Oral Findings in Hemodialyzed Patients Diagnosed with Diabetes Mellitus and/or Hypertension—A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
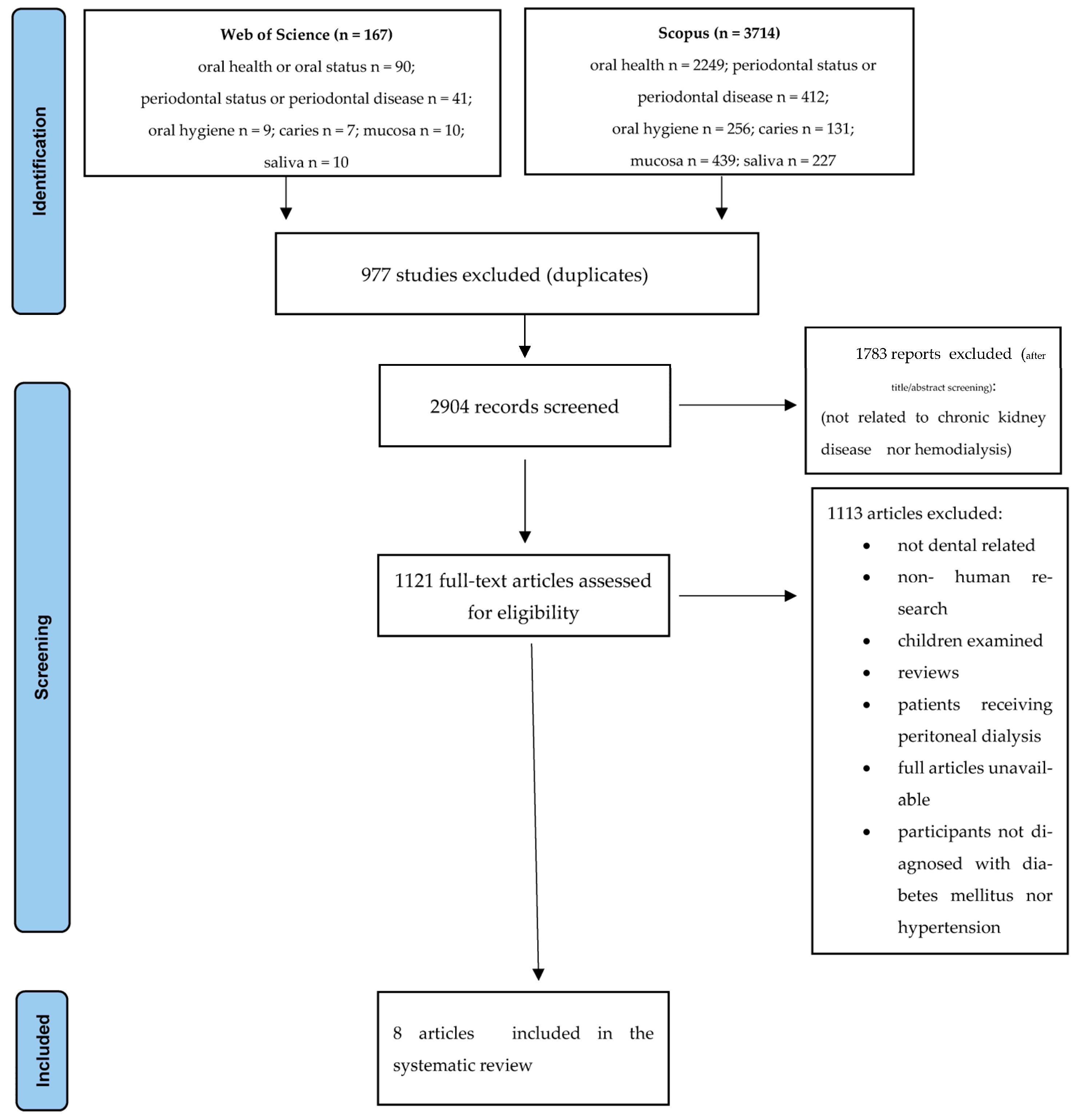
2.1. Strategy of Searching and Criteria of Material Selection
- <0.00—poor;
- 0.00–0.20—slight;
- 0.21–0.40—fair;
- 0.41–0.60—moderate;
- 0.61–0.80—substantial;
- 0.81–1.00—almost perfect.
