Abstract
Endometriosis, a chronic condition affecting around 10–14% of women, is challenging to manage, due to its complex pathogenesis and limited treatment options. Research has suggested a potential role of the gut microbiota and the endocannabinoid system in the development and progression of endometriosis. This narrative review aims to explore the role of, and any potential interactions between, the endocannabinoid system (ECS) and the gut microbiota in endometriosis. This review found that both the ECS and microbiota influence endometriosis, with the former regulating inflammation and pain perception and the latter influencing immune responses and hormonal balance. There is evidence that a dysregulation of the endocannabinoid system and the gut microbiota influence endometriosis symptoms and progression via changes in CB1 receptor expression and increased circulating levels of endocannabinoids. Microbial imbalances in the gut, such as increases in Prevotella, have been directly correlated to increased bloating, a common endometriosis symptom, while increases in E. coli have supported the bacterial contamination hypothesis as a potential pathway for endometriosis pathogenesis. These microbial imbalances have been correlated with increases in inflammatory markers such as TNF-α and IL-6, both often raised in those with endometriosis. Protective effects of the ECS on the gut were observed by increases in endocannabinoids, including 2-AG, resulting in decreased inflammation and improved gut permeability. Given these findings, both the ECS and the gut microbiota may be targets for therapeutic interventions for endometriosis; however, clinical studies are required to determine effectiveness.
1. Introduction
Endometriosis is a disease defined by the presence of endometrial-like tissue outside of the uterus [1]. Endometriosis is estimated to occur in up to 10% of women and those assigned female at birth during their reproductive years [2,3], with 11% of women being diagnosed by age 44 in Australia [4]. Endometriosis-related chronic pelvic pain (CPP) refers to a variety of pain symptoms including dysmenorrhea (period pain), dyspareunia (pain during sexual intercourse), fatigue, dyschezia (pain on bowel motions) and dysuria (pain on urination) [5,6,7]. People with endometriosis are often diagnosed with various comorbidities including irritable bowel syndrome, rheumatoid arthritis, psoriasis, anxiety, depression [8] and chronic fatigue syndrome [9]. The debilitating symptoms of endometriosis impact social activities, work and career progression, finances, academic studies, mental health, emotional health and sexual/romantic relationships, ultimately impacting quality of life [5,10,11,12,13]. Furthermore, the economic burden of endometriosis impacts the Australian economy, costing AUD 30,000 per woman per year, with the most predominant factor being lost productivity, directly correlated with pain severity. Internationally, this varies depending on different national economies, from USD 1459 to USD 20,239 per year.
One of the known mechanisms through which endometriosis symptoms occur is a result of repetitive deposition of endometrial-like tissue and active breakdown of endometriotic lesions, which in turn leads to an inflammatory cascade alongside the development of adhesions and scar tissue, as well as other factors, causing pelvic pain [1,14]. The inflammation-mediated pain has been correlated with high levels of cyclooxygenase-2 (Cox-2) and tumour necrosis factor alpha (TNF-α) in spinal cords and brains, alongside peripheral macrophages in a murine model [15].
Endometriosis is often characterized by the altered efficiency of progesterone and estrogen hormones, leading to progesterone resistance, as observed in people with endometriosis not responding to the use of progestins, and excess estrogen levels. This depicts an imbalance that initiates local infiltration of immune cells and inflammation [16,17]. Newly established cells resulting from inflammation activate various pathways of cell proliferation, angiogenesis, metastasis and invasion. As estrogen and progesterone receptors are responsible for proliferation and differentiation in the endometrium, imbalances result in changes in the expression of estrogen-metabolising enzymes, promoting progesterone resistance in endometriotic lesions [18].
Current treatments for endometriosis include surgical and/or medical management. Common treatments include analgesics (both opioid and non-opioid), hormonal treatments and anti-neuropathics. Hormonal medications include progestins, combined oral contraceptives, gonadotropin-releasing hormone (GnRH) agonists [19] or antagonists [20] and androgen analogues [21]. These modify the endocrine environment in both eutopic endometrium and ectopic lesions and hinder the inflammatory pathway involved in pelvic pain [21]. Commonly used anti-neuropathics include amitriptyline, pregabalin and gabapentin [22], the use of which is common among those experiencing chronic pelvic pain. Previous clinical trials [22] have shown the effectiveness of gabapentin on CPP, but currently, its effectiveness in endometriosis may not be effective due to the fact that endometriosis-associated pain tends not to be neuropathic [21]. There is currently limited evidence for the use of anti-neuropathics for endometriosis specifically; the use of anti-neuropathic medication is not recommended for the endometriosis cohort as endometriosis-associated pain should not be treated as neuropathic pain [21].
Overall, medical treatments are generally considered suboptimal by those with the disease [23,24] with concerns about the lack of effectiveness and problematic side effects of many medications for pelvic pain [25]. Opioid analgesics are not recommended for CPP due to both a lack of efficacy and safety concerns with respect to ongoing use [26]. However, despite this, they continue to be prescribed; people with endometriosis have a four times greater risk of chronic opioid use compared to those without [27], and opioids are often prescribed alongside benzodiazepines [28]. Both opioids [29] and benzodiazepines [30] present a significant risk of cognitive impairment, addiction and severe withdrawal symptoms, with a combination of these drugs significantly increasing the risk of overdose [31]. Surgery is considered a viable and common effective treatment [32] but often has significant costs, long waiting times [33] and substantial recurrence rates, even with expert endometriosis surgeons [34]. Access to surgery is even more reduced in developing countries and remote and rural locations [35].
Due to these issues, novel pain management options are considered an urgent research and clinical priority in endometriosis [36,37,38]. Limited access to surgery often leads to people with endometriosis employing self-management strategies. The use of cannabis, either illicitly or legally, is becoming a relatively popular self-management strategy in those with endometriosis, with substantial self-report data on the reduction in symptoms [39]. This review explores the potential mechanisms of action by which cannabis may modulate endometriosis symptoms, in the hope of exploring a new and effective therapeutic avenue.
The growing interest in the gut microbiota and its influence on various metabolic and inflammatory diseases poses the question of gut microbiota involvement in the modulation and/or pathogenesis of endometriosis. Studies have also reported interactions between gut microbes and the endocannabinoid system (ECS). Both of these aspects will be explored in this review.
1.1. Endometriosis and the Endocannabinoid System
Research into the Cannabis genus during the 1990s contributed to the scientific discovery of the ECS [40]. The ECS is a complex signalling system that comprises three major components: G-protein-coupled cannabinoid receptors (CB1, CB2, endocannabinoids (endogenously produced cannabinoids), including anandamide (AEA) and 2-arachidonoyl glycerol (2-AG)), ion channel transient receptor potential vanilloid 1 (TRPV1) [41] and the enzymes involved in the synthesis and catabolism of endocannabinoids [42,43,44]. Table 1 summarises the key endocannabinoids to be discussed in this paper.

Table 1.
Key endocannabinoids and related information.
Research to date has demonstrated that the ECS is involved in homeostasis and regulation via neuromodulatory activity, physiological processes such as digestion [46], immune function [47], nociception [48], appetite regulation [49], cardiovascular and respiratory function [50] and sleep–wake cycles [51].
The ECS influences pain modulation, making it a potential target for the treatment of chronic pain conditions such as endometriosis (Figure 1). Activation of CB1 and CB2 receptors by endocannabinoids or exogenous cannabinoids can suppress nociceptive processing and induce analgesia [52,53,54,55]. Similar observations were made in preclinical studies showing the analgesic effects of cannabinoid agonists on neuropathic pain [56,57,58]. However, clinical trials have presented mixed results in terms of the efficacy of cannabinoids in pain modulation. In a prospective randomized placebo-controlled trial, smoked cannabis (3.56% delta-9-tetrahydrocannabinol (THC)—participants smoked three cigarettes daily over a 4-day period) reduced daily pain experienced by adults with HIV-associated sensory neuropathy [59]. In contrast, the administration of Sativex, a low-dose THC (2.7 mg/100 μL) and cannabidiol (CBD) (2.5 mg/100 μL) combination oro-mucosal spray (used over an 8-week study period—drugs were titrated per patient) in adults with multiple sclerosis showed non-significant differences between the treatment and placebo groups [60]. As studies often employ different modes of administration, which affects bioavailability, the resulting effect of cannabis varies, and therefore, further studies will be required to fully understand the mechanisms and therapeutic potential of the ECS.
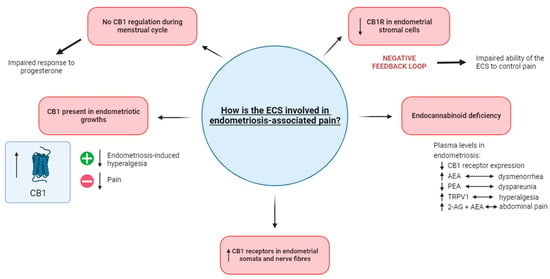
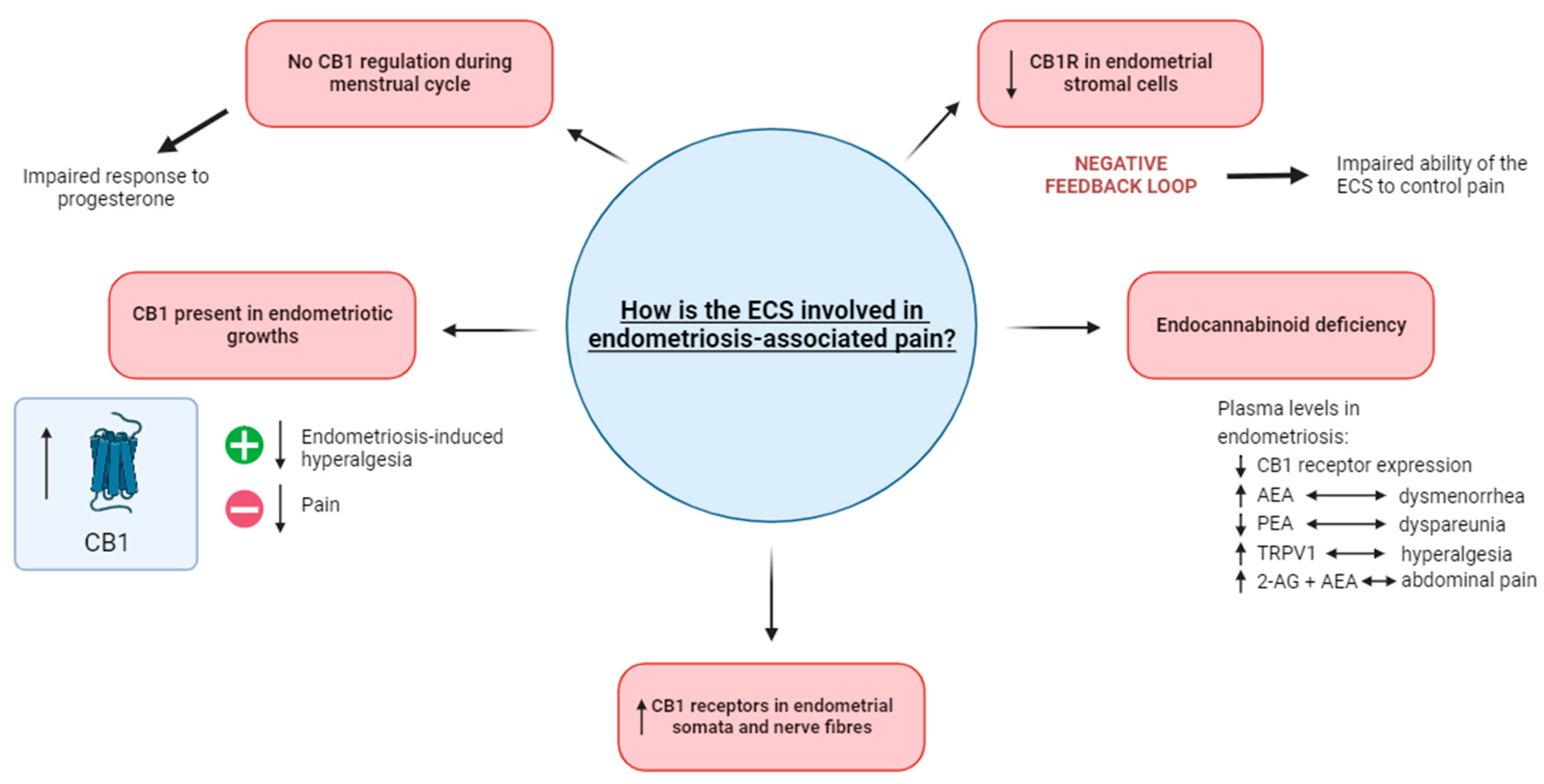
Figure 1.
