Drug Clearance in Patients with Inflammatory Bowel Disease Treated with Biologics
Abstract
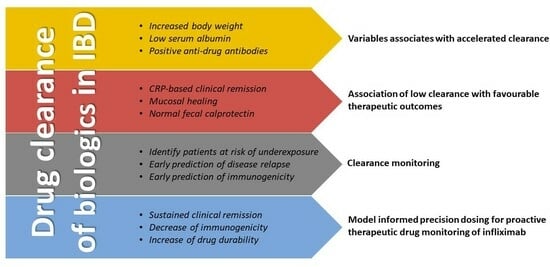
:1. Introduction
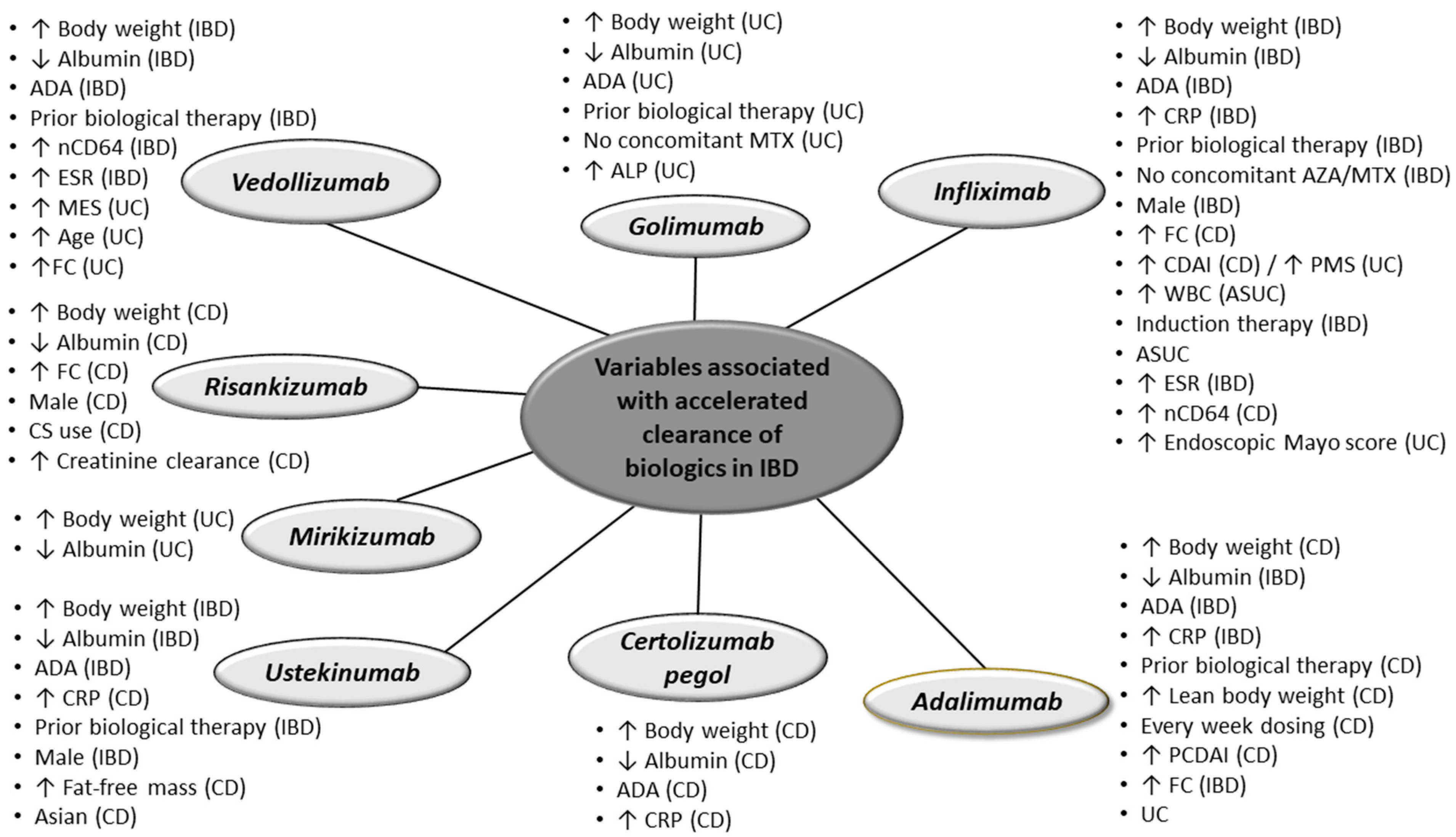
2. Variables Affecting Clearance of Biologics
2.1. Infliximab
2.2. Adalimumab
2.3. Certolizumab Pegol
2.4. Golimumab
2.5. Vedolizumab
2.6. Ustekinumab
2.7. Risankizumab
2.8. Mirikizumab
3. Association of Clearance with Therapeutic Outcomes
4. Clearance Monitoring and Model Informed Precision Dosing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papamichael, K.; Gils, A.; Rutgeerts, P.; Levesque, B.G.; Vermeire, S.; Sandborn, W.J.; Casteele, V. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: Evolution in the definition and management of primary nonresponse. Inflamm. Bowel Dis. 2015, 21, 182–197. [Google Scholar] [CrossRef]
- Miligkos, M.; Papamichael, K.; Vande Casteele, N.; Mantzaris, G.M.; Gils, A.; Levesque, B.G.; Zintzaras, E. Efficacy and safety profile of anti-tumor necrosis factor-α versus anti-integrin agents for the treatment of Crohn’s disease: A network meta-analysis of indirect comparisons. Clin. Ther. 2016, 38, 1342–1358. [Google Scholar] [CrossRef]
- Kantasiripitak, W.; Wang, Z.; Spriet, I.; Ferrante, M.; Dreesen, E. Recent advances in clearance monitoring of monoclonal antibodies in patients with inflammatory bowel diseases. Exp. Rev. Clin. Pharmacol. 2021, 14, 1455–1466. [Google Scholar] [CrossRef]
- Edlund, H.; Steenholdt, C.; Ainsworth, M.A.; Goebgen, E.; Brynskov, J.; Thomsen, O.; WHuisinga, W.; Kloft, C. Magnitude of increased infliximab clearance imposed by anti-infliximab antibodies in Crohn’s disease is determined by their concentration. AAPS J. 2017, 19, 223–233. [Google Scholar] [CrossRef]
- Fasanmade, A.A.; Adedokun, O.J.; Ford, J.; Hernandez, D.; Johanns, J.; Hu, C.; Davis, H.M.; Zhou, H. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur. J. Clin. Pharmacol. 2009, 65, 1211–1228. [Google Scholar] [CrossRef]
- Fasanmade, A.A.; Adedokun, O.J.; Blank, M.; Zhou, H.; Davis, H.M. Pharmacokinetic Properties of infliximab in children and adults with Crohn’s disease: A retrospective analysis of data from 2 phase III clinical trials. Clin. Ther. 2011, 33, 946–964. [Google Scholar] [CrossRef]
- Fasanmade, A.A.; Adedokun, O.J.; Olson, A.; Strauss, R.; Davis, H.M. Serum albumin concentration: A predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int. J. Clin. Pharmacol. Ther. 2010, 48, 297–308. [Google Scholar] [CrossRef]
- Colman, R.J.; Xiong, Y.; Mizuno, T.; Hyams, J.S.; Noe, J.D.; Boyle, B.; D’Haens, G.R.; van Limbergen, J.; Chun, K.; Yang, J.; et al. Antibodies-to-infliximab accelerate clearance while dose intensification reverses immunogenicity and recaptures clinical response in paediatric Crohn’s disease. Aliment. Pharmacol. Ther. 2022, 55, 593–603. [Google Scholar] [CrossRef]
- Grisic, A.M.; Eser, A.; Huisinga, W.; Reinisch, W.; Kloft, C. Quantitative relationship between infliximab exposure and inhibition of C-reactive protein synthesis to support inflammatory bowel disease management. Br. J. Clin. Pharmacol. 2021, 87, 2374–2384. [Google Scholar] [CrossRef]
