Recent Advances of Adipose-Tissue-Derived Mesenchymal Stem Cell-Based Therapy for Retinal Diseases
Abstract
1. Introduction
2. Stem Cells in Ophthalmology
3. Adipose-Derived Mesenchymal Stem Cells (ADSCs)
4. Methods
5. Adipose-Derived Stem Cells (ADSCs) in Retinal Diseases
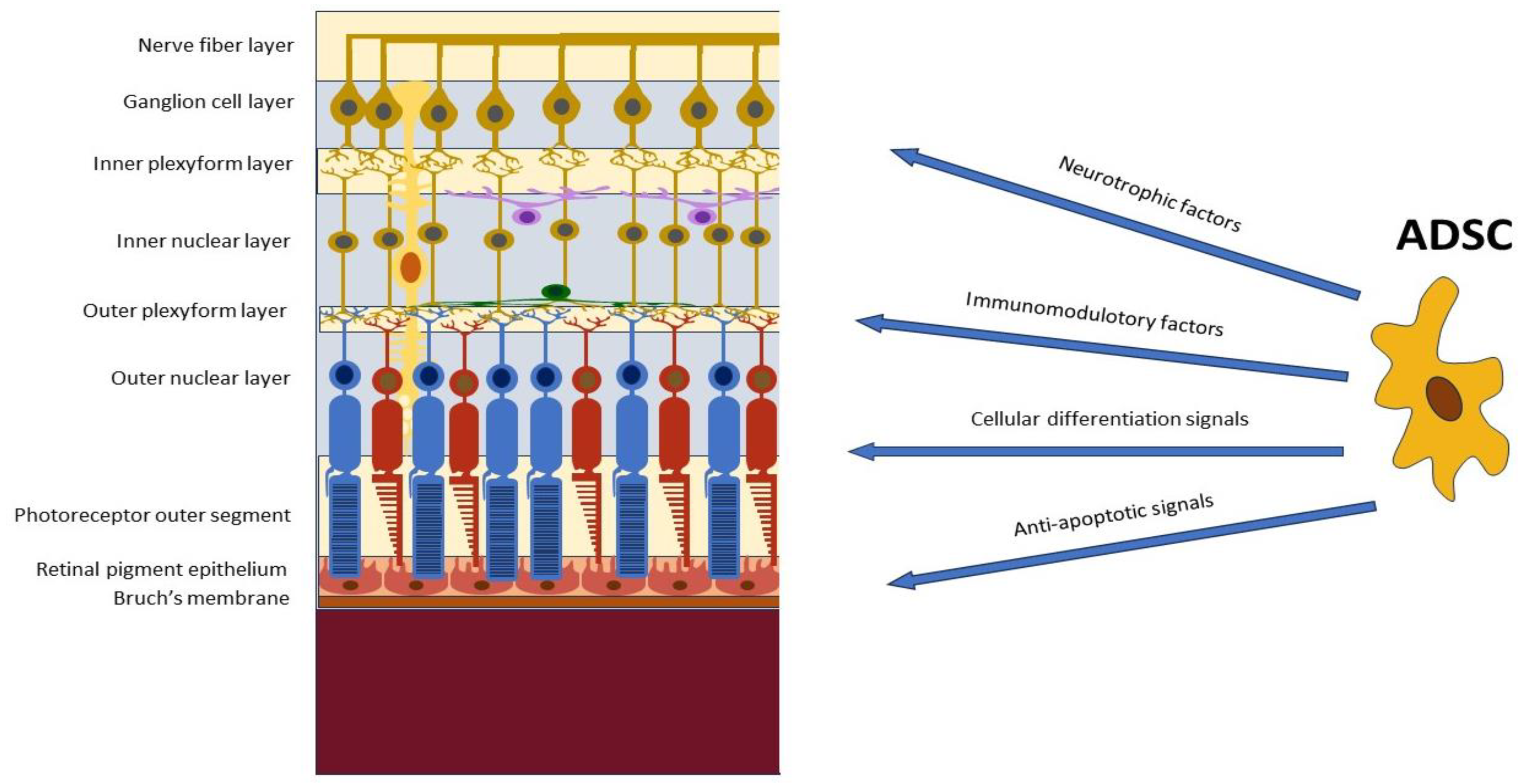
5.1. Modulation of Retinal Inflammation and Immune Responses
5.2. Promotion of Retinal Cell Survival and Regeneration
5.3. Modulation of Retinal Vascularization and Neuroprotection
6. In Vitro Studies
6.1. Evaluation of Trophic and Paracrine Effects on Retinal Cells
6.2. Exploration of ADSC-Mediated Immunomodulation
7. Animal Studies
8. Human Studies
8.1. Clinical Trials Investigating ADSC Transplantation in Retinal Disease
8.2. Safety and Feasibility Assessments
8.3. Visual Outcomes and Functional Improvements
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Blindness and Vision Impairment Collaborators. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.K.; Lu, B.; Girman, S.; Wang, S. Cell-based therapeutic strategies for replacement and preservation in retinal degenerative diseases. Prog. Retin. Eye Res. 2017, 58, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.L.S.; Kumar, S.; Mok, P.L. Cellular Reparative Mechanisms of Mesenchymal Stem Cells for Retinal Diseases. Int. J. Mol. Sci. 2017, 18, 1406. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Miotti, G.; Parodi, P.C.; Zeppieri, M. Stem cell therapy in ocular pathologies in the past 20 years. World J. Stem Cells 2021, 13, 366–385. [Google Scholar] [CrossRef]
- Wu, S.M.; Hochedlinger, K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat. Cell Biol. 2011, 13, 497–505. [Google Scholar] [CrossRef]
- Baker, P.S.; Brown, G.C. Stem-cell therapy in retinal disease. Curr. Opin. Ophthalmol. 2009, 20, 175–181. [Google Scholar] [CrossRef]
- Nazari, H.; Zhang, L.; Zhu, D.; Chader, G.J.; Falabella, P.; Stefanini, F.; Rowland, T.; Clegg, D.O.; Kashani, A.H.; Hinton, D.R.; et al. Stem cell based therapies for age-related macular degeneration: The promises and the challenges. Prog. Retin. Eye Res. 2015, 48, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, N.; Fernández-Sánchez, L.; McGill, T.J.; Lu, B.; Wang, S.; Lund, R.; Huhn, S.; Capela, A. Phagocytosis of Photoreceptor Outer Segments by Transplanted Human Neural Stem Cells as a Neuroprotective Mechanism in Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6745–6756. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Moisseiev, E.; Bauer, G.; Anderson, J.D.; Grant, M.B.; Zam, A.; Zawadzki, R.J.; Werner, J.S.; Nolta, J.A. Advances in bone marrow stem cell therapy for retinal dysfunction. Prog. Retin. Eye Res. 2017, 56, 148–165. [Google Scholar] [CrossRef]
- Ziaei, M.; Zhang, J.; Patel, D.V.; McGhee, C.N. Umbilical cord stem cells in the treatment of corneal disease. Surv. Ophthalmol. 2017, 62, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Xu, Y.; Yang, E.; Wang, Y.; Du, Y. Fidelity of long-term cryopreserved adipose-derived stem cells for differentiation into cells of ocular and other lineages. Exp. Eye Res. 2019, 189, 107860. [Google Scholar] [CrossRef] [PubMed]
- Zeppieri, M.; Salvetat, M.L.; Beltrami, A.P.; Cesselli, D.; Bergamin, N.; Russo, R.; Cavaliere, F.; Varano, G.P.; Alcalde, I.; Merayo, J.; et al. Human Adipose-Derived Stem Cells for the Treatment of Chemically Burned Rat Cornea: Preliminary Results. Curr. Eye Res. 2013, 38, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Syed-Picard, F.N.; Du, Y.; Lathrop, K.L.; Mann, M.M.; Funderburgh, M.L.; Funderburgh, J.L. Dental Pulp Stem Cells: A New Cellular Resource for Corneal Stromal Regeneration. Stem Cells Transl. Med. 2015, 4, 276–285. [Google Scholar] [CrossRef]
- Roozafzoon, R.; Lashay, A.; Vasei, M.; Ai, J.; Khoshzaban, A.; Keshel, S.H.; Barabadi, Z.; Bahrami, H. Dental pulp stem cells differentiation into retinal ganglion-like cells in a three dimensional network. Biochem. Biophys. Res. Commun. 2015, 457, 154–160. [Google Scholar] [CrossRef]
- Salero, E.; Blenkinsop, T.A.; Corneo, B.; Harris, A.; Rabin, D.; Stern, J.H.; Temple, S. Adult Human RPE Can Be Activated into a Multipotent Stem Cell that Produces Mesenchymal Derivatives. Cell Stem Cell 2012, 10, 88–95. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, S.; Musayeva, A.; Pfeiffer, N.; Gericke, A. Corneal Epithelial Stem Cells–Physiology, Pathophysiology and Therapeutic Options. Cells 2021, 10, 2302. [Google Scholar] [CrossRef]
- Gu, S.; Xing, C.; Han, J.; Tso, M.O.; Hong, J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol. Vis. 2009, 15, 99–107. [Google Scholar]