The involvement of the ECS in endometriosis-associated pain. The impaired response to progesterone in endometriosis potentially occurs as a result of CB1 regulation not occurring during menstruation. When present in endometriotic growths, the activation of CB1 decreases endometriosis-induced hyperalgesia while its blockage results in decreased pain. Increased levels of CB1 receptors have been noted in the endometrial somata and nerve fibres. An endocannabinoid deficiency in endometriosis is demonstrated in plasma levels, where there is an increased level of TRPV1 (linked with hyperalgesia) and 2-AG and AEA (linked with abdominal pain). In contrast, decreased levels of CB1 receptor expression and PEA (linked with dyspareunia) are also recognized as the defining features of an endocannabinoid deficiency. The decreased level of CB1 receptors in endometrial stromal cells is suggested to cause a negative feedback loop, possibly impairing the ability of the ECS to control pain. Abbreviations: ECS = endocannabinoid system; CB1 = cannabinoid receptor 1; 2-AG = 2-arachidonoyl glycerol; AEA = anandamide; PEA = palmitoylethanolamide.
Endocannabinoid receptors have been found throughout female reproductive tissue [44], and the use of exogenous cannabinoids may alleviate endometriosis-associated pain [61,62]. The ECS also regulates endometriosis mechanisms such as inflammation, angiogenesis, apoptosis, endometrial hyperproliferation and fibrosis [44,61,62,63,64,65,66,67], and this may be a potential pathway by which endometriosis lesions re-occur post-surgery.
Cannabinoid receptors, specifically CB1 receptors, are expressed on both the somata and fibres of sensory and sympathetic neurons that innervate endometriotic growths [68]. Activation of CB1 receptors has been linked to a reduction in endometriosis-induced hyperalgesia, while blocking CB1 receptors increases pain [69]. Moreover, elevated levels of endocannabinoids, such as AEA and 2-AG, have been observed in people with endometriosis [44]. However, the expression of CB1 receptors in endometrial stromal cells is lower in endometriosis compared to healthy controls, suggesting a negative feedback loop that may impair the ability of endocannabinoids to control pain [70]. The levels of endocannabinoids in people with endometriosis vary across the menstrual cycle, and this is thought to be influenced by the on-demand synthesis and degradation of enzymes [71]. In the secretory phase, however, CB1 regulation is absent, reflecting an impaired response to progesterone levels [72], highlighting the complex nature of this disease. Table 2 outlines the findings of these key studies.
1.1.1. Endocannabinoid Deficiency in Endometriosis
Endometriosis has been described by some researchers as an “endocannabinoid deficiency” [72]. Plasma levels of endocannabinoid ligands AEA, 2-AG, N-oleoylethanolamine (OEA) and N-palmitoylethanolamine (PEA) fluctuate in people with endometriosis [72]. When associating these endocannabinoids with endometriosis-associated pain severity, a correlation was noted between increased endocannabinoid ligands in plasma and decreased local CB1 receptor expression in people with endometriosis [72]. These levels were studied in association with common endometriosis symptoms, where it was identified that elevated levels of AEA were linked with moderate-to-severe dysmenorrhea while elevated levels of PEA were found in people with moderate-to-severe dyspareunia [72], demonstrating an interesting contrast that requires further insight. This indicates a potential dysregulation in the ECS, suggesting an altered signalling process in response to endometriosis-related pain.
1.1.2. Potential Interplay of the ECS and Endometriosis
The peritoneal microenvironment is often studied in light of the establishment and progression of endometriosis. Inflammatory cytokines (interleukin-1β (IL-1β), interleukin-6 (IL-6), insulin-like growth factor (IGF-1) and tumour necrosis factor-α (TNF-α) are observed to be at a higher concentration in the peritoneal fluid of people with endometriosis [43,73,74,75]. The production of proinflammatory cytokines may occur as a result of disease-modified macrophages producing IGF-1, as high concentrations of IGF-1 were found in the peritoneal fluid of people with endometriosis [15]. This in turn leads to people experiencing hyperalgesia, which has been correlated with altered expression of TRPV1 in the peritoneum of people with endometriosis [76]. Levels of 2-AG and AEA have also been observed to be significantly higher in the peritoneal fluid of people with endometriosis, and this was associated with relatively higher abdominal pain [77]. The protective role of the ECS was observed in people with endometriosis, where immunohistochemistry revealed that cannabinoid agonists inhibit endometrial cell proliferation [65]. In contrast, previous studies have also shown the involvement of the ECS in endometriosis modulation and progression (Figure 1). ECS modulation in the innervation of ectopic uterine growths was demonstrated through the abundance of CB1 receptors on sensory and sympathetic fibres innervating ectopic growths, dorsal root ganglia and coeliac ganglia [44]. This was also observed in the epithelial cells in ovarian endometriotic lesions [78]. Such studies demonstrate the complex and contradictory role of the ECS in pain modulation, warranting more research in this area to understand the mechanisms behind the interaction of the ECS and endometriosis.
1.1.3. Exogenous Cannabinoids
The intricate and multifaceted role of the ECS in pain modulation has spurred investigations into the potential impact of exogenous cannabinoids on pain management. There is currently only one trial on medicinal cannabis for endometriosis, where a CBD isolate oil (100 mg/mL) is being compared to CBD and vaporized THC cannabis flower (ACTRN12622001560785). This is a randomized, controlled feasibility study, assessing the usefulness of medicinal cannabis on people with endometriosis, who present to the emergency department. While clinical trials are yet to be conducted, preclinical studies have explored the effects of the exogenous cannabinoid delta-9-tetrahydrocannabinol (THC) on pain management. In murine models of endometriosis, repetitive administration of botanically derived THC demonstrated protective properties [79]. It hindered the growth of ectopic endometrial tissue while alleviating mechanical hypersensitivity in the caudal abdominal region. In an experimental mouse model, varying concentrations of cannabidiol (CBD) were found to significantly reduce endometriotic implant surface area, alongside proinflammatory cytokine levels, including IL-6 and TNF-α [80]. A similar study showed that CBD administration reduced endometriotic lesion diameter, volume and area in vivo, demonstrating antioxidant effects by reducing lipid peroxidation [81]. These promising findings highlight the potential therapeutic benefits of CBD and THC for endometriosis-associated pain, warranting the need for human studies.

Table 2.
A summary of recent studies on endometriosis and the endocannabinoid system.
Table 2.
A summary of recent studies on endometriosis and the endocannabinoid system.
| Author | Year | Type of Study | Endocannabinoid/Cannabinoid | Methodology | Findings |
|---|---|---|---|---|---|
| Andrieu et al. [77] | 2022 | Human—endometriosis | 2-AG and AEA | LCMS analysis of endocannabinoids ELISA analysis of cytokines | Increased abdominal pain associated with a high level of 2-AG and a low level of AEA in the peritoneal fluid of people with endometriosis. |
| Sanchez et al. [72] | 2016 | Human—endometriosis | CB1 | PCR analysis of menstrual cups of people with endometriosis | The absence of CB1 regulation in endometriosis in the secretory phase might reflect an impaired response to progesterone. |
| Rocha et al. [76] | 2011 | Human—endometriosis | TRPV1 | Immunohistochemistry analysis of rectouterine peritoneum | Correlation identified between greater expression of TRPV1 in the peritoneum of people with endometriosis who experience CPP compared to those who do not. |
| Bilgic et al. [65] | 2017 | Human endometrial archive samples | Cannabinoid agonists | Immunohistochemistry analysis | Cannabinoid agonists inhibit endometriotic cell proliferation, modulating apoptosis of endometriotic cells. |
| Okten et al. [80] | 2023 | Female Wistar albino female rats | CBD | Immunohistochemical staining | CBD reduced endometriotic implant surface area and proinflammatory cytokine levels. |
| Escudero-Lara et al. [79] | 2020 | Female C57Bl/6J mice | THC | Immunostaining | Repetitive administration of THC hindered ectopic endometriotic growths, alleviating hypersensitivity. |
| Genovese et al. [81] | 2022 | Sprague Dawley rats | CBD | Histological analysis ELISA | CBD reduced endometriotic lesion diameter and demonstrated antioxidant effects, as viewed by downregulated expression of MMP-9, iNOS and TGFβ. |
| ACTRN12622001560785 | 2023 | Human | THC + CBD | Various questionnaires being used | Medicinal cannabis and endometriosis trial registration. |
Abbreviations: 2-AG = 2-arachidonoyl glycerol; AEA = anandamide; CB1 = cannabinoid receptor 1; TRPV1 = transient receptor vanilloid 1; chronic pelvic pain (CPP); CBD = cannabidiol; THC = delta-9-tetrahydrocannabinol; LCMS = liquid chromatography-mass chromatography; ELISA = enzyme-linked immunosorbent assay; PCR = polymerase chain reaction; MMP-9 = matrix metallopeptidase-9; iNOS = inducible nitric oxide synthase; TGFβ = transforming growth factor beta.
1.2. The Endocannabinoid System and the Gut Microbiota
The gut microbiota refers to the collection of trillions of microorganisms, including bacteria, archaea, viruses and fungi. The gut microbiota plays a crucial role in human health and disease, impacting immunomodulation and inflammatory processes [82].
In humans, the gut microbiota is dominated by four bacterial phyla: Firmicutes, Bacteriodetes, Actinobacteria and Proteobacteria [83]. As the phylotypic composition of these can vary amongst individuals [84], it can be implied that each host has a unique biological relationship with its gut microbiota, thereby influencing the risk of disease [85,86]. The composition of the gut microbiota in physiological processes also changes with age, implicating long-term health outcomes.
Given the nascent nature of this field of research, there is currently a scarcity of studies examining the relationship between the ECS, the gut microbiota and endometriosis. However, studies have shown the involvement of the ECS in the gut microbiota in metabolic and inflammatory disorders in a bidirectional manner.
The gut is part of the ECS. CB1 receptors have been localised in the gut epithelium, smooth muscle, submucosal myenteric plexus and myenteric ganglia [87,88]. CB2 receptors have been detected in the plasma cells and macrophages of the gastrointestinal (GI) mucosa and submucosa, as well as intestinal epithelial cells in the GI mucosa [87,89]. While CB1 receptor activation is associated with appetite regulation and relief from nausea and vomiting, CB2 receptor activation mediates inflammation [90]. Endocannabinoids are known to be synthesised in various parts of the gut and their levels fluctuate, based on metabolic and inflammatory status. Endocannabinoids and exogenous cannabinoids exert opposite effects on gut permeability. For instance, when examining decreased permeability as a result of inflammation, it was demonstrated that 2-AG and AEA increased permeability, while THC and CBD decreased permeability [91,92].
