Digital Evaluation of Vertical Ridge Augmentation with the Modified Shell Technique Using a Xenogeneic Bone Lamina: A Case Series
Abstract
1. Introduction
2. Materials and Methods
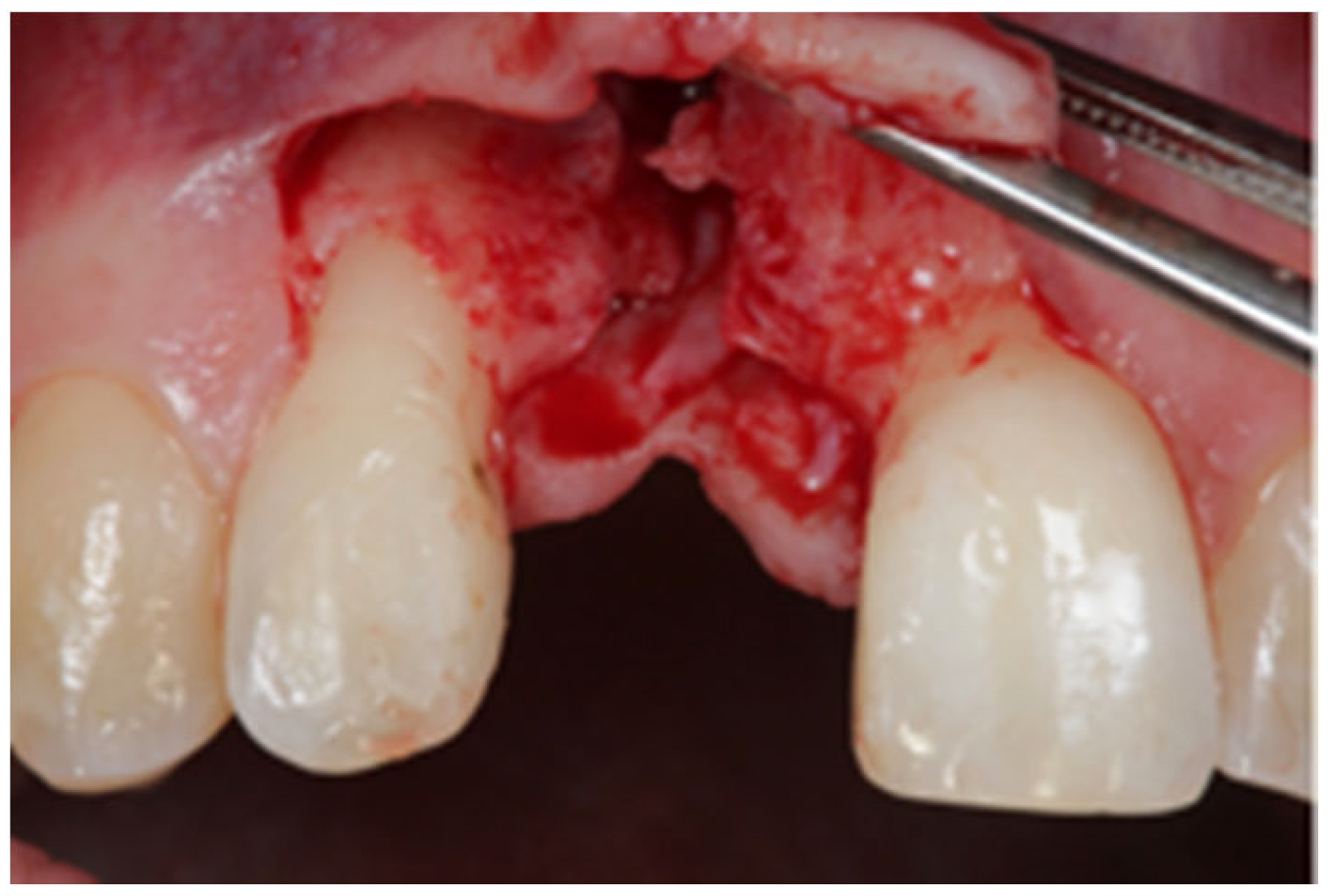
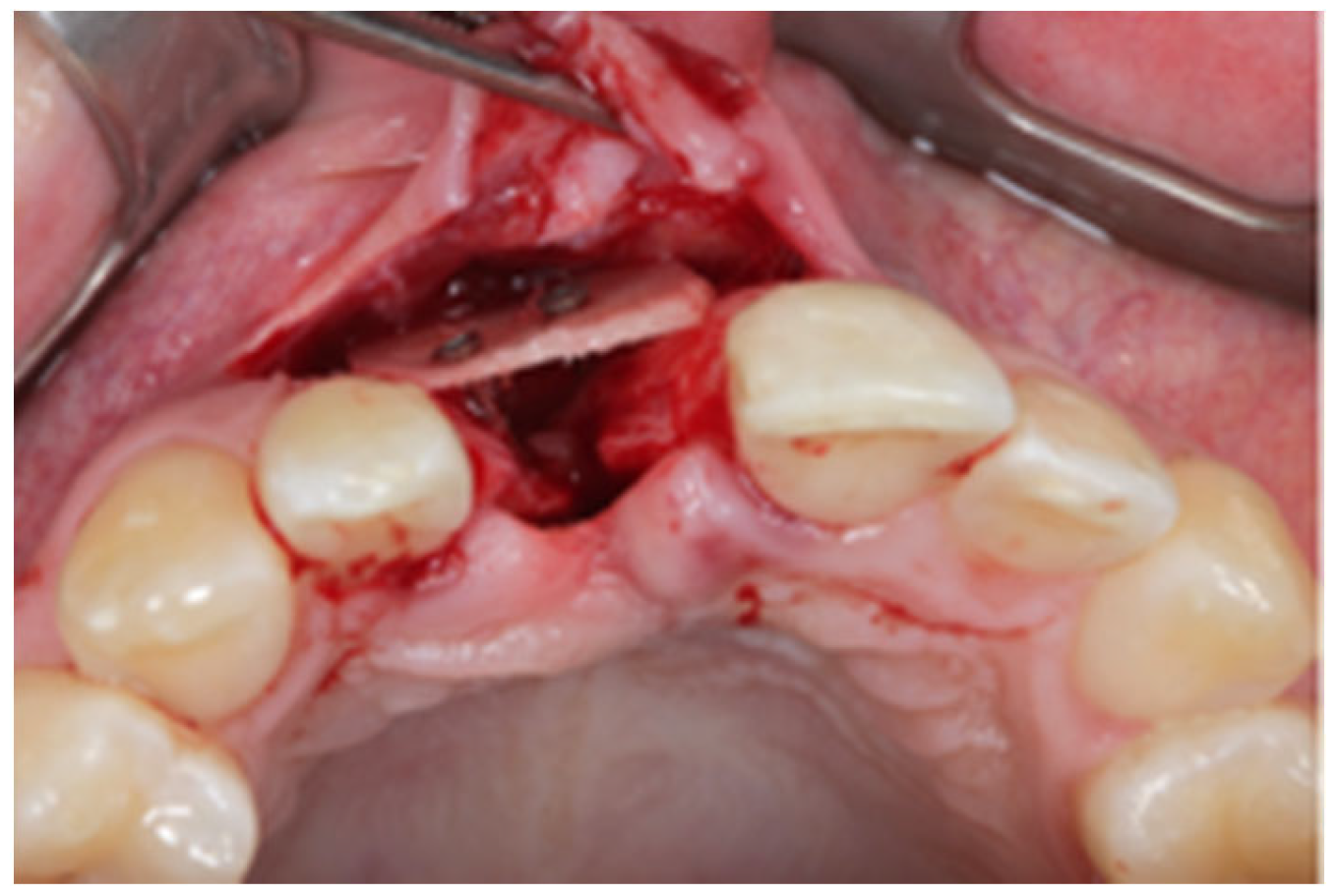
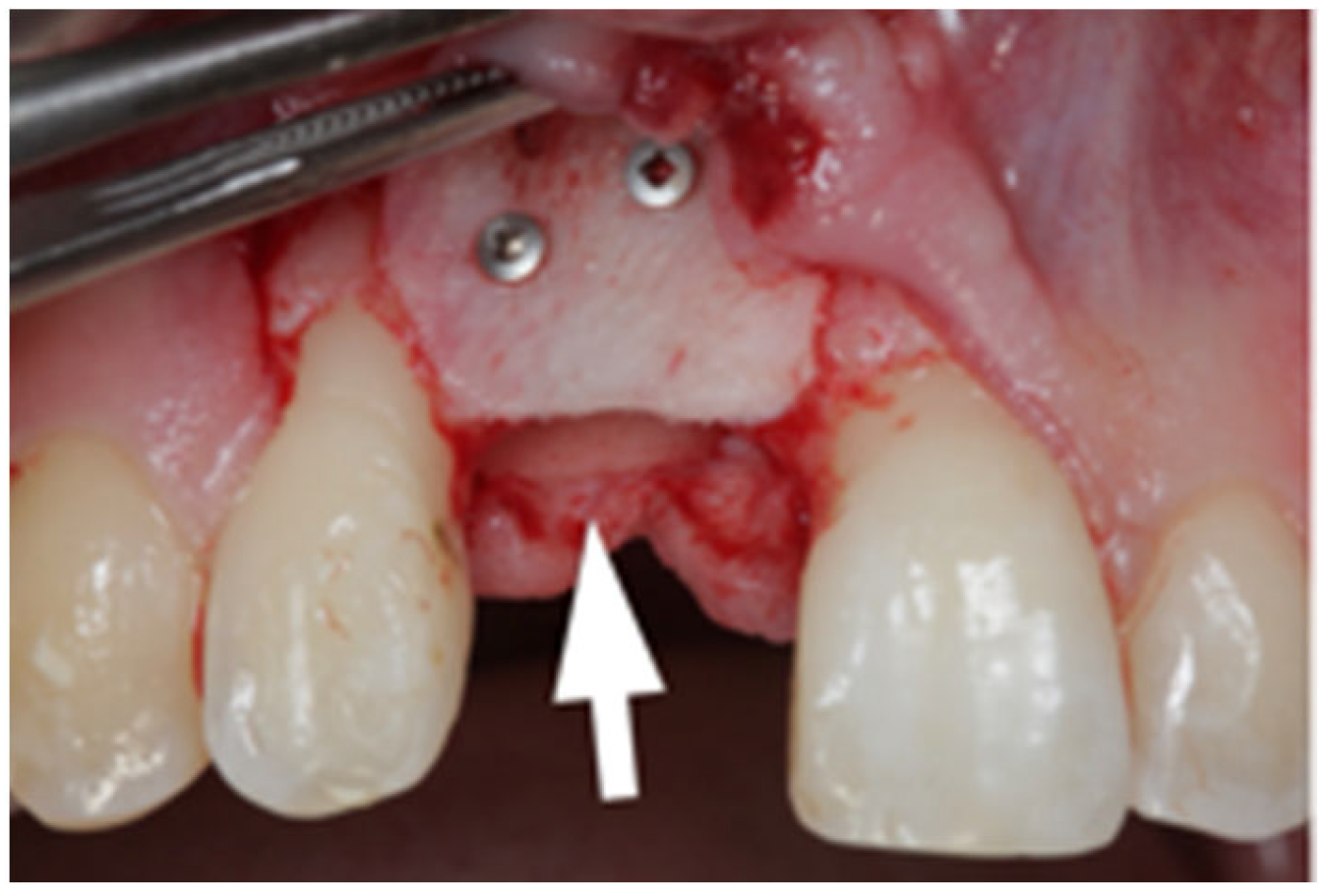