- Calonge, M.; Pérez, I.; Galindo, S.; Nieto-Miguel, T.; López-Paniagua, M.; Fernández, I.; Alberca, M.; García-Sancho, J.; Sánchez, A.; Herreras, J.M. A proof-of-concept clinical trial using mesenchymal stem cells for the treatment of corneal epithelial stem cell deficiency. Transl. Res. 2018, 206, 18–40. [Google Scholar] [CrossRef] [PubMed]
- Gokoffski, K.K.; Lam, P.; Alas, B.F.; Peng, M.G.; Ansorge, H.R.R. Optic Nerve Regeneration: How Will We Get There? J. Neuro-Ophthalmol. 2020, 40, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.J.; Zhu, W.; Cook, A.C.; Anfinson, K.R.; Tucker, B.A.; Kuehn, M.H. Induction of Trabecular Meshwork Cells From Induced Pluripotent Stem Cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7065–7072. [Google Scholar] [CrossRef]
- Minteer, D.; Marra, K.G.; Rubin, J.P. Adipose-derived mesenchymal stem cells: Biology and potential applications. Adv. Biochem. Eng. Biotechnol. 2013, 129, 59–71. [Google Scholar] [CrossRef]
- Del Barrio, J.L.A.; De la Mata, A.; De Miguel, M.P.; Arnalich-Montiel, F.; Nieto-Miguel, T.; El Zarif, M.; Cadenas-Martín, M.; López-Paniagua, M.; Galindo, S.; Calonge, M.; et al. Corneal Regeneration Using Adipose-Derived Mesenchymal Stem Cells. Cells 2022, 11, 2549. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Scheller, E.L.; MacDougald, O.A. Adipose tissue stem cells: Biology and clinical implications for regenerative medicine. Nat. Rev. Endocrinol. 2014, 10, 536–546. [Google Scholar] [CrossRef]
- Tsuji, W.; Rubin, J.P.; Marra, K.G. Adipose-derived stem cells: Implications in tissue regeneration. World J. Stem Cells 2014, 6, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, S.; Bouffi, C.; Djouad, F.; Jorgensen, C.; Noel, D. Immunosuppression by mesenchymal stem cells: Mechanisms and clinical applications. Stem Cell Res. Ther. 2010, 1, 2. [Google Scholar] [CrossRef]
- Ferraro, G.A.; De Francesco, F.; Nicoletti, G.; Paino, F.; Desiderio, V.; Tirino, V.; D’Andrea, F. Human adipose CD34+CD90+ stem cells and collagen scaffold constructs grafted in vivo fabricate loose connective and adipose tissues. J. Cell. Biochem. 2012, 114, 1039–1049. [Google Scholar] [CrossRef]
- Rochefort, G.Y.; Delorme, B.; Lopez, A.; Hérault, O.; Bonnet, P.; Charbord, P.; Eder, V.; Domenech, J.; Domenech, J. Multipotential Mesenchymal Stem Cells Are Mobilized into Peripheral Blood by Hypoxia. Stem Cells 2006, 24, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Eggenhofer, E.; Benseler, V.; Kroemer, H.; Popp, F.; Geissler, E.; Schlitt, H.; Baan, C.; Dahlke, M.; Hoogduijn, M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef]
- Holan, V.; Palacka, K.; Hermankova, B. Mesenchymal Stem Cell-Based Therapy for Retinal Degenerative Diseases: Experimental Models and Clinical Trials. Cells 2021, 10, 588. [Google Scholar] [CrossRef]
- Huang, H.; Kolibabka, M.; Eshwaran, R.; Chatterjee, A.; Schlotterer, A.; Willer, H.; Bieback, K.; Hammes, H.-P.; Feng, Y. Intravitreal injection of mesenchymal stem cells evokes retinal vascular damage in rats. FASEB J. 2019, 33, 14668–14679. [Google Scholar] [CrossRef]
- Kahraman, N.S. Umbilical cord derived mesenchymal stem cell implantation in retinitis pigmentosa: A 6-month follow-up results of a phase 3 trial. Int. J. Ophthalmol. 2020, 13, 1423–1429. [Google Scholar] [CrossRef]
- Oumlzmert, E.; Arslan, U. Management of retinitis pigmentosa by Wharton’s jelly-derived mesenchymal stem cells: Prospective analysis of 1-year results. Stem Cell Res. Ther. 2020, 11, 353. [Google Scholar] [CrossRef]
- Dov, M.B.; Krief, B.; Benhamou, M.; Klein, A.; Schwartz, S.; Loewenstein, A.; Barak, A.; Barzelay, A. Regenerative Effect of Adipose Derived Mesenchymal Stem Cells on Ganglion Cells in the Hypoxic Organotypic Retina Culture. Int. J. Stem Cells 2022, 16, 244–249. [Google Scholar] [CrossRef]
- Barzelay, A.; Weisthal Algor, S.; Niztan, A.; Katz, S.; Benhamou, M.; Nakdimon, I.; Azmon, N.; Gozlan, S.; Mezad-Koursh, D.; Neudorfer, M.; et al. Adipose-Derived Mesenchymal Stem Cells Migrate and Rescue RPE in the Setting of Oxidative Stress. Stem Cells Int. 2018, 2018, 9682856. [Google Scholar] [CrossRef]
- Safwat, A.; Sabry, D.; Ragiae, A.; Amer, E.; Mahmoud, R.H.; Shamardan, R.M. Adipose mesenchymal stem cells–derived exosomes attenuate retina degeneration of streptozotocin-induced diabetes in rabbits. J. Circ. Biomark. 2018, 7, 1849454418807827. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.B.; Roycik, M.D.; Jin, Y.; Schwartz, M.A.; Lively, T.J.; Zorio, D.A.; Sang, Q.A. A new synthetic matrix metalloproteinase inhibitor reduces human mesenchymal stem cell adipogenesis. PLoS ONE 2017, 12, e0172925. [Google Scholar] [CrossRef] [PubMed]