- Xiong, Y.; Mizuno, T.; Colman, R.; Hyams, J.; Noe, J.D.; Boyle, B.; Tsai, Y.T.; Dong, M.; Jackson, K.; Punt, N.; et al. Real-World infliximab pharmacokinetic study informs an electronic health record-embedded dashboard to guide precision dosing in children with Crohn’s disease. Clin. Pharmacol. Ther. 2021, 109, 1639–1647. [Google Scholar] [CrossRef]
- Dreesen, E.; Berends, S.; Laharie, D.; D’Haens, G.; Vermeire, S.; Gils, A.; Mathôt, R. Modelling of the relationship between infliximab exposure, faecal calprotectin and endoscopic remission in patients with Crohn’s disease. Br. J. Clin. Pharmacol. 2021, 87, 106–118. [Google Scholar] [CrossRef]
- Adedokun, O.J.; Xu, Z.; Padgett, L.; Blank, M.; Johanns, J.; Griffiths, A.; Ford, J.; Zhou, H.; Guzzo, C.; Davis, H.M.; et al. Pharmacokinetics of infliximab in children with moderate-to-severe ulcerative colitis: Results from a randomized, multicenter, open-label, phase 3 study. Inflamm. Bowel Dis. 2013, 19, 2753–2762. [Google Scholar] [CrossRef]
- Hanzel, J.; Bukkems, L.H.; Gecse, K.B.; D’Haens, G.R.; Mathôt, R.A. Population pharmacokinetics of subcutaneous infliximab CT-P13 in Crohn’s disease and ulcerative colitis. Aliment. Pharmacol. Ther. 2021, 54, 1309–1319. [Google Scholar] [CrossRef]
- Kevans, D.; Murthy, S.; Mould, D.R.; Silverberg, M.S. Accelerated clearance of infliximab is associated with treatment failure in patients with corticosteroid-refractory acute ulcerative colitis. J. Crohn’s Colitis 2018, 12, 662–669. [Google Scholar] [CrossRef]
- Matsuoka, K.; Hamada, S.; Shimizu, M.; Nanki, K.; Mizuno, S.; Kiyohara, H.; Arai, M.; Sugimoto, S.; Iwao, Y.; Ogata, H.; et al. Factors contributing to the systemic clearance of infliximab with long-term administration in Japanese patients with Crohn’s disease: Analysis using population pharmacokinetics. Int. J. Clin. Pharmacol. Ther. 2020, 58, 89. [Google Scholar] [CrossRef]
- Petitcollin, A.; Leuret, O.; Tron, C.; Lemaitre, F.; Verdier, M.C.; Paintaud, G.; Bouguen, G.; Willot, S.; Bellissant, E.; Ternant, D. Modeling immunization to infliximab in children with Crohn’s disease using population pharmacokinetics: A pilot study. Inflamm. Bowel Dis. 2018, 24, 1745–1754. [Google Scholar] [CrossRef]
- Petitcollin, A.; Brochard, C.; Siproudhis, L. Pharmacokinetic parameters of infliximab influence the rate of relapse after de-escalation in adults with inflammatory bowel diseases. Clin. Pharmacol. Ther. 2019, 106, 605–615. [Google Scholar] [CrossRef]
- Buurman, D.J.; Maurer, J.M.; Keizer, R.J.; Kosterink, J.G.W.; Dijkstra, G. Population pharmacokinetics of infliximab in patients with inflammatory bowel disease: Potential implications for dosing in clinical practice. Aliment. Pharmacol. Ther. 2015, 42, 529–539. [Google Scholar] [CrossRef]
