The Importance of Adverse Childhood Experiences in Depressive Symptoms and Their Biological Consequences in Healthy Adults: Results of a Polish University Student Study
Abstract
:1. Introduction
- (1)
- The severity of ACEs is related to a greater severity of depression;
- (2)
- ACEs affect biological processes, playing a role in mood symptom development and/or maintenance;
- (3)
- Physiological stress is an additional factor explaining experiences of depressive symptoms.
2. Materials and Methods
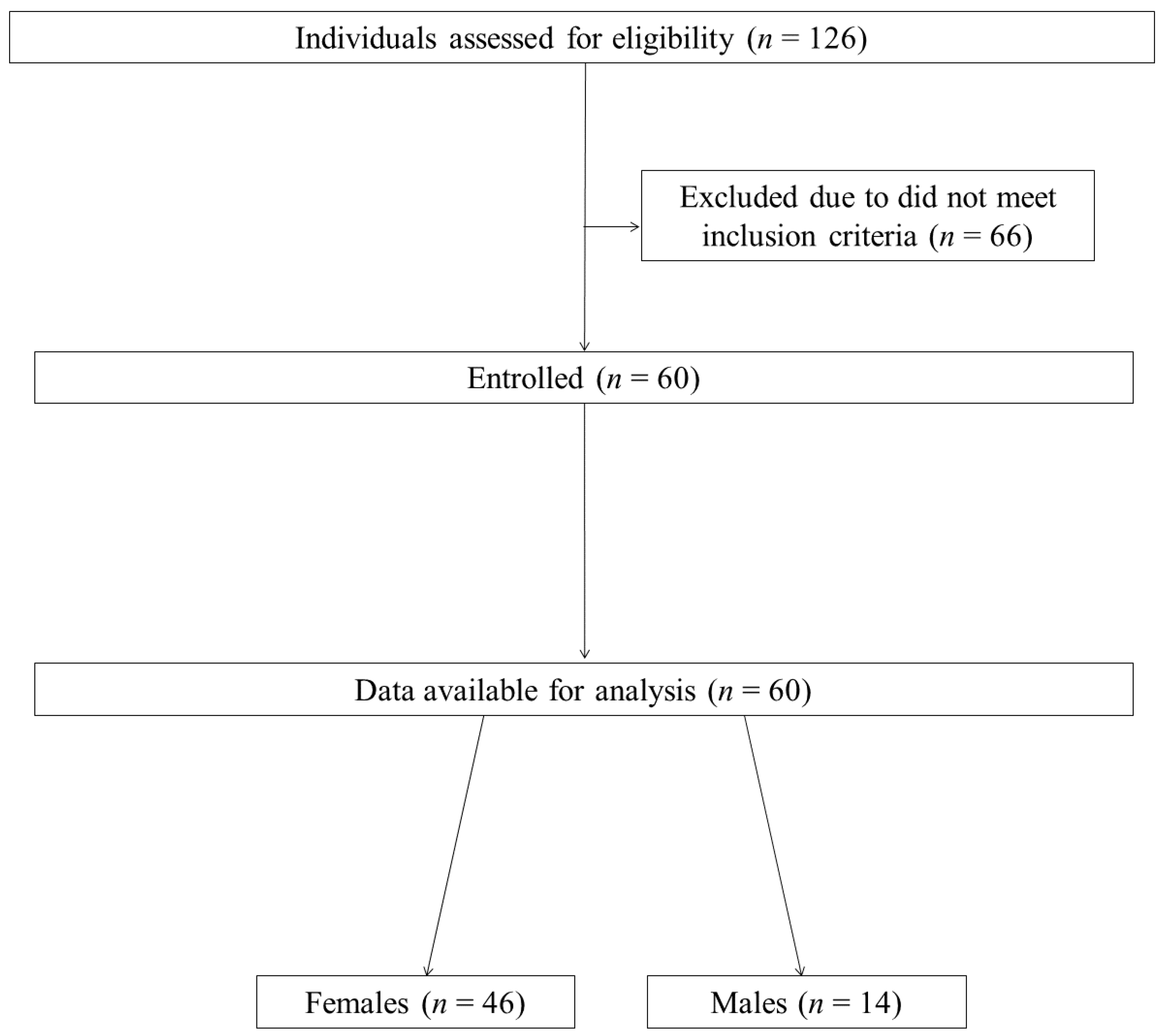
2.1. Study Group
2.2. Sociodemographic and Health-Related Data
2.3. Severity of Stress and Depressive Symptoms
2.4. Adverse Childhood Experiences
2.5. Food Intake
2.6. Blood Assessment
- for gut barrier intestinal integrity assessment: Lipopolysaccharide Binding Protein (LBP; SEB406Hu, Cloud-Clone Corp., Wuhan, China), Occludin (OCLN; SEC228Hu, Cloud-Clone Corp., Wuhan, China), Tight Junction Protein 1 (TJP1, SEC262Hu, Cloud-Clone Corp., Wuhan, China), Anti-zonulin antibody (ZON-ab; E01A4280), histamine (E-EL-0032, BIOZOL, Eching, Germany), and Immunoglobulin G (IgG; E-EL-H0169, BIOZOL, Eching, Germany);
- for pro-/anti-inflammatory homeostasis assessment: Interleukin 1β (IL-1β; 850.006.096, Diaclone SAS, Besancon, France), Interleukin 10 (IL-10, 950.060.096, Diaclone SAS, Besancon Cedex France), and Tumour necrosis factor-α (TNF-α, 950.090.096, Diaclone SAS, Besancon, France);
- for metabolic function assessment: Insulin (10-1113-01, Mercodia, Uppsala, Sweden), Cortisol (CORT; DKO001, DiaMetra, Spello–Perugia, Italy), and Adiponectin (ADP; E-EL-H6122, BIOZOL, Eching, Germany).
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Examined Population
3.2. Characteristics of the Students’ Mental Health and Differences in Examined Factors Based on Mental Health Outcomes
3.3. Relationship between Mood Disorder Symptoms and Examined Variables
3.4. Relationship between Mood Disorder Symptoms and ACEs
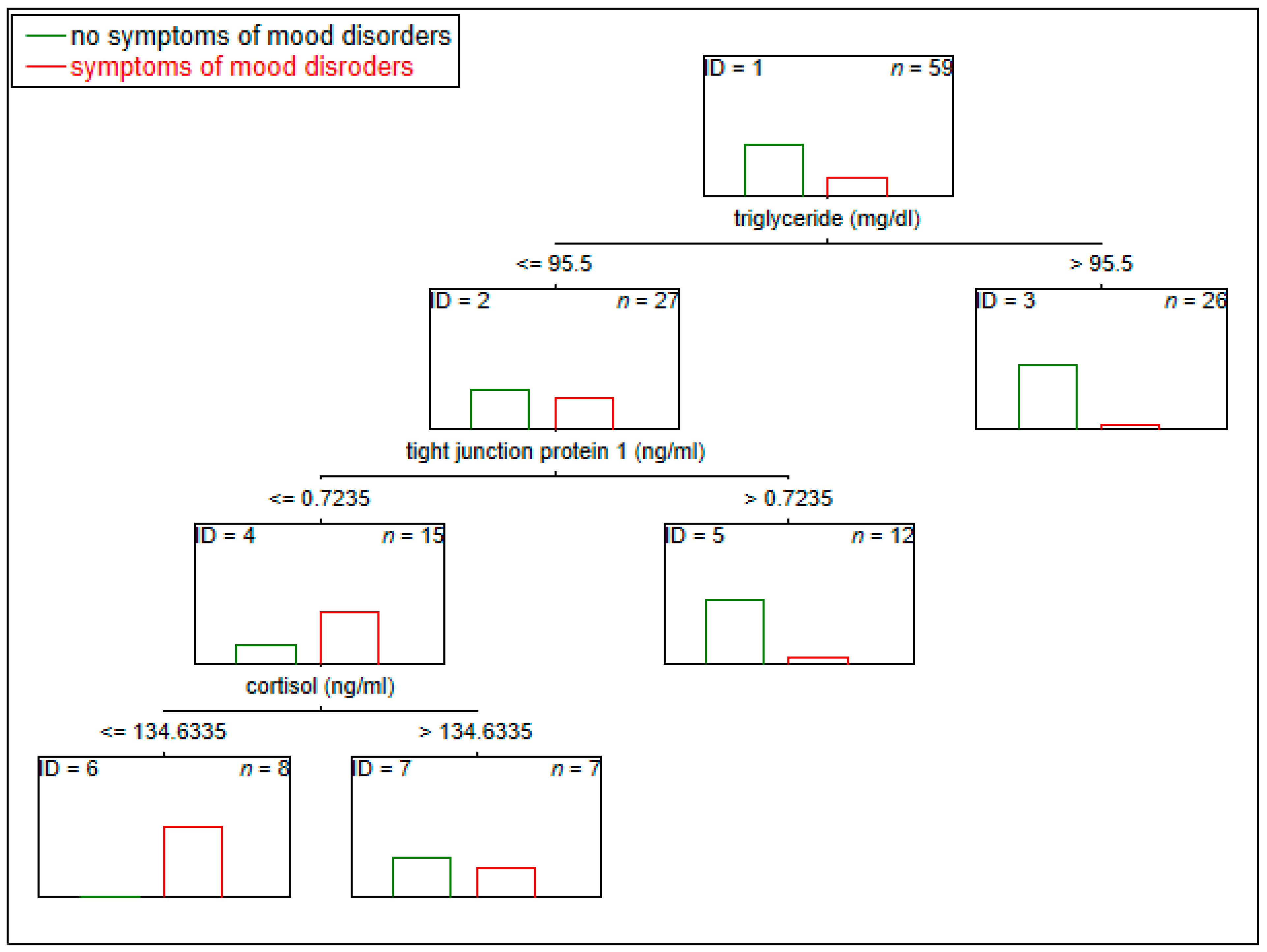
3.5. The Potential Multi-Panel Biological Markers of Experiencing Symptoms of Depression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sinanović, O.; Muftić, M.; Sinanović, S. COVID-19 Pandemia: Neuropsychiatric Comorbidity and Consequences. Psychiatr. Danub. 2020, 32, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Hamza Shuja, K.; Aqeel, M.; Jaffar, A.; Ahmed, A. COVID-19 pandemic and impending global mental health implications. Psychiatr. Danub. 2020, 32, 32–35. [Google Scholar] [CrossRef]
- Cooke, J.E.; Eirich, R.; Racine, N.; Madigan, S. Prevalence of Posttraumatic and General Psychological Stress during COVID-19: A Rapid Review and Meta-Analysis. Psychiatry Res. 2020, 292, 113347. [Google Scholar] [CrossRef] [PubMed]