- Tobita, M.; Tajima, S.; Mizuno, H. Adipose tissue-derived mesenchymal stem cells and platelet-rich plasma: Stem cell transplantation methods that enhance stemness. Stem Cell Res. Ther. 2015, 6, 215. [Google Scholar] [CrossRef]
- Bai, L.; Lennon, D.P.; Caplan, A.I.; DeChant, A.; Hecker, J.; Kranso, J.; Zaremba, A.; Miller, R.H. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat. Neurosci. 2012, 15, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yu, P.; Han, X.; Du, L.; Gan, J.; Wang, Y.; Shi, Y. TGF-β Promotes Immune Responses in the Presence of Mesenchymal Stem Cells. J. Immunol. 2014, 192, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.; Zeppieri, M.; Enaholo, E.S.; Salati, C.; Parodi, P.C. Adipose Stem Cells in Modern-Day Ophthalmology. Clin. Pract. 2023, 13, 230–245. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, J.; Liu, L.; Xu, W. Differentiation of human adipose-derived stem cells into functional retinal pigment epithelium cells. J. Cell. Mol. Med. 2017, 21, 3053–3064. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Ng, T.K.; Chen, C.B.; Huang, B.; Liang, J.J.; Pang, C.P.; Zhang, M.Z. Notch Signaling Activation Enhances Human Adipose-Derived Stem Cell Retinal Differentiation. Stem Cells Int. 2018, 2018, 9201374. [Google Scholar] [CrossRef]
- Amirpour, N.; Amirizade, S.; Hashemibeni, B.; Kazemi, M.; Hadian, M.; Salehi, H. Differentiation of eye field neuroectoderm from human adipose-derived stem cells by using small-molecules and hADSC-conditioned medium. Ann. Anat.-Anat. Anz. 2018, 221, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, D.D.; Wei, W.; Shen, B.Q.; Wang, Y.Y.; Zhang, Y.J.; Zhang, Y.D.; Ji, J.; Sun, H.; Luo, M.; et al. Effects of RPE-conditioned medium on the differentiation of hADSCs into RPE cells, and their proliferation and migration. Exp. Ther. Med. 2017, 14, 3699–3707. [Google Scholar] [CrossRef][Green Version]
- Rezanejad, H.; Soheili, Z.-S.; Haddad, F.; Matin, M.M.; Samiei, S.; Manafi, A.; Ahmadieh, H. In vitro differentiation of adipose-tissue-derived mesenchymal stem cells into neural retinal cells through expression of human PAX6 (5a) gene. Cell Tissue Res. 2014, 356, 65–75. [Google Scholar] [CrossRef]
- Yañez, R.; Lamana, M.L.; García-Castro, J.; Colmenero, I.; Ramírez, M.; Bueren, J.A. Adipose Tissue-Derived Mesenchymal Stem Cells Have In Vivo Immunosuppressive Properties Applicable for the Control of the Graft-Versus-Host Disease. Stem Cells 2006, 24, 2582–2591. [Google Scholar] [CrossRef]
- Fan, X.-L.; Zhang, Y.; Li, X.; Fu, Q.-L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef]
- Lin, L.; Du, L. The role of secreted factors in stem cells-mediated immune regulation. Cell. Immunol. 2018, 326, 24–32. [Google Scholar] [CrossRef]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Bunnell, B.A. Adipose Tissue-Derived Stem Cells: Immunomodulatory Effects and Therapeutic Potential. Physiology 2020, 35, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, Y.; Zhou, J.; Cao, K. Adipose-derived mesenchymal stem cell exosomes: A novel pathway for tissues repair. Cell Tissue Bank. 2019, 20, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Reis, R.L.; Sousa, N.; Gimble, J.M. Adipose Tissue Derived Stem Cells Secretome: Soluble Factors and Their Roles in Regenerative Medicine. Curr. Stem Cell Res. Ther. 2010, 5, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Waldner, M.; Zhang, W.; James, I.B.; Allbright, K.; Havis, E.; Bliley, J.M.; Almadori, A.; Schweizer, R.; Plock, J.A.; Washington, K.M.; et al. Characteristics and Immunomodulating Functions of Adipose-Derived and Bone Marrow-Derived Mesenchymal Stem Cells Across Defined Human Leukocyte Antigen Barriers. Front. Immunol. 2018, 9, 1642. [Google Scholar] [CrossRef] [PubMed]
- Mun, C.H.; Kang, M.-I.; Shin, Y.D.; Kim, Y.; Park, Y.-B. The Expression of Immunomodulation-Related Cytokines and Genes of Adipose- and Bone Marrow-Derived Human Mesenchymal Stromal Cells from Early to Late Passages. Tissue Eng. Regen. Med. 2018, 15, 771–779. [Google Scholar] [CrossRef]
- Yoshizumi, Y.; Yukawa, H.; Iwaki, R.; Fujinaka, S.; Kanou, A.; Kanou, Y.; Yamada, T.; Nakagawa, S.; Ohara, T.; Nakagiri, K.; et al. Immunomodulatory Effects of Adipose Tissue-Derived Stem Cells on Concanavalin A-Induced Acute Liver Injury in Mice. Cell Med. 2016, 9, 21–33. [Google Scholar] [CrossRef]
- DelaRosa, O.; Lombardo, E.; Beraza, A.; Mancheño-Corvo, P.; Ramirez, C.; Menta, R.; Rico, L.; Camarillo, E.; García, L.; Abad, J.L.; et al. Requirement of IFN-γ–Mediated Indoleamine 2,3-Dioxygenase Expression in the Modulation of Lymphocyte Proliferation by Human Adipose–Derived Stem Cells. Tissue Eng. Part A 2009, 15, 2795–2806. [Google Scholar] [CrossRef]