- Brandse, J.F.; Mould, D.; Smeekes, O.; Ashruf, Y.; Kuin, S.; Strik, A.; van den Brink, G.R.; D’Haens, G.R. A real-life population pharmacokinetic study reveals factors associated with clearance and immunogenicity of infliximab in inflammatory bowel disease. Inflamm. Bowel Dis. 2017, 23, 650–660. [Google Scholar] [CrossRef]
- Schreiber, S.; Ben-Horin, S.; Leszczyszyn, J.; Dudkowiak, R.; Lahat, A.; Gawdis-Wojnarska, B.; Pukitis, A.; Horynski, M.; Farkas, K.; Kierkus, J.; et al. Randomized controlled trial: Subcutaneous vs intravenous infliximab CT-P13 maintenance in inflammatory bowel disease. Gastroenterology 2021, 160, 2340–2353. [Google Scholar] [CrossRef]
- Whaley, K.G.; Xiong, Y.; Karns, R.; Hyams, J.S.; Kugathasan, S.; Boyle, B.M.; Walters, T.D.; Kelsen, J.; LeLeiko, N.; Shapiro, J.; et al. Multicenter cohort study of infliximab pharmacokinetics and therapy response in pediatric acute severe ulcerative colitis. Clin. Gastroenterol. Hepatol. 2023, 21, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; D’Haens, G.; Laharie, D.; Dreesen, E.; Rabizadeh, S.; Jain, A.; Spencer, E.A.; Panetta, J.C.; Dubinsky, M.; Dervieux, T. Infliximab clearance pre-therapy and during induction predicts long term disease control in inflammatory bowel disease. Gastroenterology 2022, 162, S45–S46. [Google Scholar] [CrossRef]
- Le Tilly, O.; Bejan-Angoulvant, T.; Paintaud, G.; Ternant, D. Letter to Dreesen et al. on their article “Modelling of the Relationship Between Infliximab Exposure, Faecal Calprotectin, and Endoscopic Remission in Patients with Crohn’s Disease”—A comprehensive review of infliximab population pharmacokinetic modelling publications. Br. J. Clin. Pharmacol. 2021, 87, 1594–1595. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.; Carroll, M.; Almeida, P.; Petrova, A.; Isaac, D.; Mould, D.; Wine, E.; Huynh, H. Early infliximab clearance predicts remission in children with Crohn’s disease. Dig. Dis. Sci. 2023, 68, 1995–2005. [Google Scholar] [CrossRef]
- Bauman, L.E.; Xiong, Y.; Mizuno, T.; Minar, P.; Fukuda, T.; Dong, M.; Rosen, M.J.; Vinks, A.A. Improved population pharmacokinetic model for predicting optimized infliximab exposure in pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2020, 26, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Vande Casteele, N.; Baert, F.; Bian, S.; Dreesen, E.; Compernolle, G.; Van Assche, G.; Ferrante, M.; Vermeire, S.; Gils, A. Subcutaneous absorption contributes to observed interindividual variability in adalimumab serum concentrations in Crohn’s disease: A prospective multicentre study. J. Crohn’s Colitis 2019, 13, 1248–1256. [Google Scholar] [CrossRef]
- Berends, S.E.; Strik, A.S.; Van Selm, J.C.; Löwenberg, M.; Ponsioen, C.Y.; D’Haens, G.R.; Mathôt, R.A. Explaining interpatient variability in adalimumab pharmacokinetics in patients with Crohn’s disease. Ther. Drug Monit. 2018, 40, 202–211. [Google Scholar] [CrossRef]
- Ternant, D.; Karmiris, K.; Vermeire, S.; Desvignes, C.; Azzopardi, N.; Bejan-Angoulvant, T.; Van Assche, G.; Paintaud, G. Pharmacokinetics of adalimumab in Crohn’s disease. Eur. J. Clin. Pharmacol. 2015, 71, 1155–1157. [Google Scholar] [CrossRef]