2.2. Substantive Analysis
2.3. Quality Assessment
- 9–10 points: very good quality;
- 7–8 points: good quality;
- 5–6 points: satisfactory quality;
3. Results
3.1. Study Characteristics
3.2. Oral Hygiene
3.3. Periodontal Status
3.4. Mucosa and Saliva
3.5. Oral Candidiasis
3.6. Quality Assessment
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dande, R.; Gadball, A.R.; Sarode, S.; Gadbal, M.P.M.; Gondivkar, S.M.; Gawande, M. Oral manifestations in diabetic and nondiabetic chronic renal faiure patients receiving hemodialysis. J. Contemp. Dent. Pract. 2018, 19, 398–403. [Google Scholar] [PubMed]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Preserving kidney function in people with chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Woo, K.; Yi, J.A. Epidemiology of end-stage kidney disease. Semin. Vasc. Surg. 2021, 34, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.K.; Okpechi, I.G.; Osman, M.A.; Cho, Y.; Htay, H.; Jha, V.; Wainstein, M.; Johnson, D. Epidemiology of haemodialysis outcomes. Nat. Rev. Nephrol. 2022, 18, 378–395. [Google Scholar] [CrossRef]
- Gautam, N.R.; Gautam, N.S.; Rao, T.H.; Koganti, R.; Agarwal, R.; Alamanda, M. Effect of end- stage renal disease on oral health in patients undergoing renal dialysis: A cross-sectional study. J. Int. Soc. Prev. Community Dent. 2014, 4, 164–169. [Google Scholar] [CrossRef]
- Wallace, K.; Shafique, S.; Piamjariyakul, U. The relationship between oral health and hemodialysis treatment among adults with chronic kidney disease: A systematic review. Nephrol. Nurs. J. 2019, 46, 375–394. [Google Scholar]
- Mainali, A.; Chettri, P.K. Oral manifestations in hemodialysis patients and their knowledge and attitude towards oral health. Nepal Med. Coll. J. 2020, 22, 217–222. [Google Scholar] [CrossRef]
- Acharya, A.K.; Chinna, S.K.; Bankur, R.; Margabandhy, S.; Babu, B.A.; Ismail, N. Assessment of oral health status and treatment needs in hemodialysis patients at Raichur district, Karnataka, India. Int. J. Prev. Clin. Dent. Res. 2021, 8, 47–51. [Google Scholar]
- Rohani, B. Oral manifestations in patients with diabetes mellitus. World J. Diabetes 2019, 10, 485–489. [Google Scholar] [CrossRef]
- Kumar, P.; Mastan, K.M.K.; Chowdhary, R.; Shanmugam, K. Oral manifestations in hypersensitive patients: A clinical study. J. Oral. Maxillofac. Pathol. 2012, 16, 215–221. [Google Scholar] [CrossRef]
- Lalvay Armijos, D.A.; Castaneda Espin, A.O.; Cobos Carrera, D.F. Antihyperstensive medication and its adverse reactions in the oral cavity. An integrative review. Res. Soc. Dev. 2022, 11, e202111032624. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cohrane Handbook for Systematic Reviews of Interventions; The Cohrane Collaboration: London, UK, 2011. [Google Scholar]
- Hasanpou Dehkordi, A.; Mazaheri, E.; Ibrahim, H.A.; Dalvand, S.; Ghanei Gheshlagh, R. How to write a systematic review: A narrative review. Int. J. Prev. Med. 2021, 12, 27. [Google Scholar]
- MacLure, K.; Paudyal, V.; Stewart, D. Reviewing the literature, how systematic is systematic? Int. J. Clin. Pharm. 2016, 38, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, R. PICO: Model for clinical questions. Evid. Based Med. Pract. 2018, 3, 2. [Google Scholar]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Herzgo, R.; Alvaez-Pasquin, M.J.; Diaz, C.; Del Barrio, J.L.; Estrada, J.M.; Gil, A. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013, 13, 154. [Google Scholar] [CrossRef]