1.2.1. Interactions between Gut Microbial Communities and Endocannabinoids
While the interactions between gut microbiota and endocannabinoids have mainly been investigated in preclinical models, studies have linked these interactions to beneficial effects in disease states such as inflammatory bowel disorder (IBD). The administration of Lactobacillus acidophilus in mice led to an increase in CB2 expression in intestinal epithelial cells, resulting in analgesic effects, thus decreasing visceral pain [89]. Furthermore, the administration of a probiotic mixture containing Bifidobacteria, Lactobacilli spp. and Streptococcus thermophilus in zebrafish resulted in an upregulation of CB1 and CB2 expression, which then led to anti-inflammatory effects [93,94].
Akkermansia muciniphila is a Gram-negative anaerobic mucus-degrading bacterium, abundantly found in healthy intestinal mucosa. A. muciniphila has been found to modulate gut barrier integrity. Its protective effects have been cited in relation to the ECS. A study administering Akkermansia muciniphila in mice fed a high-fat diet led to an increase in 2-AG, 2-OG and 2-PG levels [95]. A. muciniphila has been shown to regulate CB1 mRNA in Caco-2 cells through increased production in outer membrane vesicles, which prevent the development of metabolic disorders such as obesity [96]. Moreover, a decrease in CB1 activity resulted in reduced circulating lipopolysaccharide (LPS) levels, thus improving the inflammatory cytokine profile and intestinal permeability. This occurred alongside enhanced A. muciniphila and Lachnospiraceae levels in the gut. The gut microbiota profile of obesity is characterised by a Firmicutes:Bacteriodetes ratio, whereby there is an increased abundance of Firmicutes and a reduced abundance of Bacteriodetes. When chronically treating obesogenic mice with THC, this ratio was shifted, and an increase in the abundance of A. muciniphila was observed, demonstrating the protective effects of exogenous cannabinoids [97]. Further investigation is required to understand how A. muciniphila interacts with endocannabinoids and the outcomes of this on inflammation-associated disorders.
Studies have also examined the relationship between the ECS and gut microbial metabolites. For instance, endocannabinoids have been found to mediate the anti-inflammatory effects of SCFAs. This association was observed in an exercise intervention [98] where an increase in SCFAs (including butyrate) and SCFA-producing bacteria (such as Bifidobacterium) was correlated with a decrease in proinflammatory cytokines TNF-α and IL-6. Furthermore, an increased abundance of SCFA-producing bacteria, such as Bifidobacterium, was positively associated with increases in endocannabinoids AEA, PEA and OEA, which were correlated with CB1 receptor levels. Table 3 illustrates the imbalances of SCFAs currently reported in endometriotic faeces. These studies provided an indication of how the association between the ECS and the gut microbiota can be potentially utilised to improve gut inflammation. The recent literature on the link between the ECS and gut microbiota is summarised in Table 4.

Table 3.
A comparison of SCFAs found in healthy faeces and in endometriotic faeces, showing imbalances observed in faecal samples.
1.2.2. The ECS–Gut–Brain Axis
As CB1 receptors are found in the CNS, the ECS is involved in signalling, modulating various physiologic and homeostatic processes. While the ECS–gut–brain axis has not been studied extensively, recent studies have considered the involvement of the ECS–gut–brain axis in exercise interventions, processes of food intake and disease states such as Alzheimer’s disease.
While investigating the microbiota-dependent gut–brain pathway on exercise, it was shown that the CB1-expressing TRPV1 sensory neurons are triggered by fatty acid amide metabolites from gut bacteria such as Lachnospiraceae and Eubacterium [99]. The protective effects of Lachnospiraceae have been well-studied in various diseases, including ulcerative colitis [100,101]. This led to analgesic effects observed after exercise, demonstrating the interaction of intestinal microbial colonization and peripheral CB1 signalling.
Endocannabinoids such as AEA and CB2 receptors and enzymes such as fatty acid amide hydrolase (FAAH) influence retrograde signalling (observed in Alzheimer’s disease) in the brain. Here, inhibitory feedback regulates neurotransmitter release [45]. Moreover, as AEA induces gut permeability, its overexpression is thought to cause a “leaky” gut, resulting in metabolic endotoxemia [102]. As this condition leads to the release of toxins such as LPS which may cross the epithelial barrier, an inflammatory signalling pathway may develop, impacting the CNS and resulting in neuroinflammation [103].
CB1 binding leads to increased food intake. CB1 receptors are present in cells of the lining of the intestinal epithelium. When CB1 receptor activity is heightened, in the small-intestinal epithelium, the release of cholecystokinin-8 (CCK-8) is inhibited, resulting in delayed satiation and overeating (in diet-induced obesity) [104], highlighting the indirect control mechanism of endocannabinoids in gut–brain neurotransmission.
1.2.3. The ECS in IBD
The use of cannabis for abdominal pain relief and other IBD symptoms is prevalent, with studies reporting use amongst 70–95% of people with IBD [90,105,106,107]. However, several studies have reported similar results when using endocannabinoids and/or exogenous cannabinoids in therapies for IBD. In an analysis of biopsies of paediatric patients, AEA levels were found to be significantly decreased in inflamed IBD mucosa [108]. A significant increase in the expression of CB2 receptors has also been observed in the biopsies of Crohn’s disease ileum and in rectum biopsies of ulcerative colitis, colocalised with T-lymphocyte infiltration [109]. Similarly, murine IBD studies demonstrated that increasing the availability of the endogenous CB1 and CB2 receptor agonists diminishes visceral pain [110,111,112]. Nonetheless, the issue remains that such implications have not translated to clinical trials, as noted in one study where people with IBD using cannabis reported higher incidences of abdominal pain and arthralgias [113]. Further research is required to develop insights into what may be causing these negative side effects and utilisation of the ECS as a therapy for IBD.
There is a high prevalence of inflammatory bowel syndrome (IBS) and its associated symptoms in people with endometriosis; in one study, 52% (194/373) of those with endometriosis had diagnosed IBS [114], where those experiencing minimal–mild endometriosis often report more severe IBS symptoms, as compared to those with moderate–severe endometriosis. Similarly, of 160 people with diagnosed IBS, 59 had a history or recent diagnosis of endometriosis [115]. People with endometriosis are reported to have a threefold increase in the likelihood of developing IBS [116]. Such studies demonstrate the importance of understanding the mechanisms through which inflammatory bowel diseases and endometriosis interact and thus the need for treatments targeting both diseases [116].

Table 4.
A summary of recent literature on endocannabinoids and gut microbes.
Table 4.
A summary of recent literature on endocannabinoids and gut microbes.
| Year | Type of Study | Endocannabinoid/Cannabinoid + Microbiota | Methodology | Findings | |
|---|---|---|---|---|---|
| Strisciuglio et al. [109] | 2023 | Human—Crohn’s disease | CB2 receptors | Western blot immunofluorescence | Increased expression of CB2 receptors in ileum of people with Crohn’s disease. |
| Vijay et al. [98] | 2021 | People with knee osteoarthritis | 2-AG, OEA, AEA, PEA Bifidobacterium, Coprococcus, Faecalibacterium, Colinsella | Metabolomic analysis Gut microbiome analysis Gene expression assay | An association between increased levels of SCFAs with circulating levels of endocannabinoids, higher microbiome diversity and low levels of proinflammatory Colinsella. |
| Pagano et al. [117] | 2019 | Pediatric patients with ulcerative colitis and male adult CD1 mice | Cannabidivarin TRPA1 | RT-PCR | In a TRPA1 antagonist manner, cannabidivarin regulates systemic inflammation and intestinal permeability. |
| Di Sabatino et al. [108] | 2011 | Human—Crohn’s disease Mucosal samples | AEA | HPLC-MS Wound healing scratch assay Immunohistochemistry | Significantly low levels of AEA in inflamed gut mucosa. |
| Grill et al. [88] | 2019 | C57BL/6 mice | CB1 receptors | In situ hybridisation Immunohistochemistry | Changes in gene expression of CB1 and CB2 receptors, GPR-55 and monoglycerol lipase in the gut after LPS treatment, demonstrating involvement in intestinal and systemic inflammation. These were observed in comparison to CB1 and MGL knockout mice. High expression of CB1 receptors in the submucosal and myenteric plexus. Reduced MGL expression in the ileum following LPS treatment. GPR-55 mRNA present alongside T-cell and macrophage markers in the ileum of healthy and treated mice. |
| Argueta and DiPatrizio [104] | 2017 | Male C57BL/6Tac mice | CB1 receptor, 2-AG and AEA | LCMSGene expression analysis | In diet-induced obesity, the increase in CB1 receptor activity inhibits CCK-8, resulting in delayed satiation and overeating. |
| Mehrpouya-Bahrami et al. [118] | 2017 | Male C57BL/6J mice | CB1 receptor Akkermansia muciniphila, Lachnospiraceae, Erysipelotrichaceae, | 16s RNA metagenomics | Blocking CB1 receptor activity resulted in decreased LPS activity, enhancing anti-inflammatory effects by increasing the abundance of A. muciniphia, Lachnospiraceae and Erysipelotrichaceae. |
| Cluny et al. [119] | 2015 | Male C57BL/6N mice | Firmicutes, Bacteriodetes A. muciniphila THC | qPCR | Chronic administration of THC in obesogenic mice increased the Firmicutes:Bacteriodetes ratio and the abundance of A. muciniphila. |
| Sakin et al. [112] | 2015 | Adult male Balb-C mice and Sprague Dawley rats | CB1 and CB2 receptors | Colorectal distension test Nociceptive testing | Availability of CB1 and CB2 receptors diminishes visceral pain. |
| Kiran, Rakib, Moore and Singh [120] | 2022 | Female C57BL/6 mice | CB2 inverse agonist SMM-189 | Flow cytometry analysis Western blot analysis Histology | CB2 inverse agonist SMM-189 suppressed colitis, while ameliorating the loss of body weight, reducing the inflammatory disease score and disease severity. |
| Dohnalova et al. [99]. | 2022 | C57BL/6J mice | CB1 | Fibre photometry analysis DRG extraction, culture and calcium imaging Amplex fluorometry analysis PCR, qPCR, RNA-seqTranscriptional profiling | Fatty acid amide metabolites trigger CB1-expressing TRPV1 sensory neurons, thus elevating dopamine levels during exercise. |
| Jamontt, Molleman, Pertwee and Parsons [121] | 2010 | Male Charles River Wister rats Distal colon tissue | CBD + THC | In vitro evaluation MPO assay BCA protein assay | CBD and THC reduced inflammation and functional disturbances by reducing the release of TNFa, IFNγ and nitric oxide in vitro and in vivo. |
| Borelli et al. [122] | 2009 | Male ICR mice | CBD | Western blot ELISA LCMS | CBD reduced colon injury and decreased expression of inflammatory markers, including nitric oxide synthase and reactive oxygen species. |
| Alhamoruni et al. [92] | 2012 | Caco-2 cells | CB1 and CB2 receptors, TRPV1, PPARγ and PPARα THC and CBD | Measurements of transepithelial electrical resistance | THC and CBD accelerated recovery of cytokine-induced intestinal permeability. |
| Distrutti et al. [94] | 2014 | Zebrafish | Bifidobacteria, Lactobacilli spp. and Streptococcus thermophilus | TUNEL assay | Administration of a probiotic mixture containing Bifidobacteria, Lactobacilli spp. and Streptococcus thermophilus in zebrafish led to an increase in CB1 and CB2 expression. |
| Gioacchini et al. [93] | 2017 | Adult male zebrafish | Bifidobacteria, Lactobacilli spp. and S. thermophilus Bacteriodetes and Actinobacteria | RT-PCR Immunohistochemistry | Administration of a probiotic mixture containing Bifidobacteria, Lactobacilli spp. and S. thermophilus in aged zebrafish resulted in an increased abundance of Bacteriodetes and Actinobacteria, alongside increases in CB1, demonstrating anti-inflammatory effects. |
Abbreviations: CB2 = cannabinoid receptor 2; 2-AG = 2-arachidonoyl glycerol; OEA = N-oleoylethanolamine; AEA = anandamide; PEA = N-palmitoylethanolamine; TRPA-1 = transient receptor potential ankyrin type 1; CB1 = cannabinoid receptor 1; THC = delta-9-tetrahydrocannabinol; PPARγ = peroxisome proliferator-activated receptor gamma; PPARα = peroxisome proliferator-activated receptor alpha; CBD = cannabidiol; RNA = ribonucleic acid; HPLC-MS = high protein liquid chromatography—mass spectrometry; RT-PCR = reverse transcriptase polymerase chain reaction; PCR = polymerase chain reaction; qPCR = quantitative polymerase chain reaction; LCMS = liquid chromatography mass spectrometry; TUNEL = terminal deoxylnucleotidyl transferase dUTP nick end labelling; DRG = dorsal root ganglia; RNA-seq = RNA sequencing; SCFA = short-chain fatty acid; GPR55 = G-couple protein receptor 55; MGL = monoacyl glycerol lipase (MGL); CCK-8 = cholecystokinin-8; LPS = lipopolysaccharide; IFNγ = interferon gamma; TRPV-1 = transient receptor vanilloid 1; TNF-α = tumour necrosis factor alph.