- Cui, L.; Yin, S.; Liu, W.; Li, N.; Zhang, W.; Cao, Y. Expanded Adipose-Derived Stem Cells Suppress Mixed Lymphocyte Reaction by Secretion of Prostaglandin E2. Tissue Eng. 2007, 13, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Taha, S.; Volkmer, E.; Haas, E.; Alberton, P.; Straub, T.; David-Rus, D.; Aszodi, A.; Giunta, R.; Saller, M.M. Differences in the Inflammatory Response of White Adipose Tissue and Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2020, 21, 1086. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-S.; Park, B.-S.; Sung, J.-H. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin. Biol. Ther. 2009, 9, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Mohyeldin, A.; Garzon-Muvdi, T.; Quinones-Hinojosa, A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, S.-H.; Park, S.G.; Choi, J.-S.; Xia, Y.; Sung, J.-H.; Kim, W.-K.; Sung, Y.K.; Kwack, M.H.; Song, S.Y.; et al. The Pivotal Role of Reactive Oxygen Species Generation in the Hypoxia-Induced Stimulation of Adipose-Derived Stem Cells. Stem Cells Dev. 2011, 20, 1753–1761. [Google Scholar] [CrossRef]
- Rochette, L.; Mazini, L.; Malka, G.; Zeller, M.; Cottin, Y.; Vergely, C. The Crosstalk of Adipose-Derived Stem Cells (ADSC), Oxidative Stress, and Inflammation in Protective and Adaptive Responses. Int. J. Mol. Sci. 2020, 21, 9262. [Google Scholar] [CrossRef]
- Zhang, X.; Hassan, M.G.; Scheller, E.L. Neural regulation of bone marrow adipose tissue. Best Pr. Res. Clin. Endocrinol. Metab. 2021, 35, 101522. [Google Scholar] [CrossRef]
- Yao, P.; Zhou, L.; Zhu, L.; Zhou, B.; Yu, Q. Mesenchymal Stem Cells: A Potential Therapeutic Strategy for Neurodegenerative Diseases. Eur. Neurol. 2020, 83, 235–241. [Google Scholar] [CrossRef]
- Harrell, C.R.; Djonov, V.; Volarevic, V. The Cross-Talk between Mesenchymal Stem Cells and Immune Cells in Tissue Repair and Regeneration. Int. J. Mol. Sci. 2021, 22, 2472. [Google Scholar] [CrossRef]
- Oh, J.Y.; Lee, R.H. Mesenchymal stromal cells for the treatment of ocular autoimmune diseases. Prog. Retin. Eye Res. 2021, 85, 100967. [Google Scholar] [CrossRef]
- Rao, D.; Huang, D.; Sang, C.; Zhong, T.; Zhang, Z.; Tang, Z. Advances in Mesenchymal Stem Cell-Derived Exosomes as Drug Delivery Vehicles. Front. Bioeng. Biotechnol. 2022, 9, 797359. [Google Scholar] [CrossRef] [PubMed]
- Hajinejad, M.; Sahab-Negah, S. Neuroinflammation: The next target of exosomal microRNAs derived from mesenchymal stem cells in the context of neurological disorders. J. Cell. Physiol. 2021, 236, 8070–8081. [Google Scholar] [CrossRef]
- Nakano, M.; Fujimiya, M. Potential effects of mesenchymal stem cell derived extracellular vesicles and exosomal miRNAs in neurological disorders. Neural Regen. Res. 2021, 16, 2359–2366. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Volarevic, V. Therapeutic Use of Mesenchymal Stem Cell-Derived Exosomes: From Basic Science to Clinics. Pharmaceutics 2020, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Volarevic, V.; Djonov, V.; Volarevic, A. Therapeutic Potential of Exosomes Derived from Adipose Tissue-Sourced Mesenchymal Stem Cells in the Treatment of Neural and Retinal Diseases. Int. J. Mol. Sci. 2022, 23, 4487. [Google Scholar] [CrossRef]
- Ezquer, M.; Urzua, C.A.; Montecino, S.; Leal, K.; Conget, P.; Ezquer, F. Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem Cell Res. Ther. 2016, 7, 42. [Google Scholar] [CrossRef]
- Rajashekhar, G.; Ramadan, A.; Abburi, C.; Callaghan, B.; Traktuev, D.O.; Evans-Molina, C.; Maturi, R.; Harris, A.; Kern, T.S.; March, K.L. Regenerative Therapeutic Potential of Adipose Stromal Cells in Early Stage Diabetic Retinopathy. PLoS ONE 2014, 9, e84671. [Google Scholar] [CrossRef]
- Mendel, T.A.; Clabough, E.B.D.; Kao, D.S.; Demidova-Rice, T.N.; Durham, J.T.; Zotter, B.C.; Seaman, S.A.; Cronk, S.M.; Rakoczy, E.P.; Katz, A.J.; et al. Pericytes Derived from Adipose-Derived Stem Cells Protect against Retinal Vasculopathy. PLoS ONE 2013, 8, e65691. [Google Scholar] [CrossRef]
- Elshaer, S.L.; Evans, W.; Pentecost, M.; Lenin, R.; Periasamy, R.; Jha, K.A.; Alli, S.; Gentry, J.; Thomas, S.M.; Sohl, N.; et al. Adipose stem cells and their paracrine factors are therapeutic for early retinal complications of diabetes in the Ins2Akita mouse. Stem Cell Res. Ther. 2018, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Cronk, S.M.; Kelly-Goss, M.R.; Ray, H.C.; Mendel, T.A.; Hoehn, K.L.; Bruce, A.C.; Dey, B.K.; Guendel, A.M.; Tavakol, D.N.; Herman, I.M.; et al. Adipose-Derived Stem Cells From Diabetic Mice Show Impaired Vascular Stabilization in a Murine Model of Diabetic Retinopathy. Stem Cells Transl. Med. 2015, 4, 459–467. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-Derived Stem Cells for Regenerative Medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, D.; Wei, W.; Shen, B.; Zhang, Y.; Wang, Y.; Tang, Z.; Ni, N.; Sun, H.; Liu, J.; et al. Decellularized matrix of adipose-derived mesenchymal stromal cells enhanced retinal progenitor cell proliferation via the Akt/Erk pathway and neuronal differentiation. Cytotherapy 2018, 20, 74–86. [Google Scholar] [CrossRef]