- Al-Sarray, M.N.; Abdulla, A.B.A.S.; Al Kabe, M.A. Oral Candidiasis in chronic kidney disease. Indian J. Forensic Toxicol. 2020, 14, 1230–1234. [Google Scholar]
- Trzcionka, A.; Twardawa, H.; Mocny-Pachońska, K.; Tanasiewicz, M. Periodontal treatment needs of hemodialized patients. Healthcare 2021, 9, 139. [Google Scholar] [CrossRef]
- Trzcionka, A.; Twardawa, H.; Mocny-Pachońska, K.; Korkosz, R.; Tanasiewicz, M. Oral mucosa status and saliva parameters of multimorbid adult patients diagnosed with end-stage chronic kidney disease. Int. J. Environ. Res. Public Health 2021, 18, 12515. [Google Scholar] [CrossRef]
- Ayinampudi, B.K.; Chervu, A.R.; Raju, S.B.; Pacha, V.B. Oral Candida colonization in renal disease patients between diabetes and non-diabetes; a comparative study. Immunopathol. Persa 2018, 4, e08. [Google Scholar] [CrossRef]
- Naruishi, K.; Oishi, K.; Inagaki, Y.; Horibe, M.; Bando, M.; Ninomiya, M.; Kawahara, K.; Minakuchi, J.; Kawashima, S.; Shima, K.; et al. Association between periodontal condition and kidney dysfunction in Japanese adults: A cross-sectional study. Clin. Exp. Dent. Res. 2016, 2, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Swapna, L.A.; Reddy, R.S.; Ramesh, T.; Reddy, R.L.; Vijayalaxmi, N.; Karmakar, P.; Pradeep, K. Oral health in haemodialysis patients. JCDR 2013, 7, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- Trzcionka, A.; Twardawa, H.; Mocny-Pachońska, K.; Tanasiewicz, M. Oral cavity status of long-term hemodialized patients vs. their socio-economic status. Med. Pr. 2020, 71, 279–288. [Google Scholar] [CrossRef]
- Gaddey, H.L. Oral manifestations of systemic disease. Gen. Dent. 2017, 65, 23–29. [Google Scholar]
- Swinson, B.; Witherow, H.; Norris, P.; Lloyd, T. Oral manifestations of systemic diseases. Hosp. Med. 2014, 65, 92–99. [Google Scholar] [CrossRef]
- Mulliken, R.A.; Casner, M.J. Oral manifestations of systemic disease. Emerg. Med. Clin. N. Am. 2000, 18, 565–575. [Google Scholar] [CrossRef]
- Kinane, D.F.; Marshall, G.J. Periodontal manifestations of systemic disease. Aust. Dent. J. 2001, 46, 2–12. [Google Scholar] [CrossRef]
- Capodiferro, S.; Limongelli, L.; Favia, G. Oral and maxilla-facial manifestations of systemic diseases: An overview. Medicina 2021, 57, 271. [Google Scholar] [CrossRef]
- Albandar, J.M.; Susin, C.; Hughes, F.J. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45, s171–s189. [Google Scholar] [CrossRef]
- Proctor, R.; Kumar, N.; Stein, A.; Moles, D. Oral and dental aspects of chronic renal failure. J. Dent. Res. 2005, 84, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Oyetola, E.O.; Owotade, F.J.; Agbelusi, G.A.; Fatusi, O.; Sanusi, A.A. Oral findings in chronic kidney disease: Implications for management in developing countries. BMC Oral Health 2015, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.M.; Mahmood, M.A.; Abdulraheam, R.H.; Ahmed, S.M. Oral and dental findings in patients with end stage renal disease undergoing maintenance hemodialysis in Sulaimani City. J. Interdiscipl. Med. Dent. Sci. 2015, 3, 82. [Google Scholar] [CrossRef]