1.3. Endometriosis and the Gut Microbiota
A bidirectional relationship between endometriosis and gut microbiota has been proposed (Figure 2). The current literature on the potential role of gut microbiota in endometriosis is summarised in Table 5. As gut microbes and their metabolites are involved in various immune, metabolic and epithelial functions, imbalances in the gut microbiota can trigger an inflammatory response through specific inflammatory immune cell recruitment, proinflammatory cytokine production and compromised immune surveillance. These processes may be involved in some of the changes in inflammatory markers seen in endometriosis, including raised levels of IL-6 and dysfunction of macrophages [123,124,125,126].
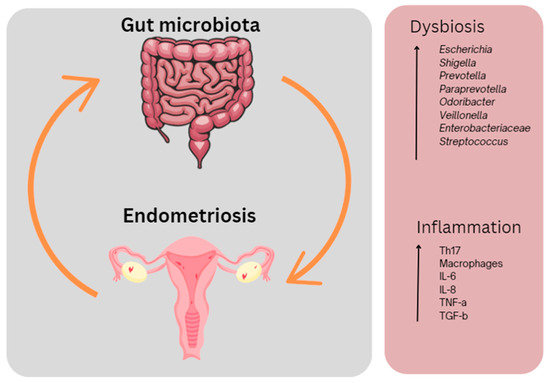
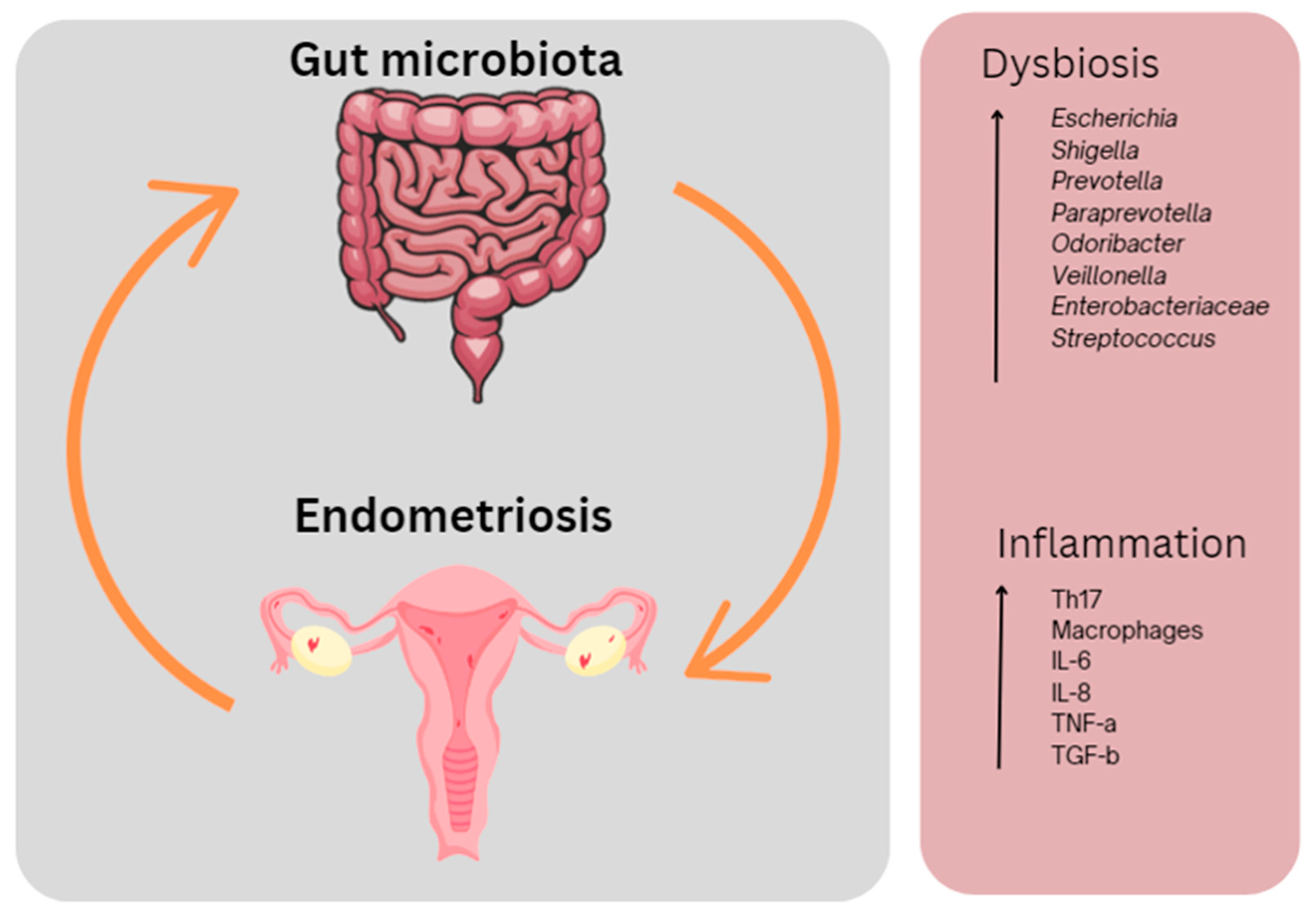
Figure 2.
The interplay between the gut microbiota and endometriosis. Dysbiosis in the gut and inflammation occurring both in the gut and in the peritoneal cavity results in endometriosis-associated symptoms including pain and fatigue. This figure was created using Canva.com (accessed on 27 September 2023).
1.3.1. Microbial Dysbiosis in Endometriosis
In the endometriotic state, larger percentages of bacterial phyla Proteobacteria, Verrucomicrobia, Streptococcus or Fusobacteria have been reported (Figure 3). Increases in Enterobacteriaceae, Streptococcus and E. coli have been identified as dominating phyla in endometriosis cohorts [127]. Recently, Fusobacterium has been suggested to contribute to the pathogenesis of endometriosis [128]. The dominance of Shigella has also been noted [129]. Such microbial communities are known to be involved in the degradation of estrogen by producing β-glucuronidase and β-glucosidase [130], inevitably resulting in the development of a high-estrogen environment, promoting the progression of endometriosis [131,132].
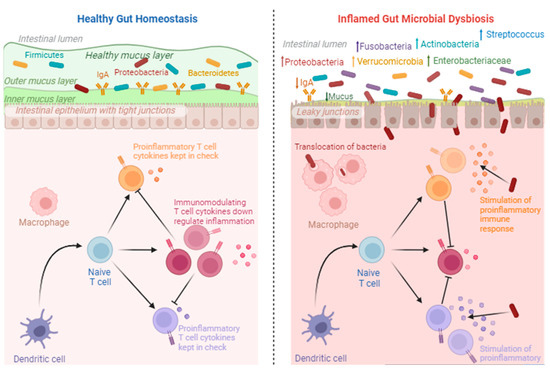
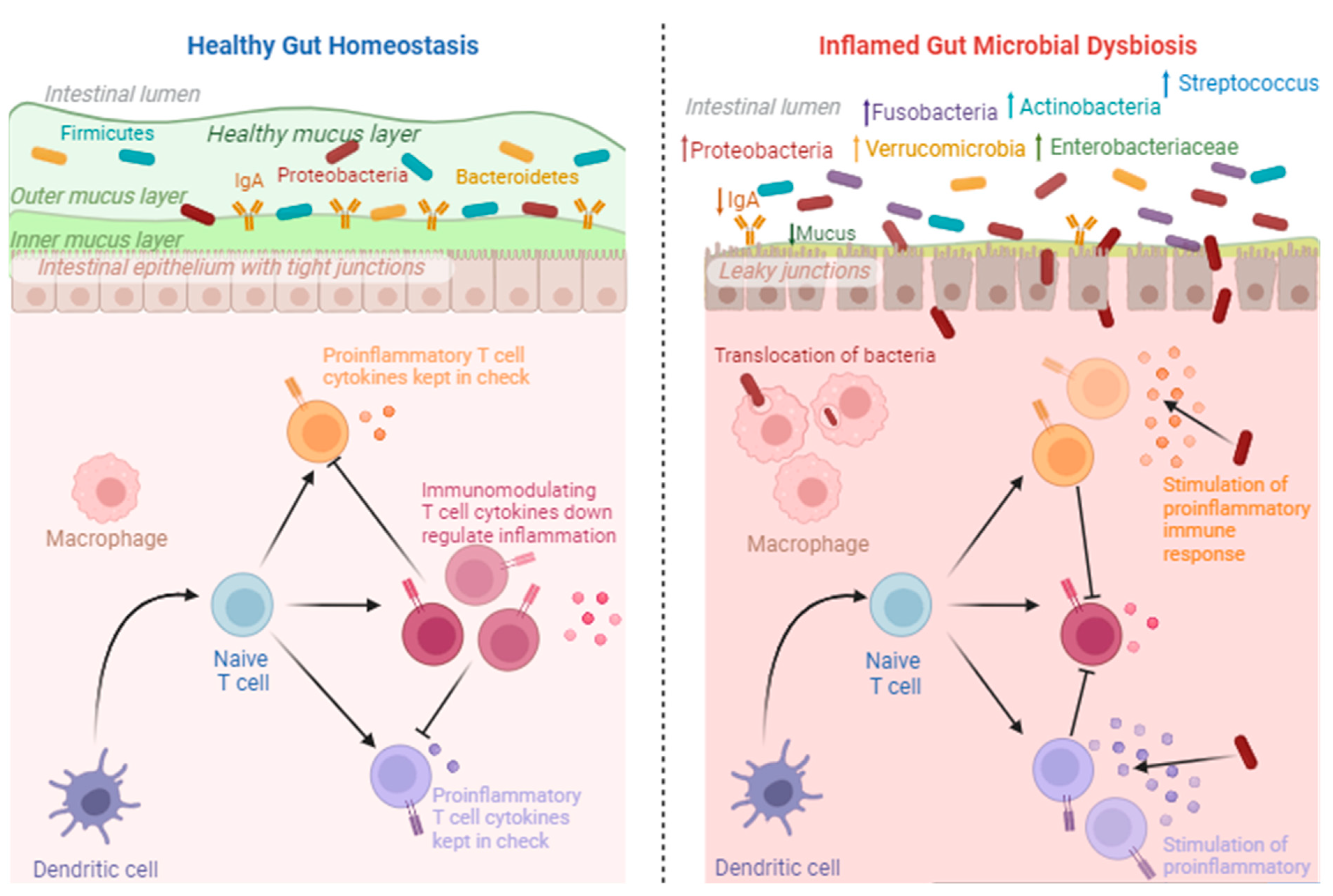
Figure 3.