- Ponce-Bobadilla, A.V.; Stodtmann, S.; Chen, M.J.; Winzenborg, I.; Mensing, S.; Blaes, J.; Haslberger, T.; Laplanche, L.; Dreher, I.; Mostafa, N.M. Assessing the impact of immunogenicity and improving prediction of trough concentrations: Population pharmacokinetic modeling of adalimumab in patients with Crohn’s disease and ulcerative colitis. Clin. Pharmacokinet. 2023, 62, 623–634. [Google Scholar] [CrossRef]
- Sharma, S.; Eckert, D.; Hyams, J.S.; Mensing, S.; Thakkar, R.B.; Robinson, A.M.; Rosh, J.R.; Ruemmele, F.M.; Awni, W.M. Pharmacokinetics and exposure–efficacy relationship of adalimumab in pediatric patients with moderate to severe Crohn’s disease: Results from a randomized, multicenter, phase-3 study. Inflamm. Bowel Dis. 2015, 21, 783–792. [Google Scholar] [CrossRef]
- Vande Casteele, N.; Mould, D.R.; Coarse, J.; Hasan, I.; Gils, A.; Feagan, B.; Sandborn, W.J. Accounting for pharmacokinetic variability of certolizumab pegol in patients with Crohn’s disease. Clin. Pharmacokinet. 2017, 56, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, P.L.C.; Dulai, P.S.; Wang, Z.; Guizzetti, L.; Feagan, B.G.; Pop, A.; Yassine, M.; Shackelton, L.M.; Jairath, V.; Sandborn, W.J.; et al. A clinical prediction model to determine probability of response to certolizumab pegol for Crohn’s disease. BioDrugs. 2022, 36, 85–93. [Google Scholar] [CrossRef]
- Dreesen, E.; Kantasiripitak, W.; Detrez, I.; Stefanović, S.; Vermeire, S.; Ferrante, M.; Bouillon, T.; Drobne, D.; Gils, A. A Population pharmacokinetic and exposure–response model of golimumab for targeting endoscopic remission in patients with ulcerative colitis. Inflamm. Bowel Dis. 2020, 26, 570–580. [Google Scholar] [CrossRef]
- Xu, Y.; Adedokun, O.J.; Chan, D.; Hu, C.; Xu, Z.; Strauss, R.S.; Hyams, J.S.; Turner, D.; Zhou, H. Population pharmacokinetics and exposure-response modeling analyses of golimumab in children with moderately to severely active ulcerative colitis. J. Clin. Pharmacol. 2019, 59, 590–604. [Google Scholar] [CrossRef]
- Rosario, M.; Dirks, N.L.; Gastonguay, M.R.; Fasanmade, A.A.; Wyant, T.; Parikh, A.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Fox, I. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment. Pharmacol. Ther. 2015, 42, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Osterman, M.T.; Rosario, M.; Lasch, K.; Barocas, M.; Wilbur, J.D.; Dirks, N.L.; Gastonguay, M.R. Vedolizumab exposure levels and clinical outcomes in ulcerative colitis: Determining the potential for dose optimisation. Aliment. Pharmacol. Ther. 2019, 49, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Dirks, N.L.; Rosario, M.; Hori, T.; Hibi, T. Population pharmacokinetics of vedolizumab in Asian and non-Asian patients with ulcerative colitis and Crohn’s disease. Intest. Res. 2021, 19, 95–105. [Google Scholar] [CrossRef]
- Hanzel, J.; Dreesen, E.; Vermeire, S.; Löwenberg, M.; Hoentjen, F.; Bossuyt, P.; Clasquin, E.; Baert, F.J.; D’Haens, G.R.; Mathôt, R. Pharmacokinetic-pharmacodynamic model of vedolizumab for targeting endoscopic remission in patients with Crohn disease: Post-hoc analysis of the LOVE-CD study. Inflamm. Bowel Dis. 2022, 28, 689–699. [Google Scholar] [CrossRef]