- Xu, W.-W.; Huang, L.; Chong, K.K.; Leung, D.S.; Li, B.F.; Yin, Z.-Q.; Huang, Y.-F.; Pang, C.P. Differentiation potential of human adipose tissue derived stem cells into photoreceptors through explants culture and enzyme methods. Int. J. Ophthalmol. 2017, 10, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Cristaldi, M.; Giurdanella, G.; Perrotta, R.E.; Furno, D.L.; Giuffrida, R.; Rusciano, D. ARPE-19 conditioned medium promotes neural differentiation of adipose-derived mesenchymal stem cells. World J. Stem Cells 2021, 13, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Rasiah, P.K.; Bajwa, A.; Rajasingh, J.; Gangaraju, R. Mesenchymal Stem Cell Induced Foxp3(+) Tregs Suppress Effector T Cells and Protect against Retinal Ischemic Injury. Cells 2021, 10, 3006. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, R.; Elshaer, S.L.; Gangaraju, R. CD140b (PDGFRβ) signaling in adipose-derived stem cells mediates angiogenic behavior of retinal endothelial cells. Regen. Eng. Transl. Med. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Tsuruma, K.; Yamauchi, M.; Sugitani, S.; Otsuka, T.; Ohno, Y.; Nagahara, Y.; Ikegame, Y.; Shimazawa, M.; Yoshimura, S.; Iwama, T.; et al. Progranulin, a Major Secreted Protein of Mouse Adipose-Derived Stem Cells, Inhibits Light-Induced Retinal Degeneration. Stem Cells Transl. Med. 2013, 3, 42–53. [Google Scholar] [CrossRef]
- Lupo, G.; Agafonova, A.; Cosentino, A.; Giurdanella, G.; Mannino, G.; Furno, D.L.; Romano, I.R.; Giuffrida, R.; D’angeli, F.; Anfuso, C.D. Protective Effects of Human Pericyte-like Adipose-Derived Mesenchymal Stem Cells on Human Retinal Endothelial Cells in an In Vitro Model of Diabetic Retinopathy: Evidence for Autologous Cell Therapy. Int. J. Mol. Sci. 2023, 24, 913. [Google Scholar] [CrossRef]
- Fontanilla, C.V.; Gu, H.Y.; Liu, Q.P.; Zhu, T.Z.; Zhou, C.W.; Johnstone, B.H.; March, K.L.; Pascuzzi, R.M.; Farlow, M.R.; Du, Y.S. Adipose-derived Stem Cell Conditioned Media Extends Survival time of a mouse model of Amyotrophic Lateral Sclerosis. Sci. Rep. 2016, 5, 16953, ARTN 20747. [Google Scholar] [CrossRef]
- Noverina, R.; Widowati, W.; Ayuningtyas, W.; Kurniawan, D.; Afifah, E.; Laksmitawati, D.R.; Rinendyaputri, R.; Rilianawati, R.; Faried, A.; Bachtiar, I.; et al. Growth factors profile in conditioned medium human adipose tissue-derived mesenchymal stem cells (CM-hATMSCs). Clin. Nutr. Exp. 2019, 24, 34–44. [Google Scholar] [CrossRef]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Paracrine-Mediated Neuroprotection and Neuritogenesis of Axotomised Retinal Ganglion Cells by Human Dental Pulp Stem Cells: Comparison with Human Bone Marrow and Adipose-Derived Mesenchymal Stem Cells. PLoS ONE 2014, 9, e109305. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Jin, J.; Chen, L.; Zhu, J.; Huang, W.; Zhao, J.; Qian, H.; Zhang, X. Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol. Int. 2006, 30, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, K.; Yan, X.; Dong, F.; Zhao, C. Amelioration of diabetic retinopathy by engrafted human adipose-derived mesenchymal stem cells in streptozotocin diabetic rats. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 1415–1422. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, H.; Wu, S.; He, Y.; Guo, K. In vitro induction and intraocular application in oxygen-induced retinopathy of adipose-derived mesenchymal stem cells. Mol. Vis. 2022, 28, 432–440. [Google Scholar]
- De Castro, L.L.; Lopes-Pacheco, M.; Weiss, D.J.; Cruz, F.F.; Rocco, P.R.M. Current understanding of the immunosuppressive properties of mesenchymal stromal cells. J. Mol. Med. JMM 2019, 97, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef]