Healthy gut homeostasis vs. inflamed gut microbial dysbiosis. The downregulation of inflammatory cytokines occurs under healthy conditions. However, under inflammatory conditions, proinflammatory cytokines are upregulated as a result of increases in pathogenic microorganisms. This image was created using Biorender.com (accessed 27 September 2023).
An increase in E. coli is attributed as a biomarker of endometriosis, and this has led to the “bacterial contamination” theory. High levels of E. coli have been found in faecal [133] and menstrual blood samples [134] of participants with endometriosis. E. coli is known to trigger TLR4-mediated growth and progression of endometriosis, resulting in pelvic inflammation [135]. This occurs through the production of LPS, triggering secretion of secondary inflammatory mediators such as NF-κB, in the peritoneal cavity, therefore, resulting in the development and progression of endometriosis.
Further research is required to unravel the intricate dynamics between the microbiota and its influence on endometriosis progression. Such investigations would not only grow the understanding of the way in which the microbiota promote endometriosis pathogenesis but also the development of targeted therapies to improve the quality of life of those with endometriosis.
1.3.2. Microbial Dysbiosis and Endometriosis Symptoms
Research has demonstrated correlations with specific microbes and bacterial phyla impacting symptoms in those with endometriosis. Previous studies have shown the estrogen–gut–brain axis, which is thought to influence the development of chronic stress in people with endometriosis via the activation of β-adrenergic signalling [136]. This has been associated with dysbiosis on a genus level, particularly a decrease in Paraprevotella, Odoribacter, Veillonella and Ruminococcus [136], which are often viewed as biomarkers of endometriosis [137]. A murine study showed that a decrease in Ruminococcus is negatively correlated with apoptosis of endometriotic epithelial cells and increased IL-6 levels, resulting in peritoneal inflammation [138]. Prevotella has been found in high abundance in people with endometriosis, especially in those experiencing gastrointestinal symptoms, and is associated with constipation, bloating, flatulence, vomiting and nausea [139]. As many studies report a correlation between gut microbiota imbalances and endometriosis, continued and extensive research is essential in understanding the full potential of harnessing the gut microbiota for the treatment of endometriosis and its associated symptoms.
The vaginal microbiota is dominated by healthy Lactobacillus, which maintains an acidic and protective environment, preventing the growth of pathogenic bacteria. However, in endometriosis, the protective environment maintained by vaginal microbiota is impacted by an abundance of Gardnerella, Escherichia, Shigella, Ureoplasma [129], Streptococcus, Moraxellae, Staphilococcus and Enterobacteria, coupled with a lowered abundance of Lactobacillus [140]. These imbalances have been correlated with endometriosis-associated pelvic pain [141,142]. An understanding of the vaginal microbiota in endometriosis, in correlation with the ECS, is required for the characterisation of treatments that may be prescribed for people with endometriosis.

Table 5.
A summary of recent literature on the gut microbiota and people with endometriosis.
Table 5.
A summary of recent literature on the gut microbiota and people with endometriosis.
| Year | Microbiota | Methodology | Findings | |
|---|---|---|---|---|
| Svensson et al. [139] | 2021 | Prevotella, Bacilli, Bacteriodia, Clostridia, Coriobacteria and Gammaproteobacter | 16s rRNA sequencing | Prevotella has been associated with constipation, flatulence, bloating, vomiting and nausea in endometriosis. High abundance of Lactococcus (Bacilli), lower abundance of Odoribacter and higher abundance of Prevotella in endometriosis. |
| Sandstrom et al. [138] | 2020 | Rumincoccus | 16s rRNA sequencing | Decrease in Ruminococcus correlated with an increase in IL-6 in a murine model of endometriosis, resulting in peritoneal inflammation. |
| Ata et al. [129] | 2019 | Complete absence of Atopobium, Gardnerella, Streptococcus, Escherichia, Shigella, Ureoplasma | PCR amplification 16s rRNA sequencing | Absence of Atopobium in vaginal and cervical microbiota. Increased Gardnerella in cervical microbiota. Dominant gut microbiota in endometriosis group—Escherichia and Shigella. Predominant population of lower genital tract—Lactobacillus. Alloprevotella significantly decreased in the cervix. |
| Xu et al. [136] | 2017 | Paraprevotella, Odoribacter, Veillonella and Ruminococcus | 16s rRNA sequencing Immunohistochemistry | The development of chronic stress in people with endometriosis occurs through the activation of β-adrenergic signalling, which occurs as a result of dysbiosis—decrease in specific unknown genus. |
| Khan et al. [134] | 2010 | E. coli | ELISA of macrophages from peritoneal fluid and epithelial/stromal cells from biopsy specimens of eutopic/ectopic endometria of women with and without endometriosis RT-PCR | Menstrual blood of people with endometriosis has a higher concentration of E. coli compared to healthy controls. An infiltration of macrophages in eutopic/ectopic endometria of people with endometriosis was noted. |
Abbreviations: rRNA = ribosomal ribonucleic acid; ELISA = enzyme-linked immunosorbent assay; RT-PCR = reverse transcriptase polymerase chain reaction; IL-6 = interleukin-6.
2. Conclusions and Future Directions
This review summarises the complex relationship between endometriosis, the ECS and the gut microbiota. While studies have not completely deciphered the molecular basis of these relationships, the current literature demonstrates the vital roles that the ECS and the gut microbiota play in potential implications in preventing the development of endometriosis as well as effective therapeutic strategies. The mechanisms of the ECS in both endometriosis and the gut microbiota were explored. This study demonstrated current knowledge on how the dysregulation of the endocannabinoid system and the gut microbiota influence the progression of endometriosis. The fluctuation of endocannabinoids in plasma, correlated with endometriosis-associated pain severity, demonstrates the dysregulation of the ECS in endometriosis. This dysregulation has been linked with innervation of ectopic uterine growths. Furthermore, variable levels of endocannabinoids prevalent in female reproductive tissue ultimately result in endometriosis-related symptoms. Here, the administration of THC and CBD depicted the protective nature of exogenous cannabinoids on endometriosis. Moreover, the protective effects of the ECS on the gut were observed by increases in endocannabinoids, including 2-AG, resulting in decreased inflammation and improved gut permeability. Microbial imbalance in the gut and menstrual blood have been directly linked to bloating in endometriosis. Importantly, increases in specific bacterial phyla were associated with increases in inflammatory markers such as TNF-α and IL-6. Further research understanding the mechanisms, influence on inflammation and analysis of endocannabinoids and exogenous cannabinoids is required for the development of treatments of various diseases including endometriosis. While the impact of cannabinoids in endometriosis has been investigated, future studies are needed to comprehend how cannabinoids influence endometriosis-related pain and symptoms and how this can be implemented in the clinic, following efficacy and safety studies. This may be done through the implementation of exogenous cannabinoids, which have been shown to exert protective effects. An understanding of various inflammatory diseases was used to show the potential therapeutic role of the gut microbiota while paving new avenues for further research. While there is currently limited understanding on the way that the ECS and gut microbes interact with each other, a comprehensive understanding of this may allow both to be harnessed in exploring therapeutic avenues. Finally, the relationship between the gut microbiota and endometriosis was explored, highlighting the need for further investigations of how cannabinoids may influence gut microbiota in endometriosis.
Author Contributions
Conceptualization, T.F., D.J.B. and M.A.; methodology, T.F., D.J.B. and M.A.; formal analysis, T.F.; writing—original draft preparation, T.F.; writing—reviewing and editing, T.F., D.J.B., M.A., M.L. (Mitchell Low), J.S. and M.L. (Mathew Leonardi); visualization, T.F., D.J.B. and M.A.; supervision, D.J.B., M.L. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
As a medical research institute, NICM Health Research Institute receives research grants and donations from foundations, universities, government agencies, individuals and industry. Sponsors and donors also provide untied funding for work to advance the vision and mission of the institute. M.A. is an advisory board member for Evolv Therapeutics and conducts research on medicinal cannabis and endometriosis that is funded by philanthropic and/or government funding. J.S. is employed by the Australian Natural Therapeutics Group and formerly sat on the scientific advisory board for BioCeuticals. J.S. is also a current member of the scientific advisory board for United in Compassion (pro bono) and a board member of the Australian Medicinal Cannabis Association (pro bono).
References
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef]
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef]
- Parazzini, F.; Esposito, G.; Tozzi, L.; Noli, S.; Bianchi, S. Epidemiology of endometriosis and its comorbidities. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 3–7. [Google Scholar] [CrossRef]
- Rowlands, I.; Abbott, J.; Montgomery, G.; Hockey, R.; Rogers, P.; Mishra, G. Prevalence and incidence of endometriosis in Australian women: A data linkage cohort study. BJOG 2020, 128, 657–665. [Google Scholar] [CrossRef]
- Armour, M.; Sinclair, J.; Ng, C.H.M.; Hyman, M.S.; Lawson, K.; Smith, C.A.; Abbott, J. Endometriosis and chronic pelvic pain have similar impact on women, but time to diagnosis is decreasing: An Australian survey. Sci. Rep. 2020, 10, 16253. [Google Scholar] [CrossRef]
- Armour, M.; Sinclair, J.; Chalmers, K.J.; Smith, C.A. Self-management strategies amongst Australian women with endometriosis: A national online survey. BMC Complement. Altern. Med. 2019, 19, 17. [Google Scholar] [CrossRef]