- Colman, R.J.; Mizuno, T.; Fukushima, K.; Haslam, D.B.; Hyams, J.S.; Boyle, B.; Noe, J.D.; D’Haens, G.R.; Van Limbergen, J.; Chun, K.; et al. Real world population pharmacokinetic study in children and young adults with inflammatory bowel disease discovers novel blood and stool microbial predictors of vedolizumab clearance. Aliment. Pharmacol. Ther. 2023, 57, 524–539. [Google Scholar] [CrossRef]
- Colman, R.; Mizuno, T.; Hyams, J.; Noe, J.; Boyle, B.; Denson, L.; Vinks, A.; Minar, P. Real-world vedolizumab pharmacokinetic study in children identifies two novel biomarkers of drug clearance. Gastroenterology 2022, 162, S100. [Google Scholar] [CrossRef]
- Wang, Z.; Verstockt, B.; Sabino, J.; Vermeire, S.; Ferrante, M.; Declerck, P.; Dreesen, E. Population pharmacokinetic-pharmacodynamic model-based exploration of alternative ustekinumab dosage regimens for patients with Crohn’s disease. Br. J. Clin. Pharmacol. 2022, 88, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Adedokun, O.J.; Xu, Z.; Gasink, C.; Kowalski, K.; Sandborn, W.J.; Feagan, B. Population pharmacokinetics and exposure–response analyses of ustekinumab in patients with moderately to severely active Crohn’s disease. Clin. Ther. 2022, 44, 1336–1355. [Google Scholar] [CrossRef]
- Aguiar Zdovc, J.; Hanžel, J.; Kurent, T.; Sever, N.; Koželj, M.; Smrekar, N.; Novak, G.; Štabuc, B.; Dreesen, E.; Thomas, D.; et al. Ustekinumab dosing individualization in crohn’s disease guided by a population pharmacokinetic–pharmacodynamic model. Pharmaceutics 2021, 13, 1587. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, C.; Chen, Y.; Miao, X.; Adedokun, O.J.; Xu, Z.; Sharma, A.; Zhou, H. Population pharmacokinetics and exposure-response modeling analyses of ustekinumab in adults with moderately to severely active ulcerative colitis. J. Clin. Pharmacol. 2020, 60, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, A.A.; Goebel, A.; Bhatnagar, S.; D’Cunha, R.; Liu, W.; Pang, Y. Population pharmacokinetic and exposure–response analyses for efficacy and safety of risankizumab in patients with active Crohn’s disease. Clin. Pharmacol. Ther. 2023, 113, 839–850. [Google Scholar] [CrossRef]
- Chua, L.; Friedrich, S.; Zhang, X.C. Mirikizumab pharmacokinetics in patients with moderately to severely active ulcerative colitis: Results from phase III LUCENT Studies. Clin. Pharmacokinet. 2023, 62, 1479–1491. [Google Scholar] [CrossRef]
- Battat, R.; Hemperly, A.; Truong, S.; Whitmire, N.; Boland, B.S.; Dulai, P.S.; Holmer, A.K.; Nguyen, N.H.; Singh, S.; Vande Casteele, N.; et al. Baseline clearance of infliximab is associated with requirement for colectomy in patients with acute severe ulcerative colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 511–518. [Google Scholar] [CrossRef]