- Patil, S.; Khandelwal, S.; Doni, B.; Rahman, F.; Kaswan, S. Oral manifestations in chronic renal failure patients attending two hospitals in north Karnataka, India. OHDM 2012, 11, 100–106. [Google Scholar]
- Teratani, G.; Awano, S.; Soh, I.; Yoshida, A.; Kinoshita, N.; Hamasaki, T.; Takata, Y.; Sonoki, K.; Nakamura, H.; Ansai, T. Oral health in patients on haemodialysis for diabetic nephropathy and chronic glomerulonephritis. Clin. Oral Investig. 2013, 17, 483–489. [Google Scholar] [CrossRef]
- Baykoucheva, S. Selectind a database for drug literature retrival: A comparison of MEDLINE, Scopus, and Web of Science. Sci. Technol. Libr. 2010, 29, 276–288. [Google Scholar] [CrossRef]
- Luchini, C.; Veronese, N.; Nottegar, A.; Shin, J.I.; Gentile, G.; Granziol, U.; Soysal, P.; Alexinschi, O.; Smith, L.; Solmi, M. Assessing the quality of studies in meta-research: Review/guidelines on the most important quality assessment tools. Pharm. Stat. 2021, 20, 185–195. [Google Scholar] [CrossRef]
| Authors, Title and Year of Publication | Country | Participants/Material | Statistical Analysis |
|---|---|---|---|
| Naruishi et al., “Association between periodontal condition and kidney dysfunction in Japanese adults: A cross-sectional study”; 2016 [23] | Japan | Group DM—48 patients diagnosed with diabetes mellitus Group Dialysis—84 hemodialyzed patients Group Dialysis with DM—32 hemodialyzed people with diabetes mellitus | mean, standard deviation, frequencies, percentages, chi-square test, analysis of variance (ANOVA), Spearman’s rank correlation coefficient, the Turkey- Kramer honest significant difference test Analyses were performed using JMP® 8 ver. 8.0.2 (SAS Institute Japan, Tokyo). |
| Swapna et al., “Oral health in diabetic and nondiabetic patients with chronic kidney disease”; 2017 [24] | Saudi Arabia | Group A—47 diabetic patients on hemodialysis Group B—54 diabetic patients with chronic kidney disease but not on hemodialysis Group C—50 nondiabetic patients on hemodialysis Group D—43 nondiabetic patients with chronic kidney disease but not hemodialyzed | Chi-square test Analyses were done with the usage of Statistical Package for Social Sciences (SPSS) ver. 15.0 (SPSS Inc., Chicago, IL, USA) and SAS 9.2. |
| Ayinampudi et al., “Oral Candida colonization in renal disease patients between diabetes and non-diabetes; a comparative study”; 2018 [22] | India | Group I—15 patients diagnosed with chronic kidney disease Group II—15 hemodialyzed patients (for at least 4 months) Group III—renal transplanted patients Each group was divided into diabetic and non-diabetic patients. | percentages, frequencies, risk ratio, and odds ratio |
| Dande et al., “Oral manifestations in diabetic and nondiabetic chronic renal failure patients receiving hemodialysis”; 2018 [1] | India | 144 patients included | percentages, frequencies, means, standard deviation, Pearson chi-square test, Fischer’s exact test, and Student’s test Analyses were performed using the Statistical Package for the Social Sciences ver. 16.0 (SPSS, Chicago, IL, USA). |
| Al-Sarray et al., “Oral candidiasis in chronic kidney disease”; 2020 [19] | Iraq | 50 patients diagnosed with chronic kidney disease | chi-square test and one-way analysis of variance (ANOVA) Analyses were performed with the Statistical Package for the Social Sciences (SPSS) ver. 21. |