- Tewhaiti-Smith, J.; Semprini, A.; Bush, D.; Anderson, A.; Eathorne, A.; Johnson, N.; Girling, J.; East, M.; Marriott, J.; Armour, M. An Aotearoa New Zealand survey of the impact and diagnostic delay for endometriosis and chronic pelvic pain. Sci. Rep. 2022, 12, 4425. [Google Scholar] [CrossRef]
- Laganà, A.S.; La Rosa, V.L.; Rapisarda, A.M.C.; Valenti, G.; Sapia, F.; Chiofalo, B.; Rossetti, D.; Frangez, H.B.; Bokal, E.V.; Vitale, S.G. Anxiety and depression in patients with endometriosis: Impact and management challenges. Int. J. Women’s Health 2017, 9, 323–330. [Google Scholar] [CrossRef]
- Ramin-Wright, A.; Schwartz AS, K.; Geraedts, K.; Rauchfuss, M.; Wölfler, M.M.; Haeberlin, F.; Leeners, B. Fatigue—A symptom in endometriosis. Hum. Reprod. 2018, 33, 1459–1465. [Google Scholar] [CrossRef]
- Sepulcri RD, P.; do Amaral, V.F. Depressive symptoms, anxiety, and quality of life in women with pelvic endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 142, 53–56. [Google Scholar] [CrossRef]
- Armour, M.; Ciccia, D.; Stoikos, C.; Wardle, J. Endometriosis and the workplace: Lessons from Australia’s response to COVID-19. Aust. N. Z. J. Obstet. Gynaecol. 2021, 62, 164–167. [Google Scholar] [CrossRef]
- Armour, M.; Lawson, K.; Wood, A.; Smith, C.A.; Abbott, J. The cost of illness and economic burden of endometriosis and chronic pelvic pain in Australia: A national online survey. PLoS ONE 2019, 14, e0223316. [Google Scholar] [CrossRef]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T.; World Endometriosis Research Foundation Global Study of Women’s Health Consortium. Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil. Steril. 2011, 96, 366–373.e8. [Google Scholar] [CrossRef]
- Cousins, F.L.; McKinnon, B.D.; Mortlock, S.; Fitzgerald, H.C.; Zhang, C.; Montgomery, G.W.; Gargett, C.E. New concepts on the etiology of endometriosis. J. Obstet. Gynaecol. Res. 2023, 49, 1090–1105. [Google Scholar] [CrossRef]
- Forster, R.; Sarginson, A.; Velichkova, A.; Hogg, C.; Dorning, A.; Horne, A.W.; Saunders, P.T.K.; Greaves, E. Macrophage-derived insulin-like growth factor-1 is a key neurotrophic and nerve-sensitizing factor in pain associated with endometriosis. FASEB J. 2019, 33, 11210–11222. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GnRH Antagonists: A Review. J. Clin. Med. 2021, 10, 1085. [Google Scholar] [CrossRef]
- Yilmaz, B.D.; Bulun, S.E. Endometriosis and nuclear receptors. Hum. Reprod. Update 2019, 25, 473–485. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. GnRH Antagonists with or without Add-Back Therapy: A New Alternative in the Management of Endometriosis? Int. J. Mol. Sci. 2021, 22, 11342. [Google Scholar] [CrossRef]
- Brown, J.; Pan, A.; Hart, R.J. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst. Rev. 2010, 2010, CD008475. [Google Scholar] [CrossRef]
- Donnez, J.; Taylor, R.N.; Taylor, H.S. Partial suppression of estradiol: A new strategy in endometriosis management? Fertil. Steril. 2017, 107, 568–570. [Google Scholar] [CrossRef]
- Abbott, J.R.M. Endometriosis Clinical Practice Guideline; RANZCOG: Melbourne, Australia, 2022; pp. 1–70. [Google Scholar]
- Andrade, M.A.; Soares, L.C.; de Oliveira, M.A.P. The Effect of Neuromodulatory Drugs on the Intensity of Chronic Pelvic Pain in Women: A Systematic Review. Rev. Bras. Hematol. Hemoter. 2022, 44, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, A.; Chalmers, K.J.; Micheal, S.; Diezel, H.; Armour, M. “A day-to-day struggle”: A comparative qualitative study on experiences of women with endometriosis and chronic pelvic pain. Fem. Psychol. 2022, 32, 482–500. [Google Scholar] [CrossRef]
- Evans, S.; Villegas, V.; Dowding, C.; Druitt, M.; O’Hara, R.; Mikocka-Walus, A. Treatment use and satisfaction in Australian women with endometriosis: A mixed-methods study. Intern. Med. J. 2021, 52, 2096–2106. [Google Scholar] [CrossRef]
- Sinaii, N.; Cleary, S.D.; Younes, N.; Ballweg, M.L.; Stratton, P. Treatment utilization for endometriosis symptoms: A cross-sectional survey study of lifetime experience. Fertil. Steril. 2007, 87, 1277–1286. [Google Scholar] [CrossRef]
- As-Sanie, S.; Soliman, A.M.; Evans, K.; Erpelding, N.; Lanier, R.; Katz, N. Healthcare utilization and cost burden among women with endometriosis by opioid prescription status in the first year after diagnosis: A retrospective claims database analysis. J. Med. Econ. 2020, 23, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Kilpatrick, R.D.; Hornstein, M.D.; Petruski-Ivleva, N.; Wegrzyn, L.R.; Dabrowski, E.C.; Velentgas, P.; Snabes, M.C.; Bateman, B.T. Chronic opioid use and complication risks in women with endometriosis: A cohort study in US administrative claims. Pharmacoepidemiol. Drug Saf. 2021, 30, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Lamvu, G.; Soliman, A.M.; Manthena, S.R.; Gordon, K.; Knight, J.; Taylor, H.S. Patterns of prescription opioid use in women with endometriosis: Evaluating prolonged use, daily dose, and concomitant use with benzodiazepines. Obstet. Gynecol. 2019, 133, 1120. [Google Scholar] [CrossRef] [PubMed]
- Els, C.; Jackson, T.D.; Kunyk, D.; Lappi, V.G.; Sonnenberg, B.; Hagtvedt, R.; Sharma, S.; Kolahdooz, F.; Straube, S. Adverse events associated with medium- and long-term use of opioids for chronic non-cancer pain: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 10, CD012509. [Google Scholar]
- Bachhuber, M.A.; Hennessy, S.; Cunningham, C.O.; Starrels, J.L. Increasing Benzodiazepine Prescriptions and Overdose Mortality in the United States, 1996–2013. Am. J. Public Health 2016, 106, 686–688. [Google Scholar] [CrossRef]
- Deering, D.E.; Adamson, S.J.; Sellman, J.D.; Henderson, C.; Sheridan, J.; Pooley, S.; Robertson, R.M.; Noller, G.; Frampton, C.M. Potential risk for fatal drug overdose perceived by people using opioid drugs. Drug Alcohol Rev. 2018, 37, S309–S313. [Google Scholar] [CrossRef]
- Leonardi, M.; Gibbons, T.; Armour, M.; Wang, R.; Glanville, E.; Hodgson, R.; Cave, A.E.; Ong, J.; Tong, Y.Y.F.; Jacobson, T.Z.; et al. When to Do Surgery and When Not to Do Surgery for Endometriosis: A Systematic Review and Meta-analysis. J. Minim. Invasive Gynecol. 2019, 27, 390–407.e3. [Google Scholar] [CrossRef] [PubMed]
- Armour, M.; Avery, J.; Leonardi, M.; Van Niekerk, L.; Druitt, M.L.; A Parker, M.; E Girling, J.; McKinnon, B.; Mikocka-Walus, A.; Ng, C.H.M.; et al. Lessons from implementing the Australian National Action Plan for Endometriosis. Reprod. Fertil. 2022, 3, C29–C39. [Google Scholar] [CrossRef] [PubMed]
- Roman, H.; Chanavaz-Lacheray, I.; Hennetier, C.; Tuech, J.-J.; Dennis, T.; Verspyck, E.; Merlot, B. Long-term risk of repeated surgeries in women managed for endometriosis: A 1,092 patient-series. Fertil. Steril. 2023, 120, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Fisher, J.; Kirkman, M. Endometriosis and fertility: Women’s accounts of healthcare. Hum. Reprod. 2016, 31, 554–562. [Google Scholar] [CrossRef]
- As-Sanie, S.; Black, R.; Giudice, L.C.; Valbrun, T.G.; Gupta, J.; Jones, B.; Laufer, M.R.; Milspaw, A.T.; Missmer, S.A.; Norman, A.; et al. Assessing research gaps and unmet needs in endometriosis. Am. J. Obstet. Gynecol. 2019, 221, 86–94. [Google Scholar] [CrossRef]
- Australian Government Department of Health. National Action Plan for Endometriosis. 2018. Available online: http://www.health.gov.au/internet/main/publishing.nsf/Content/endometriosis (accessed on 3 October 2023).
- Armour, M.; Ciccia, D.; Yazdani, A.; Rombauts, L.; Van Niekerk, L.; Schubert, R.; Abbott, J. Endometriosis research priorities in Australia. Aust. N. Z. J. Obstet. Gynaecol. 2023, 63, 594–598. [Google Scholar] [CrossRef]
- Armour, M.; Sinclair, J. Cannabis for endometriosis-related pain and symptoms: It’s high time that we see this as a legitimate treatment. Aust. N. Z. J. Obstet. Gynaecol. 2023, 63, 118–120. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Nie, Y.; Tian, Y.; Xiao, X.; Yang, F. Endocannabinoid activation of the TRPV1 ion channel is distinct from activation by capsaicin. J. Biol. Chem. 2021, 297, 101022. [Google Scholar] [CrossRef]
- Rodríguez de Fonseca, F.; Del Arco, I.; Bermudez-Silva, F.J.; Bilbao, A.; Cippitelli, A.; Navarro, M. The endocannabinoid system: Physiology and pharmacology. Alcohol Alcohol. 2005, 40, 2–14. [Google Scholar] [CrossRef]
- Lingegowda, H.; Williams, B.J.; Spiess, K.G.; Sisnett, D.J.; Lomax, A.E.; Koti, M.; Tayade, C. Role of the endocannabinoid system in the pathophysiology of endometriosis and therapeutic implications. J. Cannabis Res. 2022, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, N.; Nagabukuro, H.; Resuehr, D.; Zhang, G.; McAllister, S.L.; McGinty, K.A.; Mackie, K.; Berkley, K.J. Endocannabinoid involvement in endometriosis. Pain 2010, 151, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid Signaling and Synaptic Function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Cuddihey, H.; MacNaughton, W.K.; Sharkey, K.A. Role of the Endocannabinoid System in the Regulation of Intestinal Home-ostasis. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 947–963. [Google Scholar] [CrossRef]
- Kienzl, M.; Kargl, J.; Schicho, R. The Immune Endocannabinoid System of the Tumor Microenvironment. Int. J. Mol. Sci. 2020, 21, 8929. [Google Scholar] [CrossRef]
- Dos Santos, R.S.; Veras, F.P.; Netto, G.P.; Sorgi, C.A.; Faccioli, L.H.; Vilela, L.R.; Galdino, G. Cannabidiol reduces lipopolysaccharide-induced nociception via endocannabinoid system activation. Basic Clin. Pharmacol. Toxicol. 2023, 133, 16–28. [Google Scholar] [CrossRef]
- Rakotoarivelo, V.; Sihag, J.; Flamand, N. Role of the Endocannabinoid System in the Adipose Tissue with Focus on Energy Metabolism. Cells 2021, 10, 1279. [Google Scholar] [CrossRef]
- Smith, P.J.W.; McQueen, D.S. Anandamide induces cardiovascular and respiratory reflexes via vasosensory nerves in the anaesthetized rat. Br. J. Pharmacol. 2001, 134, 655–663. [Google Scholar] [CrossRef]
- Murillo-Rodriguez, E.; Poot-Ake, A.; Arias-Carrion, O.; Pacheco-Pantoja, E.; de la Fuente-Ortegon, A.; Arankowsky-Sandoval, G. The Emerging Role of the Endocannabinoid System in the Sleep-Wake Cycle Modulation. Cent. Nerv. Syst. Agents Med. Chem. 2011, 11, 189–196. [Google Scholar] [CrossRef]
- Woodhams, S.G.; Chapman, V.; Finn, D.P.; Hohmann, A.G.; Neugebauer, V. The cannabinoid system and pain. Neuropharmacology 2017, 124, 105–120. [Google Scholar] [CrossRef]
- Fine, P.G.; Rosenfeld, M.J. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Med. J. 2013, 4, e0022. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Hohmann, A.G. The Endocannabinoid System and Pain. CNS Neurol. Disord. Drug Targets 2009, 8, 403–421. [Google Scholar] [CrossRef] [PubMed]