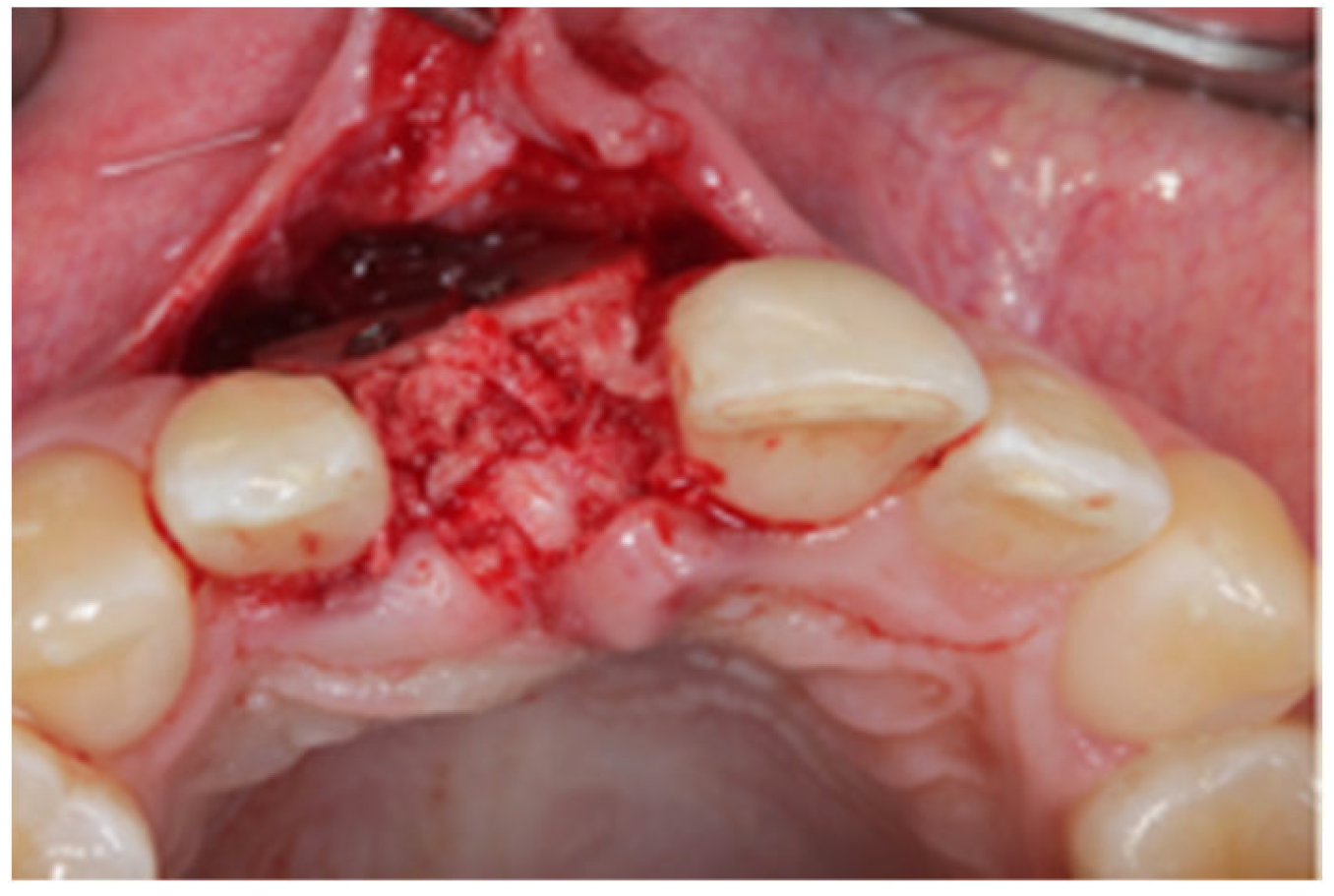
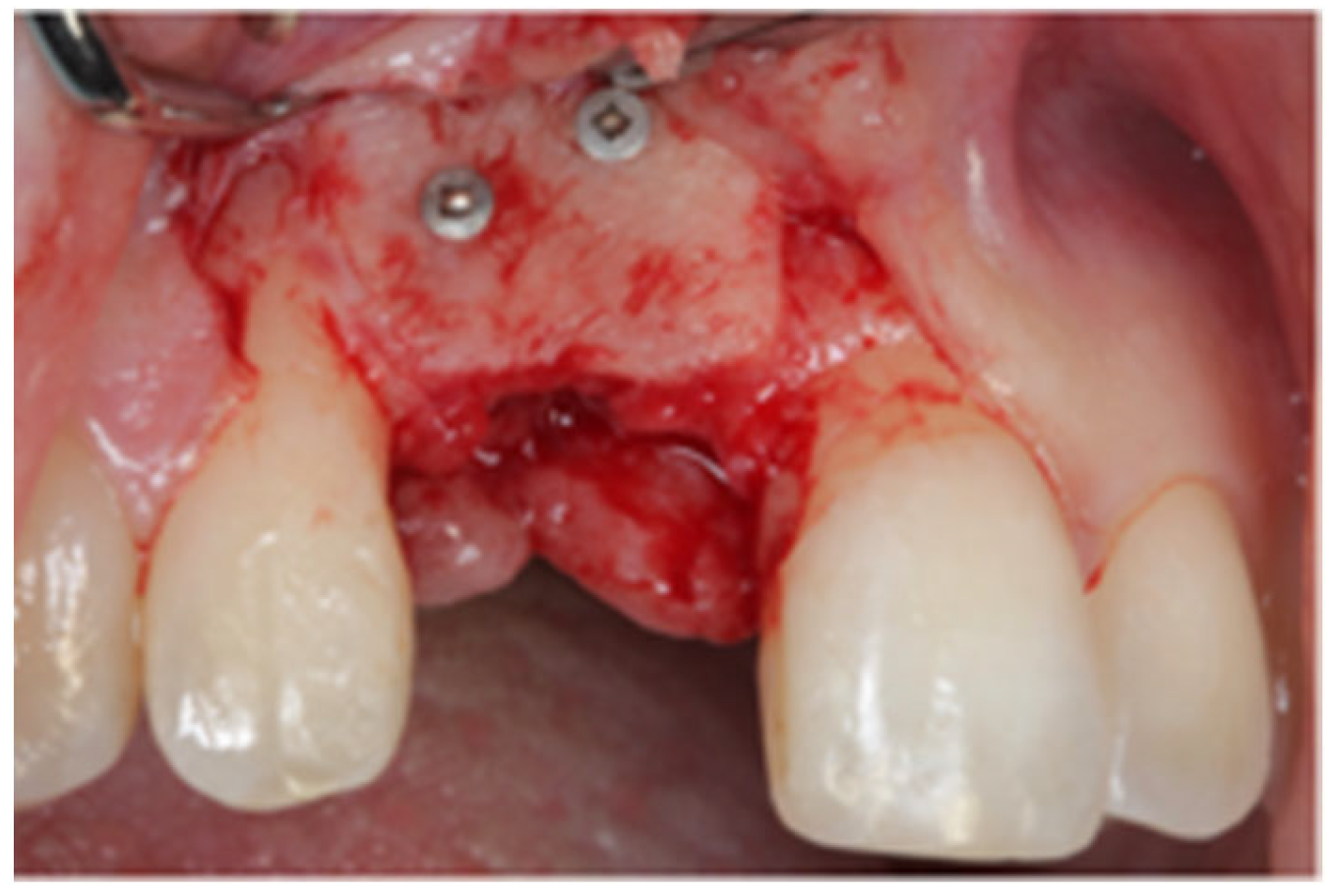