| Trzcionka et al., “Oral cavity status of long-term hemodialyzed patients vs. their socio-economic status”; 2020 [25] | Poland | Examined group: R—hemodialyzed patients (42) R + H—hemodialyzed with hypertension (79) R + D—hemodialyzed with diabetes (16) R + H + D—hemodialyzed with hypertension and diabetes (43) Control group—48 patients not diagnosed with end-stage chronic kidney disease, hypertension, or diabetes | Shapiro–Wilk test, Kruskal–Wallis test, and Mann–Whitney U test Analyses were performed using IBM’s SPSS Statistics 23 program (IBM, Armonk, NY, USA). |
| Trzcionka et al., “Periodontal treatment needs of hemodialyzed patients”; 2021 [20] | Poland | Examined group: R—hemodialyzed patients (42) R + H—hemodialyzed with hypertension (79) R + D—hemodialyzed with diabetes (16) R + H + D—hemodialyzed with hypertension and diabetes (43) Control group—48 patients not diagnosed with end-stage chronic kidney disease, hypertension, or diabetes | Kruskal–Wallis test, Mann–Whitney U test, test chi-quadrat Analyses were performed using IBM’s SPSS Statistics 23 program (IBM, Armonk, NY, USA). |
| Trzcionka et al., “Oral mucosa status and saliva parameters of multimorbid adult patients diagnosed with end-stage chronic kidney disease”; 2021 [21] | Poland | Examined group: R—hemodialyzed patients (42) R + H—hemodialyzed with hypertension (79) R + D—hemodialyzed with diabetes (16) R + H + D—hemodialyzed with hypertension and diabetes (43) Control group—48 patients not diagnosed with end-stage chronic kidney disease, hypertension, or diabetes | Kruskal–Wallis test, Mann–Whitney U test and chi-square test Analyses were performed using IBM’s SPSS Statistics 23 program (IBM, Armonk, NY, USA). |
| Reference | Group | Result | Conclusions |
|---|---|---|---|
| Naruishi et al. [23] | Hemodialyzed with diabetes mellitus | Not presented | No significant differences in the OHI score among the groups (p = 0.84, hemodialysis vs. hemodialysis with DM). |
| Dande et al. [1] | Hemodialyzed non-diabetic patients | Poor OHI 54.05% 40/74 | p = 0.000 The diabetic group revealed significantly higher levels of poor oral hygiene |
| Hemodialyzed with diabetes mellitus | poor OHI 88.57% 62/70 | ||
| Trzcionka et al. [25] | Hemodialyzed | API = 74.55 OHI-S = 1.70 | The statistical analysis of s OHI with the usage of the Kruskall–Wallis test showed statistically significant differences (p < 0.001). Test Mann–Whitney U proved that s OHI values were significantly lower in healthy patients and higher in hemodialyzed patients with diabetes mellitus and hypertension than in hemodialyzed and hemodialyzed with hypertension and higher in hemodialyzed with diabetes mellitus than in hemodialyzed. Kruskall–Wallis test also showed statistically significant differences in API values (p < 0.001)—the control group presented significantly lower values and additionally hemodialyzed with diabetes and hypertension than only hemodialyzed. |
| Hemodialyzed with hypertension | API = 69.40 OHI-S = 2.27 | ||
| Hemodialyzed with diabetes mellitus | API = 95.71 OHI-S = 3.11 | ||
| Hemodialyzed with hypertension and diabetes mellitus | API = 85.63 OHI-S = 3.64 | ||
| Control group | API = 26.68 OHI-S = 1.11 |
| Reference | Group | Results | Conclusions |
