.; Kohnen, T.; Schmack, I. Corneal Densitometry After Secondary Descemet Membrane Endothelial Keratoplasty. Cornea 2019, 38, 1083–1092. [Google Scholar] [CrossRef]
- Schaub, F.; Gerber, F.; Adler, W.; Enders, P.; Schrittenlocher, S.; Heindl, L.M.; Cursiefen, C.; Bachmann, B.O. Corneal Densitometry as a Predictive Diagnostic Tool for Visual Acuity Results After Descemet Membrane Endothelial Keratoplasty. Am. J. Ophthalmol. 2019, 198, 124–129. [Google Scholar] [CrossRef]
- Hayashi, T.; Yamaguchi, T.; Yuda, K.; Kato, N.; Satake, Y.; Shimazaki, J. Topographic Characteristics after Descemet’s Membrane Endothelial Keratoplasty and Descemet’s Stripping Automated Endothelial Keratoplasty. PLoS ONE 2017, 12, e0188832. [Google Scholar] [CrossRef] [PubMed]
- Duggan, M.J.; Rose-Nussbaumer, J.; Lin, C.C.; Austin, A.; Labadzinzki, P.C.; Chamberlain, W.D. Corneal Higher-Order Aberrations in Descemet Membrane Endothelial Keratoplasty versus Ultrathin DSAEK in the Descemet Endothelial Thickness Comparison Trial: A Randomized Clinical Trial. Ophthalmology 2019, 126, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Machalińska, A.; Kuligowska, A.; Kowalska, B.; Safranow, K. Comparative Analysis of Corneal Parameters in Swept-Source Imaging between DMEK and UT-DSAEK Eyes. J. Clin. Med. 2021, 10, 5119. [Google Scholar] [CrossRef]
- Moskwa, R.; Bloch, F.; Vermion, J.C.; Zevering, Y.; Chaussard, D.; Nesseler, A.; Goetz, C.; Perone, J.M. Postoperative, but Not Preoperative, Central Corneal Thickness Correlates with the Postoperative Visual Outcomes of Descemet Membrane Endothelial Keratoplasty. PLoS ONE 2023, 18, e0282594. [Google Scholar] [CrossRef]
- Tourabaly, M.; Chetrit, Y.; Provost, J.; Georgeon, C.; Kallel, S.; Temstet, C.; Bouheraoua, N.; Borderie, V. Influence of Graft Thickness and Regularity on Vision Recovery after Endothelial Keratoplasty. Br. J. Ophthalmol. 2020, 104, 1317–1323. [Google Scholar] [CrossRef]
- Heinzelmann, S.; Böhringer, D.; Maier, P.C.; Reinhard, T. Correlation between Visual Acuity and Interface Reflectivity Measured by Pentacam Following DSAEK. Acta Ophthalmol. 2014, 92, e1–e4. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | DSAEK (n = 19) | DMEK (n = 32) | p |
|---|---|---|---|
| Demographics, mean ± SD (IQR) or n (%) | |||
| Age (years) | 69.84 ± 10 (52–88) | 74.56 ± 11.75 (46–94) | 0.150 2 |
| Sex, male/female | 11 (57.9)/8 (42.1) | 15 (46.9)/17 (53.1) | 0.447 3 |
| Surgery, mean ± SD (IQR) | |||
| Button size (mm) | 7.66 ± 1.37 (2.2–8.5) | 8.02 ± 0.37 (7.25–8.5) | 0.336 4 |
| Graft Thickness (µm) 1 | 120.81 ± 20.42 (82–197) | 11.03 ± 1.4 (8–19) | <0.001 *,4 |
| CCT (μm) 1 | 635.21 ± 80.02 (531–895) | 552.2 ± 71.10 (429–987) | <0.001 *,4 |
| BCDVA (LogMar) 1 | 0.66 ± 0.36 (0–1.3) | 0.39 ± 0.35 (0–1.3) | <0.001 *,4 |
| Ocular disease, n (%) | |||
| Fuchs endothelial dystrophy | 6 (31.6) | 16 (50) | 0.199 3 |
| Pseudophakic bullous keratopathy | 8 (42.1) | 8 (25) | 0.203 3 |
| Descemet Membrane detachment | 2 (10.5) | 2 (6.3) | 0.583 3 |
| Herpetic endotheliitis | 0 (0) | 3 (9.4) | 0.169 3 |
| Corneal decompensation | 3 (15.8) | 3 (9.4) | 0.492 3 |
| Follow-Up | Corneal Densitometry Location 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Central, GSU | I, GSU | II, GSU | III, GSU | IV, GSU | V, GSU | VI, GSU | ||
| 1 month | DSAEK | 29.25 ± 11.78 [16.1–71.8] | 30.5 ± 6.05 [21.2–48.4] | 31.38 ± 9.26 [21.4–58.8] | 25.98 ± 5.59 [17.6–36.2] | 24.28 ± 4.31 [19.2–35] | 27.45 ± 8.43 [17.9–47] | 28.89 ± 13.48 [14.6–77.4] |