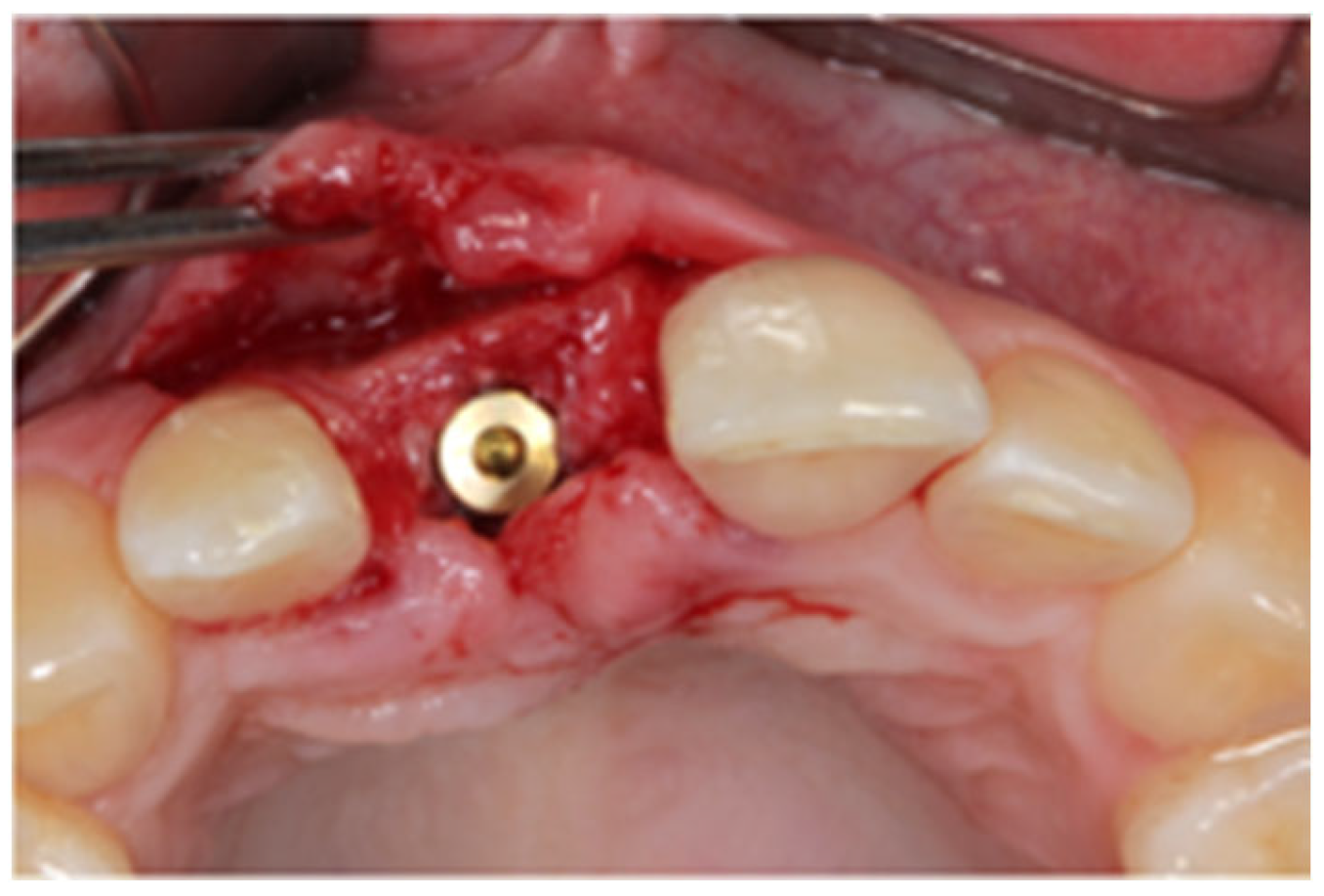
2.1. Surgical Technique
2.2. D-Evaluation Procedure