- Abuhmaidan, Y.; Al-Majali, S. The Impact of the Coronavirus Pandemic on Mental Health among Al Ain University Students in Light of Some Demographic Variables. Psychiatr. Danub. 2020, 32, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-J.; Ji, Y.; Li, Y.-H.; Pan, H.-F.; Su, P.-Y. Prevalence of Anxiety Symptom and Depressive Symptom among College Students during COVID-19 Pandemic: A Meta-Analysis. J. Affect. Disord. 2021, 292, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Kosendiak, A.; Król, M.; Ściskalska, M.; Kepinska, M. The Changes in Stress Coping, Alcohol Use, Cigarette Smoking and Physical Activity during COVID-19 Related Lockdown in Medical Students in Poland. Int. J. Environ. Res. Public Health 2021, 19, 302. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Chourdakis, M. Impact of the First COVID-19 Lockdown on Body Weight: A Combined Systematic Review and a Meta-Analysis. Clin. Nutr. 2022, 41, 3046–3054. [Google Scholar] [CrossRef] [PubMed]
- Suwalska, J.; Kolasińska, K.; Łojko, D.; Bogdański, P. Eating Behaviors, Depressive Symptoms and Lifestyle in University Students in Poland. Nutrients 2022, 14, 1106. [Google Scholar] [CrossRef]
- Zaman, R.; Hankir, A.; Jemni, M. Lifestyle Factors and Mental Health. Psychiatr. Danub. 2019, 31, 217–220. [Google Scholar] [PubMed]
- Wong, V.W.-H.; Ho, F.Y.-Y.; Shi, N.-K.; Sarris, J.; Chung, K.-F.; Yeung, W.-F. Lifestyle Medicine for Depression: A Meta-Analysis of Randomized Controlled Trials. J. Affect. Disord. 2021, 284, 203–216. [Google Scholar] [CrossRef]
- Gawlik-Kotelnicka, O.; Strzelecki, D. Adiposity in Depression or Depression in Adiposity? The Role of Immune-Inflammatory-Microbial Overlap. Life 2021, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Gregório, M.J.; Rodrigues, A.M.; Eusébio, M.; Sousa, R.D.; Dias, S.; André, B.; Grønning, K.; Coelho, P.S.; Mendes, J.M.; Graça, P.; et al. Dietary Patterns Characterized by High Meat Consumption Are Associated with Other Unhealthy Life Styles and Depression Symptoms. Front. Nutr. 2017, 4, 25. [Google Scholar] [CrossRef]
- Bayes, J.; Schloss, J.; Sibbritt, D. Effects of Polyphenols in a Mediterranean Diet on Symptoms of Depression: A Systematic Literature Review. Adv. Nutr. 2020, 11, 602–615. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The Intestinal Barrier: A Fundamental Role in Health and Disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Lonnie, M.; Wadolowska, L.; Bandurska-Stankiewicz, E. Dietary-Lifestyle Patterns Associated with Adiposity and Metabolic Abnormalities in Adult Men under 40 Years Old: A Cross-Sectional Study (MeDiSH Project). Nutrients 2020, 12, 751. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Salagre, E.; Enduru, N.; Grande, I.; Vieta, E.; Zhao, Z. Insulin Resistance in Depression: A Large Meta-Analysis of Metabolic Parameters and Variation. Neurosci. Biobehav. Rev. 2022, 139, 104758. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.; Milaneschi, Y.; Lamers, F.; Vogelzangs, N. Understanding the Somatic Consequences of Depression: Biological Mechanisms and the Role of Depression Symptom Profile. BMC Med. 2013, 11, 129. [Google Scholar] [CrossRef]
- Balaji, S.; Sankaranarayanan, A. The Association between Adverse Childhood Experiences and Metabolic Syndrome in Severe Mental Illness: A Literature Review. Australas Psychiatry 2023, 31, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Zhong, S.; Liu, X.; Lai, S.; He, J.; Zhu, Y.; Song, Z.; Chen, P.; Wang, Y.; Jia, Y. Childhood Trauma History Is Linked to Abnormal Brain Metabolism of Non-Medicated Adult Patients with Major Depressive Disorder. J. Affect. Disord. 2022, 302, 101–109. [Google Scholar] [CrossRef]
- Stoltenborgh, M.; Bakermans-Kranenburg, M.J.; Alink, L.R.; Van Ijzendoorn, M.H. The Universality of Childhood Emotional Abuse: A Meta-Analysis of Worldwide Prevalence. J. Aggress. Maltreatment Trauma 2012, 21, 870–890. [Google Scholar] [CrossRef]
- Sahle, B.W.; Reavley, N.J.; Li, W.; Morgan, A.J.; Yap, M.B.H.; Reupert, A.; Jorm, A.F. The Association between Adverse Childhood Experiences and Common Mental Disorders and Suicidality: An Umbrella Review of Systematic Reviews and Meta-Analyses. Eur. Child Adolesc. Psychiatry 2022, 31, 1489–1499. [Google Scholar] [CrossRef]
- Tsehay, M.; Necho, M.; Mekonnen, W. The Role of Adverse Childhood Experience on Depression Symptom, Prevalence, and Severity among School Going Adolescents. Depress. Res. Treat. 2020, 2020, e5951792. [Google Scholar] [CrossRef] [PubMed]
- Womersley, J.S.; Nothling, J.; Toikumo, S.; Malan-Müller, S.; van den Heuvel, L.L.; McGregor, N.W.; Seedat, S.; Hemmings, S.M.J. Childhood Trauma, the Stress Response and Metabolic Syndrome: A Focus on DNA Methylation. Eur. J. Neurosci. 2022, 55, 2253–2296. [Google Scholar] [CrossRef]
- Vega-Beyhart, A.; Iruarrizaga, M.; Pané, A.; García-Eguren, G.; Giró, O.; Boswell, L.; Aranda, G.; Flores, V.; Casals, G.; Alonso, C.; et al. Endogenous Cortisol Excess Confers a Unique Lipid Signature and Metabolic Network. J. Mol. Med. 2021, 99, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Crosswell, A.D.; Lockwood, K.G. Best Practices for Stress Measurement: How to Measure Psychological Stress in Health Research. Health Psychol. Open 2020, 7, 2055102920933072. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [PubMed]