- Burston, J.J.; Woodhams, S.G. Endocannabinoid system and pain: An introduction. Proc. Nutr. Soc. 2014, 73, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.; Baños, J.E.; Cabañero, D. The endocannabinoid system and neuropathic pain. Pain 2016, 157, S23–S32. [Google Scholar] [CrossRef]
- Curto-Reyes, V.; Boto, T.; Hidalgo, A.; Menéndez, L.; Baamonde, A. Antinociceptive effects induced through the stimulation of spinal cannabinoid type 2 receptors in chronically inflamed mice. Eur. J. Pharmacol. 2011, 668, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, T.; Farthing, J.N.; Zvonok, A.M.; Makriyannis, A.; Hohmann, A.G. Activation of peripheral cannabinoid CB1 and CB2 receptors suppresses the maintenance of inflammatory noci-ception: A comparative analysis. Br. J. Pharmacol. 2007, 150, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.I.; Jay, C.A.; Shade, S.B.; Vizoso, H.; Reda, H.; Press, S.; Kelly, M.E.; Rowbotham, M.C.; Petersen, K.L. Cannabis in painful HIV-associated sensory neuropathy: A randomized placebo-controlled trial. Neurology 2007, 68, 515–521. [Google Scholar] [CrossRef]
- Conte, A.; Bettolo, C.M.; Onesti, E.; Frasca, V.; Iacovelli, E.; Gilio, F.; Giacomelli, E.; Gabriele, M.; Aragona, M.; Tomassini, V.; et al. Cannabinoid-induced effects on the nociceptive system: A neurophysiological study in patients with secondary progressive multiple sclerosis. Eur. J. Pain 2009, 13, 472–477. [Google Scholar] [CrossRef]
- Di Blasio, A.M.; Vignali, M.; Gentilini, D. The endocannabinoid pathway and the female reproductive organs. J. Mol. Endocrinol. 2012, 50, R1–R9. [Google Scholar] [CrossRef]
- Walker, O.S.; Holloway, A.C.; Raha, S. The role of the endocannabinoid system in female reproductive tissues. J. Ovarian Res. 2019, 12, 3. [Google Scholar] [CrossRef]
- Clemenza, S.; Sorbi, F.; Noci, I.; Capezzuoli, T.; Turrini, I.; Carriero, C.; Buffi, N.; Fambrini, M.; Petraglia, F. From pathogenesis to clinical practice: Emerging medical treatments for endometriosis. Best Pr. Res. Clin. Obstet. Gynaecol. 2018, 51, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, M.; Laezza, C.; Gazzerro, P.; Pentimalli, F. Endocannabinoids as emerging suppressors of angiogenesis and tumor invasion (Review). Oncol. Rep. 2007, 17, 813–816. [Google Scholar] [CrossRef]
- Bilgic, E.; Guzel, E.; Kose, S.; Aydin, M.C.; Karaismailoglu, E.; Akar, I.; Usubutun, A.; Korkusuz, P. Endocannabinoids modulate apoptosis in endometriosis and adenomyosis. Acta Histochem. 2017, 119, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Gentilini, D.; Besana, A.; Vigano, P.; Dalino, P.; Vignali, M.; Melandri, M.; Busacca, M.; Di Blasio, A.M. Endocannabinoid system regulates migration of endometrial stromal cells via cannabinoid receptor 1 through the activation of PI3K and ERK1/2 pathways. Fertil. Steril. 2010, 93, 2588–2593. [Google Scholar] [CrossRef]
- Leconte, M.; Nicco, C.; Ngô, C.; Arkwright, S.; Chéreau, C.; Guibourdenche, J.; Weill, B.; Chapron, C.; Dousset, B.; Batteux, F. Antiproliferative Effects of Cannabinoid Agonists on Deep Infiltrating Endometriosis. Am. J. Pathol. 2010, 177, 2963–2970. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.T. The endocannabinoid system. Pharmacotherapy 2006, 26 Pt 2, 218S–221S. [Google Scholar] [CrossRef]
- Hermanson, D.J.; Marnett, L.J. Cannabinoids, endocannabinoids, and cancer. Cancer Metastasis Rev. 2011, 30, 599–612. [Google Scholar] [CrossRef]
- Ayakannu, T.; Taylor, A.H.; Marczylo, T.H.; Willets, J.M.; Konje, J.C. The Endocannabinoid System and Sex Steroid Hormone-Dependent Cancers. Int. J. Endocrinol. 2013, 2013, 259676. [Google Scholar] [CrossRef]
- Tanaka, K.; Mayne, L.; Khalil, A.; Baartz, D.; Eriksson, L.; Mortlock, S.-A.; Montgomery, G.; McKinnon, B.; Amoako, A.A. The role of the endocannabinoid system in aetiopathogenesis of endometriosis: A potential therapeutic target. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 244, 87–94. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Cioffi, R.; Viganò, P.; Candiani, M.; Verde, R.; Piscitelli, F.; Di Marzo, V.; Garavaglia, E.; Panina-Bordignon, P. Elevated Systemic Levels of Endocannabinoids and Related Mediators Across the Menstrual Cycle in Women With Endometriosis. Reprod. Sci. 2016, 23, 1071–1079. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Vigano, P.; Mugione, A.; Panina-Bordignon, P.; Candiani, M. The molecular connections between the cannabinoid system and endometriosis. Mol. Hum. Reprod. 2012, 18, 563–571. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.M.; Belo, A.V.; Andrade, S.P.; Campos, P.P.; Ferreira MC, F.; da Silva-Filho, A.L.; Carneiro, M.M. Identification of local angiogenic and inflammatory markers in the menstrual blood of women with endometriosis. Biomed. Pharmacother. 2014, 68, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Naseri, S.; Rosenberg-Hasson, Y.; Maecker, H.T.; Avrutsky, M.I.; Blumenthal, P.D. A cross-sectional study comparing the inflammatory profile of menstrual effluent vs. peripheral blood. Health Sci. Rep. 2023, 6, e1038. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.G.; e Silva, J.C.R.; da Silva, A.R.; Dos Reis, F.J.C.; Nogueira, A.A.; Poli-Neto, O.B. TRPV1 Expression on Peritoneal Endometriosis Foci is Associated With Chronic Pelvic Pain. Reprod. Sci. 2011, 18, 511–515. [Google Scholar] [CrossRef]
- Andrieu, T.; Chicca, A.; Pellegata, D.; Bersinger, N.A.; Imboden, S.; Nirgianakis, K.; Gertsch, J.; Mueller, M.D. Association of endocannabinoids with pain in endometriosis. Pain 2022, 163, 193–203. [Google Scholar] [CrossRef]
- Allam, S.; Paris, E.; Lazcano, I.; Bitterman, P.; Basu, S.; O’donnell, J.; Barua, A. Detection of Cannabinoid Receptor Expression by Endometriotic Lesions in Women with Endometriosis as an Alternative to Opioid-Based Pain Medication. J. Immunol. Res. 2022, 2022, 4323259. [Google Scholar] [CrossRef]
- Escudero-Lara, A.; Argerich, J.; Cabañero, D.; Maldonado, R. Disease-modifying effects of natural Delta9-tetrahydrocannabinol in endometriosis-associated pain. eLife 2020, 9, e50356. [Google Scholar] [CrossRef]
- Okten, S.B.; Cetin, C.; Tok, O.E.; Guler, E.M.; Taha, S.H.; Ozcan, P.; Ficicioglu, C. Cannabidiol as a potential novel treatment for endometriosis by its anti-inflammatory, antioxidative and antiangiogenic effects in an experimental rat model. Reprod. Biomed. Online 2023, 46, 865–875. [Google Scholar] [CrossRef]
- Genovese, T.; Cordaro, M.; Siracusa, R.; Impellizzeri, D.; Caudullo, S.; Raffone, E.; Macrí, F.; Interdonato, L.; Gugliandolo, E.; Interlandi, C.; et al. Molecular and Biochemical Mechanism of Cannabidiol in the Management of the Inflammatory and Oxidative Processes Associated with Endometriosis. Int. J. Mol. Sci. 2022, 23, 5427. [Google Scholar] [CrossRef]
- Kinross, J.M.; Darzi, A.W.; Nicholson, J.K. Gut microbiome-host interactions in health and disease. Genome Med. 2011, 3, 14. [Google Scholar] [CrossRef]
- Ley, R.E.; Knight, R.; Gordon, J.I. The human microbiome: Eliminating the biomedical/environmental dichotomy in microbial ecology. Environ. Microbiol. 2007, 9, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial community variation in human body habitats across space and time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: Antibiotics, probiotics, and prebiotics. Gastroenterology 2004, 126, 1620–1633. [Google Scholar] [CrossRef]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.; Rooney, N.; Feeney, M.; Tate, J.; Robertson, D.; Welham, M.; Ward, S. Differential expression of cannabinoid receptors in the human colon: Cannabinoids promote epithelial wound healing. Gastroenterology 2005, 129, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Grill, M.; Hasenoehrl, C.; Kienzl, M.; Kargl, J.; Schicho, R. Cellular localization and regulation of receptors and enzymes of the endocannabinoid system in intestinal and systemic inflammation. Histochem. Cell Biol. 2019, 151, 5–20. [Google Scholar] [CrossRef]
- Rousseaux, C.; Thuru, X.; Gelot, A.; Barnich, N.; Neut, C.; Dubuquoy, L.; Dubuquoy, C.; Merour, E.; Geboes, K.; Desreumaux, P.; et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med. 2007, 13, 35–37. [Google Scholar] [CrossRef]
- Ahmed, W.; Katz, S. Therapeutic Use of Cannabis in Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2016, 12, 668–679. [Google Scholar]
- Alhamoruni, A.; Lee, A.C.; Wright, K.L.; Larvin, M.; O’Sullivan, S.E. Pharmacological Effects of Cannabinoids on the Caco-2 Cell Culture Model of Intestinal Permeability. Experiment 2010, 335, 92–102. [Google Scholar] [CrossRef]
- Alhamoruni, A.; Wright, K.; Larvin, M.; O’Sullivan, S. Cannabinoids mediate opposing effects on inflammation-induced intestinal permeability. Br. J. Pharmacol. 2012, 165, 2598–2610. [Google Scholar] [CrossRef]
- Gioacchini, G.; Rossi, G.; Carnevali, O. Host-probiotic interaction: New insight into the role of the endocannabinoid system by in vivo and ex vivo approaches. Sci. Rep. 2017, 7, 1261. [Google Scholar] [CrossRef] [PubMed]
- Distrutti, E.; O’Reilly, J.-A.; McDonald, C.; Cipriani, S.; Renga, B.; Lynch, M.A.; Fiorucci, S. Modulation of Intestinal Microbiota by the Probiotic VSL#3 Resets Brain Gene Expression and Ameliorates the Age-Related Deficit in LTP. PLoS ONE 2014, 9, e106503. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Ghaderi, F.; Sotoodehnejadnematalahi, F.; Hajebrahimi, Z.; Fateh, A.; Siadat, S.D. Effects of active, inactive, and derivatives of Akkermansia muciniphila on the expression of the endocannabinoid system and PPARs genes. Sci. Rep. 2022, 12, 10031. [Google Scholar] [CrossRef]
- Forte, N.; Fernández-Rilo, A.C.; Palomba, L.; Di Marzo, V.; Cristino, L. Obesity Affects the Microbiota–Gut–Brain Axis and the Regulation Thereof by Endocannabinoids and Related Mediators. Int. J. Mol. Sci. 2020, 21, 1554. [Google Scholar] [CrossRef]
- Vijay, A.; Kouraki, A.; Gohir, S.; Turnbull, J.; Kelly, A.; Chapman, V.; A Barrett, D.; Bulsiewicz, W.J.; Valdes, A.M. The anti-inflammatory effect of bacterial short chain fatty acids is partially mediated by endocannabinoids. Gut Microbes 2021, 13, 1997559. [Google Scholar] [CrossRef] [PubMed]
- Dohnalová, L.; Lundgren, P.; Carty, J.R.E.; Goldstein, N.; Wenski, S.L.; Nanudorn, P.; Thiengmag, S.; Huang, K.-P.; Litichevskiy, L.; Descamps, H.C.; et al. A microbiome-dependent gut–brain pathway regulates motivation for exercise. Nature 2022, 612, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Sasaki, K.; Inoue, J.; Sasaki, D.; Hoshi, N.; Shirai, T.; Fukuda, I.; Azuma, T.; Kondo, A.; Osawa, R. Construction of a Model Culture System of Human Colonic Microbiota to Detect Decreased Lachnospiraceae Abundance and Butyrogenesis in the Feces of Ulcerative Colitis Patients. Biotechnol. J. 2019, 14, e1800555. [Google Scholar] [CrossRef]