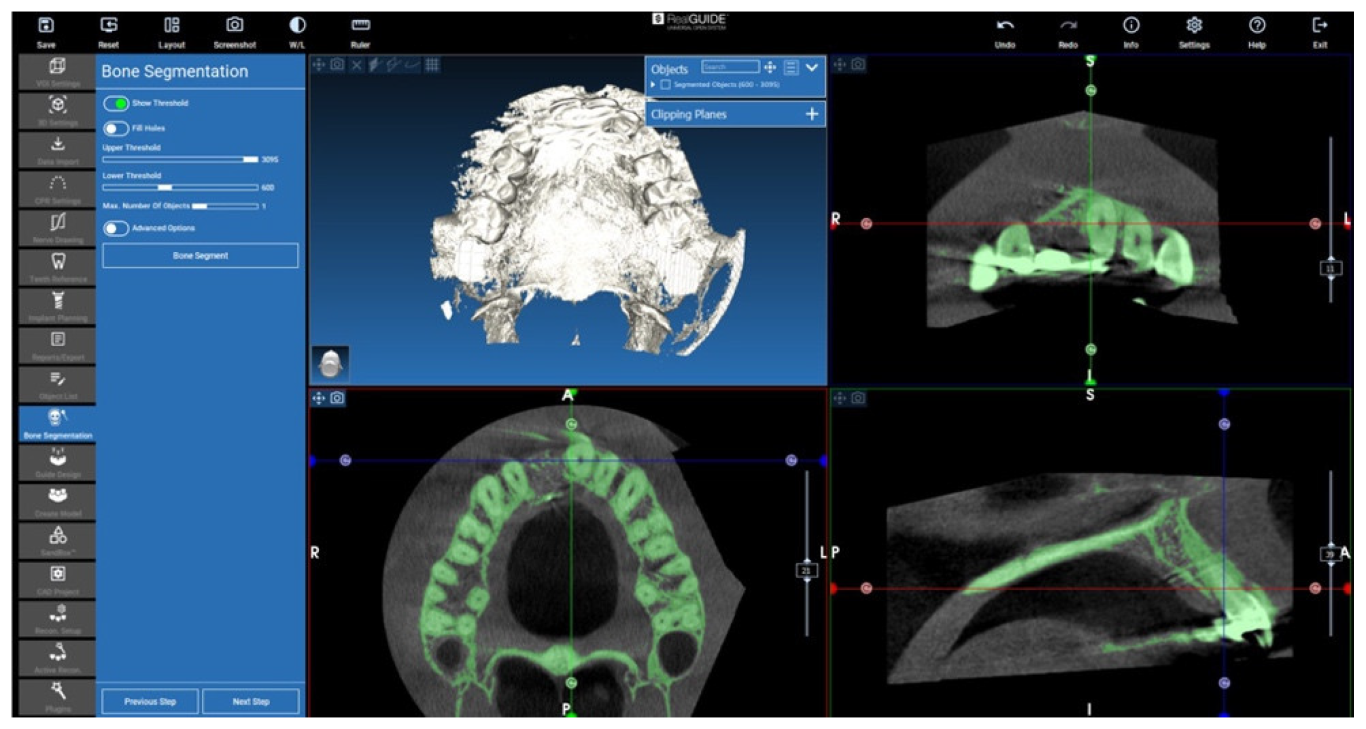
2.3. Cone Beam CT Conversion to STL by Means of Segmentation