- Juczyński, Z.; Ogińska-Bulik, N. Narzędzia Pomiaru Stresu i Radzenia Sobie Ze Stresem; Pracownia Testów Psychologicznych Polskiego Towarzystw Psychologicznego: Warsaw, Poland, 2012; ISBN 83-60733-47-3. [Google Scholar]
- Kowalska, J.; Olszowa, D.; Markowska, D.; Teplik, M.; Rymaszewska, J. Physical Activity and Childbirth Classes during a Pregnancy and the Level of Perceived Stress and Depressive Symptoms in Women after Childbirth. Psychiatr. Pol. 2014, 48, 889–900. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An Inventory for Measuring Depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Forkmann, T.; Vehren, T.; Boecker, M.; Norra, C.; Wirtz, M.; Gauggel, S. Sensitivity and Specificity of the Beck Depression Inventory in Cardiologic Inpatients: How Useful Is the Conventional Cut-off Score? J. Psychosom. Res. 2009, 67, 347–352. [Google Scholar] [CrossRef]
- Bernstein, D.P.; Stein, J.A.; Newcomb, M.D.; Walker, E.; Pogge, D.; Ahluvalia, T.; Stokes, J.; Handelsman, L.; Medrano, M.; Desmond, D.; et al. Development and Validation of a Brief Screening Version of the Childhood Trauma Questionnaire. Child Abus. Negl. 2003, 27, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Murzyn, A.; Murzyn, A. Childhood Interpersonal Trauma as a Predictor of Psychotherapy Outcome in Patients with Neurotic, Stress-Related, Somatic and Personality Disorders. Ph.D. Thesis, Wydział Lekarski, Krakov, Poland, 2012. [Google Scholar]
- Niedzwiedzka, E.; Wadolowska, L.; Kowalkowska, J. Reproducibility of A Non-Quantitative Food Frequency Questionnaire (62-Item FFQ-6) and PCA-Driven Dietary Pattern Identification in 13–21-Year-Old Females. Nutrients 2019, 11, 2183. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J. An Introduction to Classification and Regression Tree (CART) Analysis; Citeseer: University Park, PA, USA, 2000; Volume 14. [Google Scholar]
- Muller, R.; Möckel, M. Logistic Regression and CART in the Analysis of Multimarker Studies. Clin. Chim. Acta 2008, 394, 1–6. [Google Scholar] [CrossRef]
- Mandelli, L.; Petrelli, C.; Serretti, A. The Role of Specific Early Trauma in Adult Depression: A Meta-Analysis of Published Literature. Childhood Trauma and Adult Depression. Eur. Psychiatry 2015, 30, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Watters, E.R.; Aloe, A.M.; Wojciak, A.S. Examining the Associations Between Childhood Trauma, Resilience, and Depression: A Multivariate Meta-Analysis. Trauma Violence Abus. 2023, 24, 231–244. [Google Scholar] [CrossRef]