- Cani, P.D.; Plovier, H.; Van Hul, M.; Geurts, L.; Delzenne, N.M.; Druart, C.; Everard, A. Endocannabinoids—At the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 2016, 12, 133–143. [Google Scholar] [CrossRef]
- Bisogno, T.; Lauritano, A.; Piscitelli, F. The Endocannabinoid System: A Bridge between Alzheimer’s Disease and Gut Microbiota. Life 2021, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- Argueta, D.A.; DiPatrizio, N.V. Peripheral endocannabinoid signaling controls hyperphagia in western diet-induced obesity. Physiol. Behav. 2017, 171, 32–39. [Google Scholar] [CrossRef]
- Actis, G.C. The gut microbiome. Inflamm. Allergy Drug Targets 2014, 13, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Prasad, N.; Ryan, M.; Tangri, S.; Silverberg, M.S.; Gordon, A.; Steinhart, H. Cannabis use amongst patients with inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2011, 23, 891–896. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Courtwright, A.; Lucci, M.; Korzenik, J.R.; Levine, J. Marijuana Use Patterns Among Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 2809–2814. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Battista, N.; Biancheri, P.; Rapino, C.; Rovedatti, L.; Astarita, G.; Vanoli, A.; Dainese, E.; Guerci, M.; Corazza, G.R. The endogenous cannabinoid system in the gut of patients with inflammatory bowel disease. Mucosal Immunol. 2011, 4, 574–583. [Google Scholar] [CrossRef]
- Strisciuglio, C.; Creoli, M.; Tortora, C.; Martinelli, M.; Miele, E.; Paino, S.; Luongo, L.; Rossi, F. Increased expression of CB2 receptor in the intestinal biopsies of children with inflammatory bowel disease. Pediatr. Res. 2023, 93, 520–525. [Google Scholar] [CrossRef]
- Bogale, K.; Raup-Konsavage, W.; Dalessio, S.; Vrana, K.; Coates, M.D. Cannabis and Cannabis Derivatives for Abdominal Pain Management in Inflammatory Bowel Disease. Med. Cannabis Cannabinoids 2021, 4, 97–106. [Google Scholar] [CrossRef]
- Lichtman, A.H.; Naidu, P.S.; Booker, L.; Boger, D.L.; Cravatt, B.F. Targeting FAAH and COX to treat visceral pain. FASEB J. 2008, 22, 12. [Google Scholar] [CrossRef]
- Sakin, Y.S.; Dogrul, A.; Ilkaya, F.; Seyrek, M.; Ulas, U.H.; Gulsen, M.; Bagci, S. The effect of FAAH, MAGL, and Dual FAAH/MAGL inhibition on inflammatory and colorectal distension-induced visceral pain models in Rodents. Neurogastroenterol. Motil. 2015, 27, 936–944. [Google Scholar] [CrossRef]
- Coates, M.D.; Dalessio, S.; Walter, V.; Stuart, A.; Bernasko, N.; Tinsley, A.; Razeghi, S.; Williams, E.D.; Clarke, K.; Vrana, K. Symptoms and Extraintestinal Manifestations in Active Cannabis Users with Inflammatory Bowel Disease. Cannabis Cannabinoid Res. 2022, 7, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.E.; Yong, P.J.; Williams, C.; Allaire, C. Factors Associated with Severity of Irritable Bowel Syndrome Symptoms in Patients with Endometriosis. J. Obstet. Gynaecol. Can. 2018, 40, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.S.; Gibson, P.R.; Perry, R.E.; Burgell, R.E. Endometriosis in patients with irritable bowel syndrome: Specific symptomatic and demographic profile, and response to the low FODMAP diet. Aust. N. Z. J. Obstet. Gynaecol. 2017, 57, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Nabi, M.Y.; Nauhria, S.; Reel, M.; Londono, S.; Vasireddi, A.; Elmiry, M.; Ramdass, P.V.A.K. Endometriosis and irritable bowel syndrome: A systematic review and meta-analyses. Front. Med. 2022, 9, 914356. [Google Scholar] [CrossRef] [PubMed]
- Pagano, E.; Romano, B.; Iannotti, F.A.; Parisi, O.A.; D’armiento, M.; Pignatiello, S.; Coretti, L.; Lucafò, M.; Venneri, T.; Borrelli, F.; et al. The non-euphoric phytocannabinoid cannabidivarin counteracts intestinal inflammation in mice and cytokine ex-pression in biopsies from UC pediatric patients. Pharmacol. Res. 2019, 149, 104464. [Google Scholar] [CrossRef]
- Mehrpouya-Bahrami, P.; Chitrala, K.N.; Ganewatta, M.S.; Tang, C.; Murphy, E.A.; Enos, R.T.; Velazquez, K.T.; McCellan, J.; Nagarkatti, M.; Nagarkatti, P.; et al. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and di-et-induced obesity. Sci. Rep. 2017, 7, 15645. [Google Scholar] [CrossRef] [PubMed]
- Cluny, N.L.; Keenan, C.M.; Reimer, R.A.; Le Foll, B.; Sharkey, K.A. Prevention of Diet-Induced Obesity Effects on Body Weight and Gut Microbiota in Mice Treated Chronically with Delta9-Tetrahydrocannabinol. PLoS ONE 2015, 10, e0144270. [Google Scholar] [CrossRef]
- Kiran, S.; Rakib, A.; Moore, B.M.; Singh, U.P. Cannabinoid Receptor 2 (CB2) Inverse Agonist SMM-189 Induces Expression of Endogenous CB2 and Protein Kinase A That Differentially Modulates the Immune Response and Suppresses Experimental Colitis. Pharmaceutics 2022, 14, 936. [Google Scholar] [CrossRef]
- Jamontt, J.M.; Molleman, A.; Pertwee, R.G.; Parsons, M.E. The effects of Delta-tetrahydrocannabinol and cannabidiol alone and in combination on damage, inflammation and in vitro motility disturbances in rat colitis. Br. J. Pharmacol. 2010, 160, 712–723. [Google Scholar] [CrossRef]
- Borrelli, F.; Aviello, G.; Romano, B.; Orlando, P.; Capasso, R.; Maiello, F.; Guadagno, F.; Petrosino, S.; Capasso, F.; Di Marzo, V.; et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J. Mol. Med. 2009, 87, 1111–1121. [Google Scholar] [CrossRef]
- Martínez, S.; Garrido, N.; Coperias, J.; Pardo, F.; Desco, J.; García-Velasco, J.; Simón, C.; Pellicer, A. Serum interleukin-6 levels are elevated in women with minimal–mild endometriosis. Hum. Reprod. 2007, 22, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Bedaiwy, M.A.; Falcone, T.; Sharma, R.K.; Goldberg, J.M.; Attaran, M.; Nelson, D.R.; Agarwal, A. Prediction of endometriosis with serum and peritoneal fluid markers: A prospective controlled trial. Hum. Reprod. 2002, 17, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Mosbah, A.; Nabiel, Y.; Khashaba, E. Interleukin-6, intracellular adhesion molecule-1, and glycodelin A levels in serum and peritoneal fluid as biomarkers for endometriosis. Int. J. Gynecol. Obstet. 2016, 134, 247–251. [Google Scholar] [CrossRef]
- Hogg, C.; Panir, K.; Dhami, P.; Rosser, M.; Mack, M.; Soong, D.; Pollard, J.W.; Jenkins, S.J.; Horne, A.W.; Greaves, E. Macrophages inhibit and enhance endometriosis depending on their origin. Proc. Natl. Acad. Sci. USA 2021, 118, e2013776118. [Google Scholar] [CrossRef] [PubMed]
- Allaband, C.; McDonald, D.; Vázquez-Baeza, Y.; Minich, J.J.; Tripathi, A.; Brenner, D.A.; Loomba, R.; Smarr, L.; Sandborn, W.J.; Schnabl, B.; et al. Microbiome 101: Studying, Analyzing, and Interpreting Gut Microbiome Data for Clinicians. Clin. Gastroenterol. Hepatol. 2019, 17, 218–230. [Google Scholar] [CrossRef]
- Muraoka, A.; Suzuki, M.; Hamaguchi, T.; Watanabe, S.; Iijima, K.; Murofushi, Y.; Shinjo, K.; Osuka, S.; Hariyama, Y.; Kondo, Y.; et al. Fusobacterium infection facilitates the development of endometriosis through the phenotypic transition of endo-metrial fibroblasts. Sci. Transl. Med. 2023, 15, eadd1531. [Google Scholar] [CrossRef] [PubMed]
- Ata, B.; Yildiz, S.; Turkgeldi, E.; Brocal, V.P.; Dinleyici, E.C.; Moya, A.; Urman, B. The Endobiota Study: Comparison of Vaginal, Cervical and Gut Microbiota Between Women with Stage 3/4 Endometriosis and Healthy Controls. Sci. Rep. 2019, 9, 2204. [Google Scholar] [CrossRef]
- Beaud, D.; Tailliez, P.; Anba-Mondoloni, J. Genetic characterization of the beta-glucuronidase enzyme from a human intestinal bacterium, Ruminococcus gnavus. Microbiology 2005, 151 Pt 7, 2323–2330. [Google Scholar] [CrossRef]
- Kwa, M.; Plottel, C.S.; Blaser, M.J.; Adams, S. The Intestinal Microbiome and Estrogen Receptor–Positive Female Breast Cancer. J. Natl. Cancer Inst. 2016, 108, djw029. [Google Scholar]
- Possemiers, S.; Bolca, S.; Verstraete, W.; Heyerick, A. The intestinal microbiome: A separate organ inside the body with the metabolic potential to influence the bioactivity of botanicals. Fitoterapia 2011, 82, 53–66. [Google Scholar] [CrossRef]
- Leonardi, M.; Hicks, C.; El-Assaad, F.; El-Omar, E.; Condous, G. Endometriosis and the microbiome: A systematic review. BJOG 2020, 127, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Kitajima, M.; Hiraki, K.; Yamaguchi, N.; Katamine, S.; Matsuyama, T.; Nakashima, M.; Fujishita, A.; Ishimaru, T.; Masuzaki, H. Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil. Steril. 2010, 94, 2860–2863.e3. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Kitajima, M.; Imamura, T.; Hiraki, K.; Fujishita, A.; Sekine, I.; Ishimaru, T.; Masuzaki, H. Toll-like receptor 4-mediated growth of endometriosis by human heat-shock protein 70. Hum. Reprod. 2008, 23, 2210–2219. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, K.; Zhang, L.; Liu, Q.Y.; Huang, Y.K.; Kang, Y.; Xu, C.J. Dysbiosis of gut microbiota contributes to chronic stress in endometriosis patients via activating inflammatory pathway. Reprod. Dev. Med. 2017, 1, 221–227. [Google Scholar] [CrossRef]
- Iavarone, I.; Greco, P.F.; La Verde, M.; Morlando, M.; Torella, M.; de Franciscis, P.; Ronsini, C. Correlations between Gut Microbial Composition, Pathophysiological and Surgical Aspects in Endometriosis: A Review of the Literature. Medicina 2023, 59, 347. [Google Scholar] [CrossRef] [PubMed]
- Sandström, A.; Bixo, M.; Johansson, M.; Bäckström, T.; Turkmen, S. Effect of hysterectomy on pain in women with endometriosis: A population-based registry study. BJOG 2020, 127, 1628–1635. [Google Scholar] [CrossRef]
- Svensson, A.; Brunkwall, L.; Roth, B.; Orho-Melander, M.; Ohlsson, B. Associations Between Endometriosis and Gut Microbiota. Reprod. Sci. 2021, 28, 2367–2377. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Masumoto, H.; Muto, H.; Kitajima, M.; Masuzaki, H.; Kitawaki, J. Molecular detection of intrauterine microbial colonization in women with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 199, 69–75. [Google Scholar] [CrossRef]
- Chang, C.Y.-Y.; Chiang, A.-J.; Lai, M.-T.; Yan, M.-J.; Tseng, C.-C.; Lo, L.-C.; Wan, L.; Li, C.-J.; Tsui, K.-H.; Chen, C.-M.; et al. A More Diverse Cervical Microbiome Associates with Better Clinical Outcomes in Patients with Endometriosis: A Pilot Study. Biomedicines 2022, 10, 174. [Google Scholar] [CrossRef]
- Salliss, M.E.; Farland, L.V.; Mahnert, N.D.; Herbst-Kralovetz, M.M. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum. Reprod. Updat. 2021, 28, 92–131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).