- Vande Casteele, N.; Jeyarajah, J.; Jairath, V.; Feagan, B.G.; Sandborn, W.J. Infliximab exposure-response relationship and thresholds associated with endoscopic healing in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2019, 17, 1814–1821. [Google Scholar] [CrossRef]
- Abraham, B.P.; Ziring, D.A.; Dervieux, T.; Han, P.A.; Shim, A.; Battat, R. Real-world impact of infliximab precision-guided dosing on management of patients with IBD. Am. J. Manag. Care 2023, 29, S227–S235. [Google Scholar] [CrossRef]
- Wright, E.K.; Chaparro, M.; Gionchetti, P.; Hamilton, A.L.; Schulberg, J.; Gisbert, J.P.; Valerii, M.C.; Rizzello, F.; De Cruz, P.; Panetta, J.C.; et al. Adalimumab clearance, rather than trough level, may have greatest relevance to crohn’s disease therapeutic outcomes assessed clinically and endoscopically. J. Crohn’s Colitis, 2023; jjad140, Online ahead of print. [Google Scholar] [CrossRef]
- Vande Casteele, N.; Sandborn, W.J.; Feagan, B.G. Real-world multicentre observational study including population pharmacokinetic modelling to evaluate the exposure–response relationship of vedolizumab in inflammatory bowel disease: ERELATE Study. Aliment. Pharmacol. Ther. 2022, 56, 463–476. [Google Scholar] [CrossRef]
- Kantasiripitak, W.; Wicha, S.G.; Thomas, D.; Hoffman, I.; Ferrante, M.; Vermeire, S.; Karen van Hoeve, K.; Dreesen, E. A model-based tool for guiding infliximab induction dosing to maximise long-term deep remission in children with inflammatory bowel diseases. J. Crohn’s Colitis 2023, jjad009. [Google Scholar] [CrossRef]
- Wang, Z.; Dreesen, E. Therapeutic drug monitoring of anti-tumor necrosis factor agents: Lessons learned and remaining issues. Curr. Opin. Pharmacol. 2020, 55, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, M.C.; Rabizadeh, S.; Panetta, J.C.; Spencer, E.A.; Everts-van der Wind, A.; Dervieux, T. The combination of predictive factors of pharmacokinetic origin associates with enhanced disease control during treatment of pediatric Crohn’s disease with infliximab. Pharmaceutics 2023, 15, 2408. [Google Scholar] [CrossRef] [PubMed]
- Kantasiripitak, W.; Outtier, A.; Wicha, S.G.; Kensert, K.; Wang, Z.; Sabino, J.; Vermeire, S.; Thomas, D.; Ferrante, M.; Dreesen, E. Multi-model averaging improves the performance of model-guided infliximab dosing in patients with inflammatory bowel diseases. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 1045–1059. [Google Scholar] [CrossRef]
- Primas, C.; Reinisch, W.; Panetta, J.C.; Eser, A.; Mould, D.R.; Dervieux, T. Model informed precision dosing tool forecasts trough infliximab and associates with disease status and tumor necrosis factor-alpha levels of inflammatory bowel diseases. J. Clin. Med. 2022, 11, 3316. [Google Scholar] [CrossRef]
- Dubinsky, M.C.; Phan, B.L.; Singh, N.; Rabizadeh, S.; Mould, D.R. Pharmacokinetic dashboard-recommended dosing is different than standard of care dosing in infliximab-treated pediatric IBD patients. AAPS J. 2017, 19, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Eser, A.; Primas, C.; Reinisch, S.; Vogelsang, H.; Novacek, G.; Mould, D.R.; Reinisch, W. Prediction of individual serum infliximab concentrations in inflammatory bowel disease by a Bayesian dashboard system. J. Clin. Pharmacol. 2018, 58, 790–802. [Google Scholar] [CrossRef]