- Lai, C.-L.J.; Fan, Y.; Man, H.Y.; Huang, Y. Childhood Adversity and Depression in Chinese Populations: A Multilevel Meta-Analysis of Studies Using the Childhood Trauma Questionnaire (CTQ). Asian J. Psychiatry 2023, 84, 103582. [Google Scholar] [CrossRef]
- Ainiyet, B.; Rybakowski, J.K. Suicidal Behaviour and Lipid Levels in Unipolar and Bipolar Depression. Acta Neuropsychiatr. 2014, 26, 315–320. [Google Scholar] [CrossRef]
- Wei, Y.-G.; Cai, D.-B.; Liu, J.; Liu, R.-X.; Wang, S.-B.; Tang, Y.-Q.; Zheng, W.; Wang, F. Cholesterol and Triglyceride Levels in First-Episode Patients with Major Depressive Disorder: A Meta-Analysis of Case-Control Studies. J. Affect. Disord. 2020, 266, 465–472. [Google Scholar] [CrossRef]
- Aljuhani, H.E.; Alshammari, G.M.; AlHadi, A.N.; Alabdulkarem, K.B.; Albader, O.S.M.; Baig, M.B.; Yahya, M.A. Food Habits and Associated Risk Factors of Depressed Patients with Cardiovascular Disease. PLoS ONE 2022, 17, e0263519. [Google Scholar] [CrossRef]
- Pearce, M.; Garcia, L.; Abbas, A.; Strain, T.; Schuch, F.B.; Golubic, R.; Kelly, P.; Khan, S.; Utukuri, M.; Laird, Y. Association between Physical Activity and Risk of Depression: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2022, 79, 550–559. [Google Scholar] [CrossRef]
- Kuo, W.-T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight Junction Proteins Occludin and ZO-1 as Regulators of Epithelial Proliferation and Survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yuan, S.; Sui, X.; Bian, H.; Wei, M.; Chen, Z.; Shao, H.; Shi, W.; Shi, S.; Ji, X. Higher Serum Occludin after Successful Reperfusion Is Associated with Early Neurological Deterioration. CNS Neurosci. Ther. 2022, 28, 999–1007. [Google Scholar] [CrossRef]
- Yuan, S.; Ma, Q.; Hou, C.; Zhao, Y.; Liu, K.J.; Ji, X.; Qi, Z. Association of Serum Occludin Levels and Perihematomal Edema Volumes in Intracranial Hemorrhage Patients. CNS Neurosci. Ther. 2023. [Google Scholar] [CrossRef]
- Bilgiç, A.; Ferahkaya, H.; Karagöz, H.; Kılınç, İ.; Energin, V.M. Serum Claudin-5, Claudin-11, Occludin, Vinculin, Paxillin, and Beta-Catenin Levels in Preschool Children with Autism Spectrum Disorder. Nord. J. Psychiatry 2023, 77, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Akkuş, M.; Avşar, P.A. Serum Occludin Levels Are Elevated in Schizophrenia: A Case-Control Study. Neurochem. J. 2023, 17, 297–302. [Google Scholar] [CrossRef]