|---|---|---|---|
| Naruishi et al. [23] | Hemodialysis vs. hemodialysis + diabetes mellitus | Not presented | No significant differences among the groups with regard to alveolar bone loss (it tended to be higher in dialysis with DM in comparison to dialysis; p = 0.079) CPI—lower in the DM group than in others (p = 0.083 vs. hemodialysis with DM; p = 0.033 vs. hemodialysis) |
| Swapna et al. [24] | Hemodialysis + diabetes mellitus | CPI = 3.1 | In diabetics with chronic kidney disease, an increased periodontal pocket depth was observed in comparison to nondiabetics (p < 0.05). |
| Diabetics + chronic kidney disease not on hemodialysis | CPI = 3.0 | ||
| Nondiabetics on hemodialysis | CPI = 2.8 | ||
| Nondiabetics not on hemodialysis | CPI = 2.8 | ||
| Dande et al. [1] | Hemodialyzed non-diabetic patients | Gingivitis: 25.71% (18/74) periodontitis: 13.51% (10/74) | Gingivitis (p = 0.531) and periodontitis (p = 0.191) showed no statistically significant differences, but the tendency to be slightly higher in diabetics was observed. |
| Hemodialyzed with diabetes mellitus | Gingivitis: 32.43% (24/70) periodontitis: 25.71% (18/70) | ||
| Trzcionka et al. [20] | Hemodialyzed | BI—M = 49.61 CPI0—6%, CPI1—21%, CPI2—39%, CPI3—9%, CPI4—24% TNI—28%, TNII—48%, TNIII—24% | The Kruskall–Wallis test showed that the BI value in the control group was significantly lower (p < 0.001). In the control group, there were significantly more patients qualified for CPI1 and CPI2 and less for CPI3 and CPI4 than in the hemodialyzed people. Most of the patients from the control group were qualified for TNIII, and most were from the hemodialyzed TNII. P1 (p = 0.000) P2 (p = 0.533) P3 (p = 0.000) In all subgroups of the hemodialyzed patients, the percentage of people with healthy periodontium was significantly lower. In the examined patients, the highest percentage of patients with healthy periodontium was in hemodialyzed patients and hemodialyzed patients with hypertension. |
| Hemodialyzed with hypertension | BI—M = 44.73 CPI0—6%, CPI1—30%, CPI2—30%, CPI3—13%, CPI4—22% TNI-35%, TNII—42%, TNIII—22% | ||
| Hemodialyzed with diabetes mellitus | BI—M = 54.00 CPI0—0%, CPI1—11%, CPI2—67%, CPI3—0%, CPI4—22% TNI—11%, TNII—67%, TNIII—22% | ||
| Hemodialyzed with hypertension and diabetes mellitus | BI—M = 37.55 CPI0—0%, CPI1—10%, CPI2—41%, CPI3—21%, CPI4—28% TNI—10%, TNII—62%, TNIII—28% | ||
| Control group | BI—M = 5.36 CPI0—28%, CPI1—30%, CPI2—21%, CPI3—13%, CPI4—9% TNI—57%, TNII—34%, TNIII—9% |
| Reference | Group | Result | Conclusion |
|---|---|---|---|
| Swapna et al. [24] | Hemodialysis + diabetes mellitus | subjective dry mouth: 37/47 subjective dysgeusia: 37/47 mucosal pain:14/47 uremic odor: 35/47 tongue coating: 18/47 mucosal petechiae: 15/47 ecchymosis:0 mouth ulceration: 1/47 dry mouth: 47/47 | Dysgeusia was significantly more prevalent in hemodialyzed nondiabetics (p = 0.03). Statistically significant differences were also observed in the frequency of occurrence of uremic odor (p = 0.04) and mucosal petechiae (p = 0.01). |
| Diabetics + chronic kidney disease not on hemodialysis | subjective dry mouth: 40/54 subjective dysgeusia: 40/54 mucosal pain:17/54 uremic odor: 41/54 tongue coating: 18/54 mucosal petechiae: 15/54 ecchymosis:0 mouth ulceration: 1/54 dry mouth: 53/54 | ||