- Dubinsky, M.C.; Mendiolaza, M.L.; Phan, B.L.; Moran, H.R.; Tse, S.S.; Mould, D.R. Dashboard-driven accelerated infliximab induction dosing increases infliximab durability and reduces immunogenicity. Inflamm. Bowel Dis. 2022, 28, 1375–1385. [Google Scholar] [CrossRef]
- Santacana Juncosa, E.; Rodríguez-Alonso, L.; Padullés Zamora, A.; Guardiola, J.; Rodríguez-Moranta, F.; Nilsson, K.S.; Minguet, J.B.; Rego, F.M.; Codina, H.C.; Zamora, N.P. Bayes-based dosing of infliximab in inflammatory bowel diseases: Short-term efficacy. Br. J. Clin. Pharmacol. 2021, 87, 494–505. [Google Scholar] [CrossRef]
- Papamichael, K.; Cheifetz, A.S. Optimizing therapeutic drug monitoring in inflammatory bowel disease: A focus on therapeutic monoclonal antibodies. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1423–1431. [Google Scholar] [CrossRef]
- Serrano-Díaz, L.; Iniesta-Navalón, C.; Gómez-Espín, R.; Nicolás-de Prado, I.; Bernal-Morell, E.; Rentero-Redondo, L. Impact of proactive therapeutic drug monitoring of infliximab during the induction phase in IBD patients. A Bayesian Approach. Rev. Esp. Enferm. Dig. 2023, 115, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Díaz, L.S.; Navalón, C.I.; Espín, R.G.; Nicolás-de Prado, I.; Bernal-Morell, E.; Rentero-Redondo, L. Impact of proactive of infliximab monitoring using the Bayesian approach in the maintenance phase in patients with inflammatory bowel disease. Gastroenterol. Hepatol. 2023, 46, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Strik, A.S.; Löwenberg, M.; Mould, D.R.; Berends, S.E.; Ponsioen, C.I.; van den Brande, J.M.H.; Jansen, J.M.; Hoekman, D.R.; Brandse, J.F.; Duijvestein, M.; et al. Efficacy of dashboard driven dosing of infliximab in inflammatory bowel disease patients; a randomized controlled trial. Scand. J. Gastroenterol. 2021, 56, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, R.; Guo, G.; Wu, W.; Ke, M.; Ke, C.; Huang, P.; Lin, C. Physiologically-based pharmacokinetic modeling of anti-tumor necrosis factor agents for inflammatory bowel disease patients to predict the withdrawal time in pregnancy and vaccine time in infants. Clin. Pharmacol. Ther. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Colman, R.J.; Samuels, A.; Mizuno, T.; Punt, N.; Vinks, A.A.; Minar, P. Model-informed precision dosing for biologics is now available at the bedside for patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2023, 29, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Study Design | Drug | Population (N) | Estimated Clearance Time Point | Estimated Clearance Threshold, L/Day | Therapeutic Outcome (Time Point) |
|---|---|---|---|---|---|---|
| Battat (2021) [47] | Retrospective | IFX | ASUC (N = 39) | Baseline | ≥0.627 | Colectomy (6 months) |
| Vande Casteele (2019) [48] | ACT 1 and 2 RCTs | IFX | UC (N = 484) | Baseline | <0.397 | MES ≤ 1 (week 8) |