- Zengil, S.; Laloğlu, E. Evaluation of Serum Zonulin and Occludin Levels in Bipolar Disorder. Psychiatry Investig. 2023, 20, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Videlock, E.J.; Mayer, E.A.; Naliboff, B.D.; Chang, L. 1002 The Effect of Childhood Trauma and Abuse on the Development of Irritable Bowel Syndrome Is Mediated by Somatization. Gastroenterology 2010, 5, S-144. [Google Scholar] [CrossRef]
- Maes, M.; Vasupanrajit, A.; Jirakran, K.; Klomkliew, P.; Chanchaem, P.; Tunvirachaisakul, C.; Payungporn, S. Exploration of the Gut Microbiome in Thai Patients with Major Depressive Disorder Uncovered a Specific Bacterial Profile with Depletion of the Ruminococcus Genus as a Putative Biomarker. Cells 2023, 12, 1240. [Google Scholar] [CrossRef]
- van Eeden, W.A.; van Hemert, A.M.; Carlier, I.V.E.; Penninx, B.W.J.H.; Lamers, F.; Fried, E.I.; Schoevers, R.; Giltay, E.J. Basal and LPS-Stimulated Inflammatory Markers and the Course of Individual Symptoms of Depression. Transl. Psychiatry 2020, 10, 235. [Google Scholar] [CrossRef]
- Ohgi, Y.; Futamura, T.; Kikuchi, T.; Hashimoto, K. Effects of Antidepressants on Alternations in Serum Cytokines and Depressive-like Behavior in Mice after Lipopolysaccharide Administration. Pharmacol. Biochem. Behav. 2013, 103, 853–859. [Google Scholar] [CrossRef]
- Zorn, J.V.; Schür, R.R.; Boks, M.P.; Kahn, R.S.; Joëls, M.; Vinkers, C.H. Cortisol Stress Reactivity across Psychiatric Disorders: A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2017, 77, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Rohleder, N.; Joksimovic, L.; Wolf, J.M.; Kirschbaum, C. Hypocortisolism and Increased Glucocorticoid Sensitivity of Pro-Inflammatory Cytokine Production in Bosnian War Refugees with Posttraumatic Stress Disorder. Biol. Psychiatry 2004, 55, 745–751. [Google Scholar] [CrossRef]
- Kennis, M.; Gerritsen, L.; van Dalen, M.; Williams, A.; Cuijpers, P.; Bockting, C. Prospective Biomarkers of Major Depressive Disorder: A Systematic Review and Meta-Analysis. Mol. Psychiatry 2020, 25, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Kang, H.-J.; Kim, J.-W.; Choi, W.; Lee, J.-Y.; Kim, S.-W.; Shin, I.-S.; Kim, M.-G.; Chun, B.J.; Stewart, R. Multiple Serum Biomarkers for Predicting Suicidal Behaviours in Depressive Patients Receiving Pharmacotherapy. Psychol. Med. 2023, 53, 4385–4394. [Google Scholar] [CrossRef] [PubMed]
- Regehr, C.; Glancy, D.; Pitts, A. Interventions to Reduce Stress in University Students: A Review and Meta-Analysis. J. Affect. Disord. 2013, 148, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Amanvermez, Y.; Zhao, R.; Cuijpers, P.; de Wit, L.M.; Ebert, D.D.; Kessler, R.C.; Bruffaerts, R.; Karyotaki, E. Effects of Self-Guided Stress Management Interventions in College Students: A Systematic Review and Meta-Analysis. Internet Interv. 2022, 28, 100503. [Google Scholar] [CrossRef] [PubMed]