- Dostert, G.; Mesure, B.; Menu, P.; Velot, É. How Do Mesenchymal Stem Cells Influence or Are Influenced by Microenvironment through Extracellular Vesicles Communication? Front. Cell Dev. Biol. 2017, 5, 6. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, Y.; Lai, R.C.; Tan, S.S.; Choo AB, H.; Lim, S.K. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014, 23, 1233–1244. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, W. Introduction to stem cells and regenerative medicine for retinal diseases. In Retinal Diseases—Advances in Research and Treatment; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Sugitani, S.; Tsuruma, K.; Ohno, Y.; Kuse, Y.; Yamauchi, M.; Egashira, Y.; Yoshimura, S.; Shimazawa, M.; Iwama, T.; Hara, H. The potential neuroprotective effect of human adipose stem cells conditioned medium against light-induced retinal damage. Exp Eye Res. 2013, 116, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Kadkhodaeian, H.A.; Tiraihi, T.; Ahmadieh, H.; Ardakani, H.Z.; Daftarian, N.; Taheri, T. Survival and Migration of Adipose-Derived Stem Cells Transplanted in the Injured Retina. Exp. Clin. Transplant. 2018, 16, 204–211. [Google Scholar] [CrossRef]
- Gounari, E.; Komnenou, A.; Kofidou, E.; Nanaki, S.; Bikiaris, D.; Almpanidou, S.; Kouzi, K.; Karampatakis, V.; Koliakos, G. Intravitreal Administration Effect of Adipose-Derived Mesenchymal Stromal Cells Combined with Anti-VEGF Nanocarriers, in a Pharmaceutically Induced Animal Model of Retinal Vein Occlusion. Stem Cells Int. 2022, 2022, 2760147. [Google Scholar] [CrossRef] [PubMed]
- Krief, B.; Algor, S.W.; Nakdimon, I.; Elhikis, A.; Benhamou, M.; Kadmon, A.S.; Keren, S.; Ohana, O.; Feldman, I.; Ben Cnaan, R.; et al. Retinal Lineage Therapeutic Specific Effect of Human Orbital and Abdominal Adipose-Derived Mesenchymal Stem Cells. Stem Cells Int. 2021, 2021, 7022247. [Google Scholar] [CrossRef] [PubMed]
- Xuqian, W.; Kanghua, L.; WeiHong, Y.; Xi, Y.; Rongping, D.; Qin, H.; Fangtian, D.; Zhao, R.C. Intraocular Transplantation of Human Adipose-Derived Mesenchymal Stem Cells in a Rabbit Model of Experimental Retinal Holes. Ophthalmic Res. 2011, 46, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Shao, H.; Su, C.; Jiang, Y.; Chen, X.; Bai, L.; Zhang, Y.; Li, Q.; Zhang, X.; Li, X. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci. Rep. 2016, 6, srep34562. [Google Scholar] [CrossRef] [PubMed]
- Jha, K.A.; Pentecost, M.; Lenin, R.; Klaic, L.; Elshaer, S.L.; Gentry, J.; Russell, J.M.; Beland, A.; Reiner, A.; Jotterand, V.; et al. Concentrated Conditioned Media from Adipose Tissue Derived Mesenchymal Stem Cells Mitigates Visual Deficits and Retinal Inflammation Following Mild Traumatic Brain Injury. Int. J. Mol. Sci. 2018, 19, 2016. [Google Scholar] [CrossRef]
- Jha, K.A.; Gentry, J.; Del Mar, N.A.; Reiner, A.; Sohl, N.; Gangaraju, R. Adipose Tissue-Derived Mesenchymal Stem Cell Concentrated Conditioned Medium Alters the Expression Pattern of Glutamate Regulatory Proteins and Aquaporin-4 in the Retina after Mild Traumatic Brain Injury. J. Neurotrauma 2021, 38, 1702–1716. [Google Scholar] [CrossRef]
- Ballios, B.G.; Cooke, M.J.; Donaldson, L.; Coles, B.L.; Morshead, C.M.; van der Kooy, D.; Shoichet, M.S. A hyaluronan-based injectable hydrogel improves the survival and integration of stem cells in the retina of a preclinical model of retinal degeneration. Stem Cells Transl. Med. 2015, 4, 980–991. [Google Scholar]
- Da Silva-Junior, A.J.; Mesentier-Louro, L.A.; Nascimento-Dos-Santos, G.; Teixeira-Pinheiro, L.C.; Vasques, J.F.; Chimeli-Ormonde, L.; Bodart-Santos, V.; de Carvalho, L.R.P.; Santiago, M.F.; Mendez-Otero, R. Human mesenchymal stem cell therapy promotes retinal ganglion cell survival and target reconnection after optic nerve crush in adult rats. Stem Cell Res. Ther. 2021, 12, 69. [Google Scholar] [CrossRef]
- Tzameret, A.; Sher, I.; Belkin, M.; Treves, A.J.; Meir, A.; Nagler, A.; Levkovitch-Verbin, H.; Barshack, I.; Rosner, M.; Rotenstreich, Y. Transplantation of human bone marrow mesenchymal stem cells as a thin subretinal layer ameliorates retinal degeneration in a rat model of retinal dystrophy. Exp Eye Res. 2014, 118, 135–144. [Google Scholar] [CrossRef]
- Fiori, A.; Hammes, H.-P.; Bieback, K. Adipose-derived mesenchymal stromal cells reverse high glucose–induced reduction of angiogenesis in human retinal microvascular endothelial cells. Cytotherapy 2020, 22, 261–275. [Google Scholar] [CrossRef]
- Mead, B.; Tomarev, S. Extracellular vesicle therapy for retinal diseases. Prog. Retin. Eye Res. 2020, 79, 100849. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Levy, S.; Benes, S.C.; Adelman, R.A. Mesenchymal stem cell-based treatment for outer retinal diseases: An update. Stem Cells Transl. Med. 2019, 8, 637–647. [Google Scholar]
- Levy, S.; Weiss, J.N.; Malkin, A. Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: A preliminary report. Neural Regen. Res. 2015, 10, 982–988. [Google Scholar] [CrossRef]
- Weiss, J.N.; Levy, S.; Benes, S.C. Stem Cell Ophthalmology Treatment Study: Bone marrow derived stem cells in the treatment of non-arteritic ischemic optic neuropathy (NAION). Stem Cell Investig. 2017, 4, 94. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Levy, S. Stem Cell Ophthalmology Treatment Study: Bone marrow derived stem cells in the treatment of Retinitis Pigmentosa. Stem Cell Investig. 2018, 5, 18. [Google Scholar] [CrossRef]