| DMEK | 24.26 ± 12.8 [14.1–77.6] | 18.67 ± 5.1 [11.2–36.4] | 20.55 ± 7.12 [12.3–42] | 18.58 ± 7.56 [11.1–44.7] | 17.77 ± 6 [10.3–33.3] | 17.43 ± 5.43 [11.2–36.7] | 19.04 ± 5.33 [12.6–38.5] | |
| p value | 0.005 *,2 | <0.001 *,2 | <0.001 *,2 | <0.001 *, 3 | <0.001 *,2 | <0.001 *,2 | <0.001 *,2 | |
| 3 months | DSAEK | 26.32 ± 7.12 [18.8–45.5] | 23.77 ± 4.86 [14.5–31.9] | 25.56 ± 7.55 [15.1–44.1] | 22.31 ± 6.28 [14–35.5] | 21.33 ± 4.39 [16.5–32] | 24.52 ± 6.89 [16.9–44.1] | 30.62 ± 14.19 [14.1–54.9] |
| DMEK | 22.22 ± 11.89 [11–65.9] | 17.96 ± 6.5 [4.7–43.5] | 19.32 ± 7.8 [11.6–54.5] | 16.95 ± 7.53 [9.1–49.3] | 16.91 ± 7.59 [10.4–50.2] | 16.44 ± 4.85 [10.9–33.9] | 19.04 ± 7.61 [11.7–49.7] | |
| p value | 0.002 *,2 | <0.001 *,3 | 0.004 *,2 | 0.006 *,3 | <0.001 *,2 | <0.001 *,2 | <0.001 *,2 | |
| 6 months | DSAEK | 26.67 ± 11.25 [16.9–61.2] | 24.23 ± 6.32 [12.9–35.9] | 25.52 ± 7.7 [18–42.7] | 21.61 ± 6.58 [12.9–39.4] | 20.98 ± 5.28 [15.4–34] | 23.15 ± 6.89 [16.7–44] | 25.59 ± 11.31 [12.4–55.6] |
| DMEK | 18.45 ± 5.23 [11.4–32.2] | 16.31 ± 6.33 [4.5–35.4] | 18.7 ± 6.61 [11.6–46.1] | 15.49 ± 4.41 [10.2–29.5] | 17.41 ± 15.18 [10.5–98] | 15.02 ± 3.24 [11.6–26.6] | 17.31 ± 5.98 [11.4–40.4] | |
| p value | <0.001 *,2 | <0.001 *,3 | <0.001 *,2 | <0.001 *,2 | <0.001 *,2 | <0.001 *,2 | <0.001 *,2 | |
| 12 months | DSAEK | 25.94 ± 9.84 [13.7–50.2] | 25.07 ± 13.75 [12.4–75.9] | 25.21 ± 11.41 [14.3–57.3] | 22.59 ± 8.71 [11.7–40.9] | 20.08 ± 5.02 [13.6–31.7] | 21.76 ± 6.35 [13.9–34] | 25.89 ± 16.3 [13–80.6] |
| DMEK | 16.92 ± 5.23 [2.12–30.4] | 17.24 ± 4.57 [8.6–26.7] | 17.05 ± 5.06 [2.83–30.6] | 15.95 ± 5.17 [10.7–37.6] | 13.9 ± 3.89 [9.5–27.3] | 14.9 ± 5.02 [10.2–34.2] | 16.43 ± 4.11 [12.1–31.1] | |
| p value | <0.001 *,2 | 0.003 *,2 | 0.004 *,3 | 0.002 *,3 | <0.001 *,3 | <0.001 *,2 | <0.001 *,2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballesteros-Sánchez, A.; Peraza-Nieves, J.; Casablanca-Piñera, A.; Rodríguez-Calvo-De-Mora, M.; Catalán-Coronado, S.; Torras-Sanvicens, J.; Borroni, D.; Sánchez-González, J.-M.; Rocha-De-Lossada, C. Scheimpflug Corneal Densitometry Patterns at the Graft–Host Interface in DMEK and DSAEK: A 12-Month Longitudinal Comparative Study. J. Clin. Med. 2023, 12, 7133. https://doi.org/10.3390/jcm12227133
Ballesteros-Sánchez A, Peraza-Nieves J, Casablanca-Piñera A, Rodríguez-Calvo-De-Mora M, Catalán-Coronado S, Torras-Sanvicens J, Borroni D, Sánchez-González J-M, Rocha-De-Lossada C. Scheimpflug Corneal Densitometry Patterns at the Graft–Host Interface in DMEK and DSAEK: A 12-Month Longitudinal Comparative Study. Journal of Clinical Medicine. 2023; 12(22):7133. https://doi.org/10.3390/jcm12227133
Chicago/Turabian StyleBallesteros-Sánchez, Antonio, Jorge Peraza-Nieves, Anna Casablanca-Piñera, Marina Rodríguez-Calvo-De-Mora, Saray Catalán-Coronado, Josep Torras-Sanvicens, Davide Borroni, José-María Sánchez-González, and Carlos Rocha-De-Lossada. 2023. "Scheimpflug Corneal Densitometry Patterns at the Graft–Host Interface in DMEK and DSAEK: A 12-Month Longitudinal Comparative Study" Journal of Clinical Medicine 12, no. 22: 7133. https://doi.org/10.3390/jcm12227133
APA StyleBallesteros-Sánchez, A., Peraza-Nieves, J., Casablanca-Piñera, A., Rodríguez-Calvo-De-Mora, M., Catalán-Coronado, S., Torras-Sanvicens, J., Borroni, D., Sánchez-González, J.-M., & Rocha-De-Lossada, C. (2023). Scheimpflug Corneal Densitometry Patterns at the Graft–Host Interface in DMEK and DSAEK: A 12-Month Longitudinal Comparative Study. Journal of Clinical Medicine, 12(22), 7133. https://doi.org/10.3390/jcm12227133