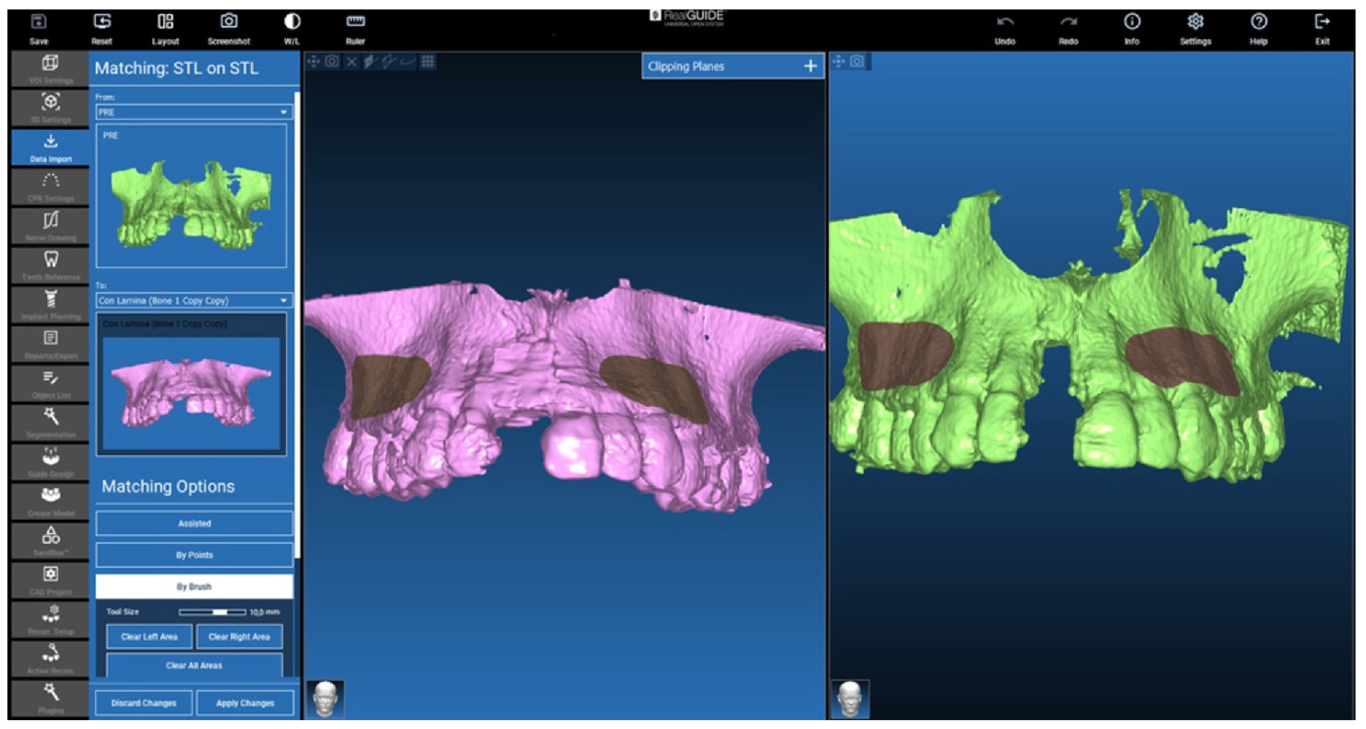
2.4. Isolation of the Augmented Area
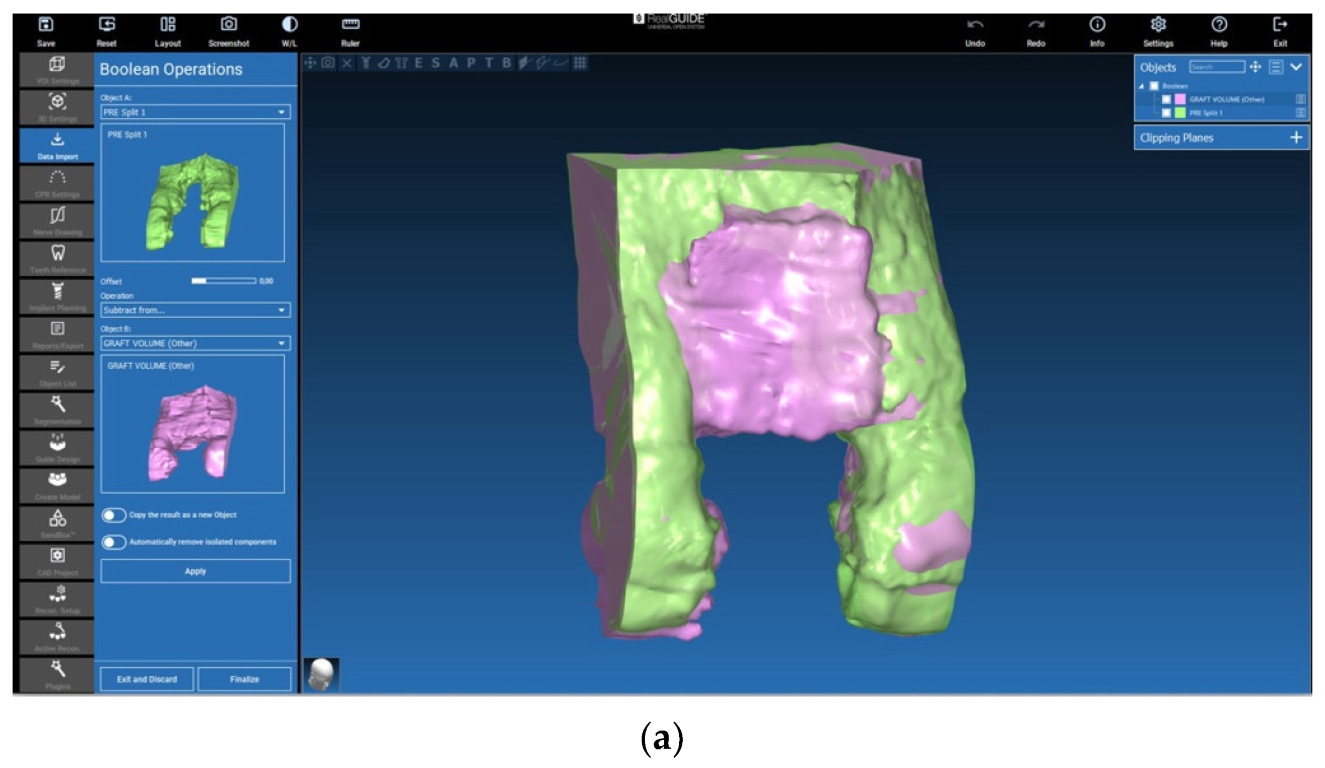