- Oner, A.; Gonen, Z.B.; Sinim, N.; Cetin, M.; Ozkul, Y. Subretinal adipose tissue-derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: A phase I clinical safety study. Stem Cell Res. Ther. 2016, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Limoli, P.G.; Vingolo, E.M.; Morales, M.U.; Nebbioso, M.; Limoli, C. Preliminary Study on Electrophysiological Changes After Cellular Autograft in Age-Related Macular Degeneration. Medicine 2014, 93, e355. [Google Scholar] [CrossRef]
- Limoli, P.G.; Limoli, C.; Vingolo, E.M.; Scalinci, S.Z.; Nebbioso, M. Cell surgery and growth factors in dry age-related macular degeneration: Visual prognosis and morphological study. Oncotarget 2016, 7, 46913–46923. [Google Scholar] [CrossRef]
- Weiss, J.N.; Levy, S.; Malkin, A. Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: A case report of improvement in relapsing auto-immune optic neuropathy. Neural Regen. Res. 2015, 10, 1507–1515. [Google Scholar]
- Adak, S.; Magdalene, D.; Deshmukh, S.; Das, D.; Jaganathan, B.G. A Review on Mesenchymal Stem Cells for Treatment of Retinal Diseases. Stem Cell Rev. Rep. 2021, 17, 1154–1173. [Google Scholar] [CrossRef]
| ADSC Origin | Retinal Disease | Effect on Retinal Diseases | References |
|---|---|---|---|
| decellularized matrix manufactured using 0.5% ammonium hydroxide Triton + 20 mmol/L NH4OH in combination with DNase solution | Not specified |
| Ji et al. [82] |
| Human-ADSCs (hASCSs) induced by RPE-conditioned medium (RPECM) | age-related macular degeneration (AMD) |
| Zhang et al. [46] |
| Human adipose tissue, the coding region of the human PAX6 (5a) gene isoform was cloned and lentiviral particles were propagated in HEK293T | Not specified |
| Razanejad et al. [49] |
| human orbital ADSCs generated by enzyme (EN) and explant (EX) culture methods | Not specified |
| Xu et al. [83] |
| adipose tissue, ADSCs cultured in a conditioned medium (CM) from an RPE cell line (ARPE-19 CM) | Not specified |
| Mannino et al. [84] |
| adipose tissue | Not specified |
| Shen et al. [45] |
| Human ADSCs | Hypoxic ex vivo organotypic cultures of retina |
| Dov et al. [37] |
| ADSCs | Not specified |
| Barzelay et al. [38] |
| Induced MSCs (iMSCs) generated by differentiating the induced pluripotent stem cells (iPSC) | Not specified |
| Agrawal et al. [85] |
| ADSCs | Not specified |
| Periasamy et al. [86] |
| mouse subcutaneous tissue | light-induced retinal damage |
| Tsuruma et al. [87] |
| pericyte-like differentiated phenotype of ADSCs (P-ASCs) | Diabetic retinopathy (human retinal endothelial cells (HRECs) in high glucose conditions [25 mM glucose, HG]) |
| Lupo et al. [88] |
| Induction of Retinal Diseases | Species | Treatment | Results | Reference |
|---|---|---|---|---|
| sodium iodate model with consequent oxidative stress, acute injury to RPE, and progressive ongoing retinal damage | Wild-type C57BL mice | subretinal transplantation of hADSCs | ADSCs are able to home in on damaged RPE cells and protect against damage to the RPE and PR layers caused by oxidative stress | Barzelay et al. [38] |
| light-induced retinal photoreceptor damage (under controlled lighting conditions [12/12 h light/dark cycle]) | Male adult ddY albino mice | Not available |
| Sugitani et al. [100] |
| light-induced retinal photoreceptor damage (under controlled lighting conditions [12/12 h light/dark cycle]) | Male adult ddY mice and C57BL/6-Tg (CAG-EGFP) mice | Murine ADSCs intravitreally injected | ADSC-CM significantly inhibited photoreceptor degeneration and retinal dysfunction after light exposure. Progranulin was identified as a major secreted protein of ASCs that showed protective effects against retinal damage | Tsuruma et al. [87] |
| Retinal pigmented epithelium damage induced by retro-orbital sinus sodium iodate injection | Sprague-Dawley albino rats | Murine ADSCs transplanted in the subretinal space | ADSCs survived for 4 weeks after transplantation and migrated into the RPE layer | Kadkhodaeian et al. [101] |
| oxygen-induced retinopathy (OIR) | Pregnant C57BL/6J mice | hADSCs intravitreally injected | five days after the hADSC intravitreal injection, the area of neovascularization was reduced by 94.83% compared with that of the OIR group | Zhou et al. [94] |
| oxygen-induced retinopathy (OIR) | NOD SCID mice (Charles River) | hADSCs intravitreally injected | ADSC-derived pericytes can integrate with retinal vasculature, adopting both pericyte morphology and marker expression, | Mendel et al. [78] |