| Nondiabetics on hemodialysis | subjective dry mouth: 31/50 subjective dysgeusia: 45/50 mucosal pain:18/50 uremic odor: 45/50 tongue coating: 9/50 mucosal petechiae: 5/50 ecchymosis:0 mouth ulceration: 0 dry mouth: 48/50 | ||
| Nondiabetics not on hemodialysis | subjective dry mouth: 28/43 subjective dysgeusia: 28/43 mucosal pain:15/43 uremic odor: 39/43 tongue coating: 9/43 mucosal petechiae: 5/43 ecchymosis:0 mouth ulceration: 0 dry mouth: 42/43 | ||
| Dande et al. [1] | Hemodialyzed non-diabetic patients | ulcers: 8.10%, dryness: 48.64%, uremic fetor: 59.45%, dry-fissured lips: 2.70%, pale mucosa: 35.13%, unpleasant taste: 35.13% | In diabetic patients, significantly more patients were diagnosed with a uremic fetor (p = 0.005), unpleasant taste (p = 0.009), dry-fissured lips (p = 0.002), and pale mucosa (p = 0.019). |
| Hemodialyzed with diabetes mellitus | ulcers: 8.77%, dryness: 60.00%, uremic fetor: 88.57%, dry-fissured lips: 28.57%, pale mucosa: 62.85%, unpleasant taste: 65.71% | ||
| Trzcionka et al. [21] | Hemodialyzed | S1 = 0.55 mL/min S2 = 0.72 mL/min Buffer capacity: VL—21.4%, L—14.3%, N—64.3% pH = 6.39 dryness: 50%, ecchymosis: 36%, candidiasis: 40%, fissured tongue: 33%, trauma-related oral lesions: 21%, ulcerations: 0, herpes simplex: 0, overgrowth of gingiva: 0, signs of operations: 0, malformations of mucosa: 36%, white patches: 17%, taste disorders: 10%, geographic tongue: 7%, halitosis: 7%, red patches: 5%, pain: 2%, burning mouth syndrome: 2% | The salivary flow rate after hemodialysis was significantly higher in healthy participants (p < 0.001). The chi-squared test showed statistically significant differences (p < 0.05) in healthy people and showed fewer participants with a very low buffer capacity. There were no statistically significant differences in pH values (p = 0.987). The percentage of healthy patients who complained about dryness was significantly lower (p = 0.002); in that group of patients, the percentage of patients with ecchymosis (p = 0.005), candidiasis (p = 0.003), fissured tongue (p = 0.000) and trauma-related oral lesions (p = 0.021) was also lower. |
| Hemodialyzed with hypertension | S1 = 0.63 mL/min S2 = 0.68 mL/min Buffer capacity: VL—31.6%, L—16.5%, N—51.9% pH = 6.22 dryness: 54%, ecchymosis: 19%, candidiasis: 37%, fissured tongue: 40%, trauma-related oral lesions: 16%, ulcerations: 1%, herpes simplex: 1%, overgrowth of gingiva: 1%, signs of operations: 1%, malformations of mucosa: 19%, white patches: 19%, taste disorders: 10%, geographic tongue: 5%, halitosis: 5%, red patches: 8%, pain: 4%, burning mouth syndrome: 1% | ||
| Hemodialyzed with diabetes mellitus | S1 = 0.40 mL/min S2 = 0.80 mL/min Buffer capacity: VL—12.5%, L—18.8%, N—68.8% pH = 5.96 dryness: 50%, ecchymosis: 31%, candidiasis: 31%, fissured tongue: 25%, trauma-related oral lesions: 25%, ulcerations: 0, herpes simplex: 0, overgrowth of gingiva: 6%, signs of operations: 0, malformations of mucosa: 36%, white patches: 12%, taste disorders: 19%, geographic tongue: 6%, halitosis: 12%, red patches: 5%, pain: 0, burning mouth syndrome: 0 | ||