- Rog, J.; Rząd, Z.; Karakuła-Juchnowicz, H. The interaction between stress and metabolic disruption in student population—Preliminary study. Curr. Probl. Psychiatry 2021, 22, 312–317. [Google Scholar] [CrossRef]
| Variable | % | n | |
|---|---|---|---|
| Year of study | I | 8 | 5 |
| II | 10 | 6 | |
| III | 13 | 8 | |
| IV | 22 | 13 | |
| V | 27 | 16 | |
| VI | 20 | 12 | |
| Dietary supplements | Vitamin D | 30 | 18 |
| Probiotics/prebiotics | 5 | 3 | |
| Hair growth | 5 | 3 | |
| Vitamin B | 2 | 1 | |
| Vitamin C | 2 | 1 | |
| Fish oil | 2 | 1 | |
| Creatine | 2 | 1 | |
| Variable | X−/Me | SD/min–max | |
| Age (years) | 23 | 1.9 | |
| Education period (years) | 16 | 13–19 | |
| PSS-10 (points) | 22 | 3.48 | |
| BDI (points) | 6 | 0–26 | |
| CTQ (points) | 34 | 25–68 | |
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Variability | 29.23% | 26.04% | 33.83% | |||
| β | p | β | p | β | p | |
| CTQ (total score) | 0.29 | 0.028 | 0.35 | 0.008 | 0.32 | 0.009 |
| PSS-10 (total score) | 0.26 | 0.049 | 0.30 | 0.027 | 0.25 | 0.04 |
| Education period (years) | −0.26 | 0.045 | −0.23 | 0.077 | −0.32 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rog, J.; Karakuła, M.; Rząd, Z.; Fitowska, A.; Brzezińska, A.; Karakula-Juchnowicz, H. The Importance of Adverse Childhood Experiences in Depressive Symptoms and Their Biological Consequences in Healthy Adults: Results of a Polish University Student Study. J. Clin. Med. 2023, 12, 7093. https://doi.org/10.3390/jcm12227093
Rog J, Karakuła M, Rząd Z, Fitowska A, Brzezińska A, Karakula-Juchnowicz H. The Importance of Adverse Childhood Experiences in Depressive Symptoms and Their Biological Consequences in Healthy Adults: Results of a Polish University Student Study. Journal of Clinical Medicine. 2023; 12(22):7093. https://doi.org/10.3390/jcm12227093
Chicago/Turabian StyleRog, Joanna, Michał Karakuła, Zuzanna Rząd, Aleksandra Fitowska, Agnieszka Brzezińska, and Hanna Karakula-Juchnowicz. 2023. "The Importance of Adverse Childhood Experiences in Depressive Symptoms and Their Biological Consequences in Healthy Adults: Results of a Polish University Student Study" Journal of Clinical Medicine 12, no. 22: 7093. https://doi.org/10.3390/jcm12227093
APA StyleRog, J., Karakuła, M., Rząd, Z., Fitowska, A., Brzezińska, A., & Karakula-Juchnowicz, H. (2023). The Importance of Adverse Childhood Experiences in Depressive Symptoms and Their Biological Consequences in Healthy Adults: Results of a Polish University Student Study. Journal of Clinical Medicine, 12(22), 7093. https://doi.org/10.3390/jcm12227093