| Vande Casteele (2019) [48] | ACT 1 and 2 RCTs | IFX | UC (N = 484) | Baseline | <0.364 | MES ≤ 1 (week 30) |
| Peticollin (2019) [17] | Prospective | IFX | IBD (N = 91) | At time of de-escalation | >0.320 | Relapse following treatment de-escalation |
| Whaley (2023) [21] | Prospective | IFX | ASUC * (N = 38) | Day 3 after drug initiation | >0.480 | Colectomy |
| Chung (2023) [24] | Retrospective | IFX | CD (N = 85) | Baseline | <0.230 | Remission (5 months) |
| Chung (2023) [24] | Retrospective | IFX | CD (N = 85) | Baseline | <0.238 | Remission (10 months) |
| Chung (2023) [24] | Retrospective | IFX | CD (N = 85) | Baseline | <0.243 | Remission (16 months) |
| Chung (2023) [24] | Retrospective | IFX | CD (N = 85) | End of induction | <0.230 | Remission (5 months) |
| Chung (2023) [24] | Retrospective | IFX | CD (N = 85) | End of induction | <0.213 | Remission (10 months) |
| Chung (2023) [24] | Retrospective | IFX | CD (N = 85) | End of induction | <0.252 | Remission (16 months) |
| Vermeire (2022) [22] | Prospective | IFX | IBD (N = 276) | Baseline, weeks 2, 6, 14 | <0.250 | CRP-based clinical remission |
| Abraham (2023) [49] | Prospective | IFX | IBD (N = 275) | Maintenance | >0.294 | Active disease, drug discontinuation |
| Wright (2023) [50] | Retrospective | ADM | CD (N = 237) | Maintenance # | <0.350 | SES-CD < 3 |
| Wright (2023) [50] | Retrospective | ADM | CD (N = 237) | Maintenance # | <0.280 | FC < 100 ug/g |
| Wright (2023) [50] | Retrospective | ADM | CD (N = 237) | Maintenance # | <0.300 | CRP-based clinical remission |
| Lefevre (2022) [32] | PRECiSE 1 and 2 RCTs | CZP | CD (N = 964) | Baseline | >0.500 | Drug TC < 36 μg/mL (week 6) |
| Vande Casteele (2022) [51] | Retrospective | VDZ | IBD (N = 695) | Baseline | <0.170 | Clinical remission (week 52) |
| Vande Casteele (2022) [51] | Retrospective | VDZ | IBD (N = 695) | Baseline | <0.160 | Deep remission (week 52) |
| Osterman (2019) [36] | GEMINI 1 RCT | VDZ | UC (N = 693) | Week 6 | <0.140 | Clinical response (week 14) |
| Colman (2022) [40] | Prospective | VDZ | IBD (N = 21) | Baseline | <0.161 | FC < 250 mg/g (week 14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deyhim, T.; Cheifetz, A.S.; Papamichael, K. Drug Clearance in Patients with Inflammatory Bowel Disease Treated with Biologics. J. Clin. Med. 2023, 12, 7132. https://doi.org/10.3390/jcm12227132
Deyhim T, Cheifetz AS, Papamichael K. Drug Clearance in Patients with Inflammatory Bowel Disease Treated with Biologics. Journal of Clinical Medicine. 2023; 12(22):7132. https://doi.org/10.3390/jcm12227132
Chicago/Turabian StyleDeyhim, Tina, Adam S. Cheifetz, and Konstantinos Papamichael. 2023. "Drug Clearance in Patients with Inflammatory Bowel Disease Treated with Biologics" Journal of Clinical Medicine 12, no. 22: 7132. https://doi.org/10.3390/jcm12227132
APA StyleDeyhim, T., Cheifetz, A. S., & Papamichael, K. (2023). Drug Clearance in Patients with Inflammatory Bowel Disease Treated with Biologics. Journal of Clinical Medicine, 12(22), 7132. https://doi.org/10.3390/jcm12227132