| Diabetes Mellitus induced by intravenous injections of streptozotocin (STZ) | Male rabbits | Rabbit ADSCs-exosomes | potency of rabbit ADSCs-derived exosomes in retinal repair | Safwat et al. [39] |
| Diabetes Mellitus induced by intraperitoneal injections of streptozotocin (STZ) | C57BL/6 and C57BL76-Tg(ACTB-EGFP)1Obs mice | Murine ADSCs intravitreally injected | increased the intraocular levels of several potent neurotrophic factors (nerve growth factor, basic fibroblast growth factor and glial cell line-derived neurotrophic factor) and reduced the oxidative damage in the diabetic retina | Ezquer et al. [76] |
| Diabetes Mellitus induced by intraperitoneal injections of streptozotocin (STZ) | Athymic nude rats (Hsd:RH-Foxn1rnu) | hADSCs intravitreally injected | ADSCs are able to rescue the neural retina from hyperglycemia-induced degeneration, resulting in importantly improved visual function | Rajashekhar et al. [77] |
| early-stage Diabetic Retinopathy mouse model | Ins2Akita heterozygote male mice and age-matched C57BL/6J (WT) male mice | hADSCs intravitreally injected | ADSCs or their secreted factors mitigate retinal complications of diabetes | Elsaher et al. [79] |
| Diabetic Retinopathy mouse model | Akimba mouse | mouse ADSCs (mADSCs)/media conditioned by mASCs intravitreally injected | ADSCs taken from diabetic sources have an impaired ability to stabilize the microvasculature in diabetic retinopathy | Cronk et al. [80] |
| intravitreal injections of MEK kinase inhibitor induced animal model of Retinal Vein Occlusion (RVO) | New Zealand rabbits | intravitreal administration of rabbit ADSCs combined with encapsulated anti-VEGF in thiolated chitosan nanocarriers (nanoThioCHI) | a stem cell-based therapy for RVO is proposed, accompanied by sustained release of anti-VEGF, in order to combine the paracrine action of ASCs and the progressive reduction of neovascularization. | Gounari et al. [102] |
| mouse model of retinal degeneration | sodium iodate mice model | Subretinal transplantation of human orbital (OADSCs) and abdominal ADSCs (ABASCs) | subretinal transplantation of OADSCs in a mouse model of retinal degeneration led to restoration of the RPE layer while transplantation of ABASCs resulted in a significant restoration of the photoreceptor layer | Krief et al. [103] |
| Rabbit Model of Experimental Retinal Holes | New Zealand rabbits | hADSCs intravitreally injected | Transplanted hAD-MSCs could engraft in the retinal hole of a rabbit model, and clearly accelerated the healing process and ameliorated injury recovery | Xuqian et al. [104] |
| Krypton laser-induced retinal injury | C57BL/6 mice | Mouse ADSC-derived exosomes (MSC-Exos) intravitreally injected | MSC-Exos ameliorate laser-induced retinal injury partially through down-regulation of monocyte chemotactic protein MCP-1 | Yu et al. [105] |
| mouse model of visual deficits following mild traumatic brain injury (mTBI) | C57BL/6 mice | ADSC concentrated conditioned medium (ADSC-CCM) from cells pre-stimulated with inflammatory cytokines (ASC-CCM) intravitreally injected | Injection of ADSC-CCM mitigates loss of visual acuity and contrast sensitivity four weeks post blast injury. | Jha et al. [106] |
| mouse model of visual deficits following mild traumatic brain injury (mTBI) | C57Bl/6 mice | Human ADSC concentrated conditioned medium (ADSC-CCM) intravitreally injected | novel neuroprotective role for ASC-CCM in the rescue of the visual deficits and pathologies of mTBI via restoration of Müller cell health | Jha et al. [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finocchio, L.; Zeppieri, M.; Gabai, A.; Spadea, L.; Salati, C. Recent Advances of Adipose-Tissue-Derived Mesenchymal Stem Cell-Based Therapy for Retinal Diseases. J. Clin. Med. 2023, 12, 7015. https://doi.org/10.3390/jcm12227015
Finocchio L, Zeppieri M, Gabai A, Spadea L, Salati C. Recent Advances of Adipose-Tissue-Derived Mesenchymal Stem Cell-Based Therapy for Retinal Diseases. Journal of Clinical Medicine. 2023; 12(22):7015. https://doi.org/10.3390/jcm12227015
Chicago/Turabian StyleFinocchio, Lucia, Marco Zeppieri, Andrea Gabai, Leopoldo Spadea, and Carlo Salati. 2023. "Recent Advances of Adipose-Tissue-Derived Mesenchymal Stem Cell-Based Therapy for Retinal Diseases" Journal of Clinical Medicine 12, no. 22: 7015. https://doi.org/10.3390/jcm12227015
APA StyleFinocchio, L., Zeppieri, M., Gabai, A., Spadea, L., & Salati, C. (2023). Recent Advances of Adipose-Tissue-Derived Mesenchymal Stem Cell-Based Therapy for Retinal Diseases. Journal of Clinical Medicine, 12(22), 7015. https://doi.org/10.3390/jcm12227015