| Hemodialyzed with hypertension and diabetes mellitus | S1 = 0.55 mL/min S2 = 0.86 mL/min Buffer capacity: VL—23.3%, L—14%, N—62.8% pH = 6.3 dryness: 48%, ecchymosis: 39%, candidiasis: 39%, fissured tongue: 39%, trauma-related oral lesions: 16%, ulcerations: 0, herpes simplex: 0, overgrowth of gingiva: 0, signs of operations: 0, malformations of mucosa: 40%, white patches: 23%, taste disorders: 10%, geographic tongue: 7%, halitosis: 2%, red patches: 12%, pain: 2%, burning mouth syndrome: 5% | ||
| Control group | S = 1.55 mL/min Buffer capacity: VL—2.1%, L—25%, N—72.9% pH = 7.00 dryness: 19%, ecchymosis: 10%, candidiasis: 8%, fissured tongue: 2%, trauma-related oral lesions: 0, ulcerations: 4%, herpes simplex: 6%, overgrowth of gingiva: 0, signs of operations: 0, malformations of mucosa: 10%, white patches: 8%, taste disorders: 6%, geographic tongue: 2%, halitosis: 0, red patches: 8%, pain: 0, burning mouth syndrome: 0 |
| Reference | Group (n) | Results | Conclusions |
|---|---|---|---|
| Ayinampudi et al. [22] | Hemodialyzed non-diabetic patients (11) | Candida observed in 3 samples (27%) | The high-risk ratio (1.774) is an indication that the presence of Candida is probably higher in diabetic patients. |
| Hemodialyzed diabetic patients (4) | Candida observed in 1 sample (25%) | ||
| Al-Sarray et al. [19] | Hemodialyzed (4) | Candida observed in 2 samples (50%) | Diabetes mellitus and hypertension do not predispose patients to oral Candidiasis. |
| Hemodialyzed with hypertension (33) | Candida observed in 15 samples (45.5%) | ||
| Hemodialyzed with diabetes mellitus (1) | Candida positive | ||
| Hemodialyzed with hypertension and diabetes mellitus (12) | Candida observed in 6 samples (50%) |
| Study Number | Selection | Comparability | Outcome | Quality | ||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | A | B | |||
| 1. | 1 | 0 | 0 | 0 | 2 | 2 | 0 | SATISFACTORY |
| 2. | 1 | 0 | 1 | 0 | 2 | 2 | 1 | GOOD |
| 3. | 1 | 1 | 0 | 0 | 2 | 2 | 1 | GOOD |
| 4. | 1 | 1 | 1 | 1 | 1 | 2 | 1 | GOOD |
| 5. | 1 | 1 | 1 | 1 | 1 | 2 | 1 | GOOD |
| 6. | 1 | 1 | 1 | 1 | 1 | 2 | 1 | GOOD |
| 7. | 1 | 1 | 1 | 1 | 2 | 2 | 1 | VERY GOOD |
| 8. | 1 | 1 | 1 | 1 | 2 | 2 | 1 | VERY GOOD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trzcionka, A.; Mączkowiak, D.; Korkosz, R.; Rahnama, M.; Duława, J.; Tanasiewicz, M. Oral Findings in Hemodialyzed Patients Diagnosed with Diabetes Mellitus and/or Hypertension—A Systematic Review. J. Clin. Med. 2023, 12, 7072. https://doi.org/10.3390/jcm12227072
Trzcionka A, Mączkowiak D, Korkosz R, Rahnama M, Duława J, Tanasiewicz M. Oral Findings in Hemodialyzed Patients Diagnosed with Diabetes Mellitus and/or Hypertension—A Systematic Review. Journal of Clinical Medicine. 2023; 12(22):7072. https://doi.org/10.3390/jcm12227072
Chicago/Turabian StyleTrzcionka, Agata, Dagmara Mączkowiak, Rafał Korkosz, Mansur Rahnama, Jan Duława, and Marta Tanasiewicz. 2023. "Oral Findings in Hemodialyzed Patients Diagnosed with Diabetes Mellitus and/or Hypertension—A Systematic Review" Journal of Clinical Medicine 12, no. 22: 7072. https://doi.org/10.3390/jcm12227072
APA StyleTrzcionka, A., Mączkowiak, D., Korkosz, R., Rahnama, M., Duława, J., & Tanasiewicz, M. (2023). Oral Findings in Hemodialyzed Patients Diagnosed with Diabetes Mellitus and/or Hypertension—A Systematic Review. Journal of Clinical Medicine, 12(22), 7072. https://doi.org/10.3390/jcm12227072