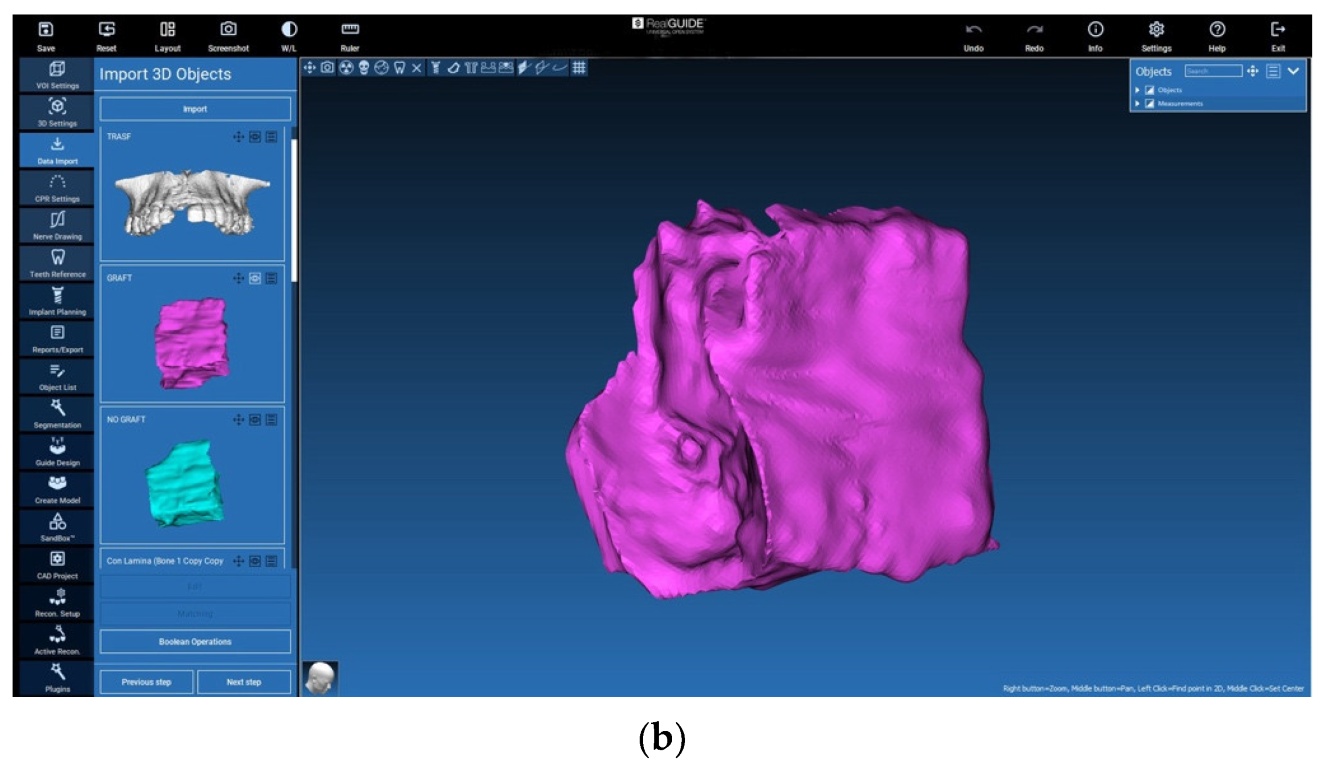
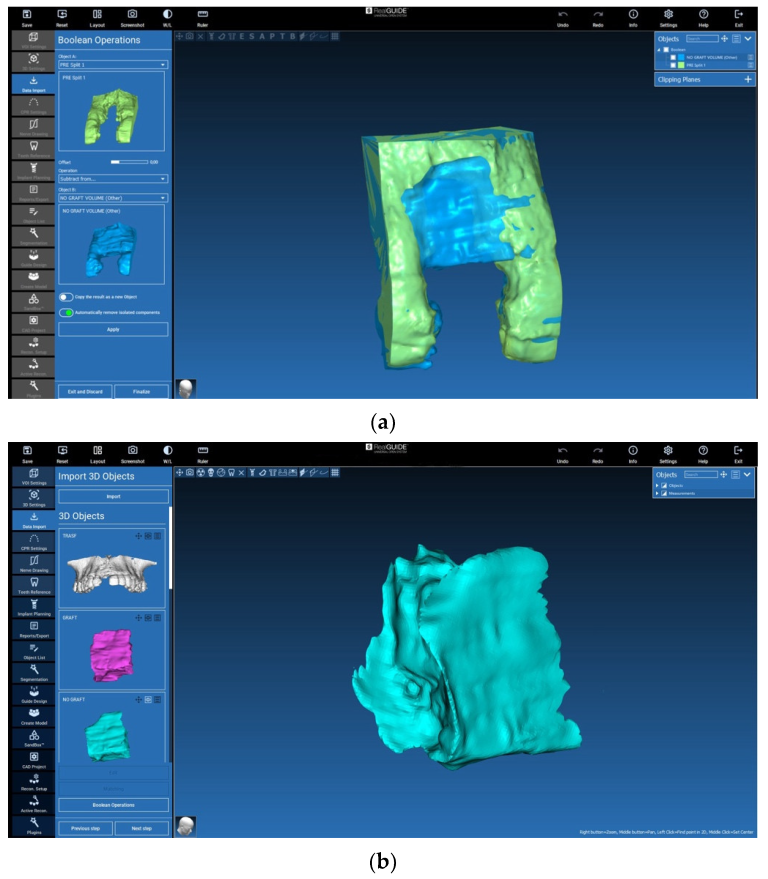
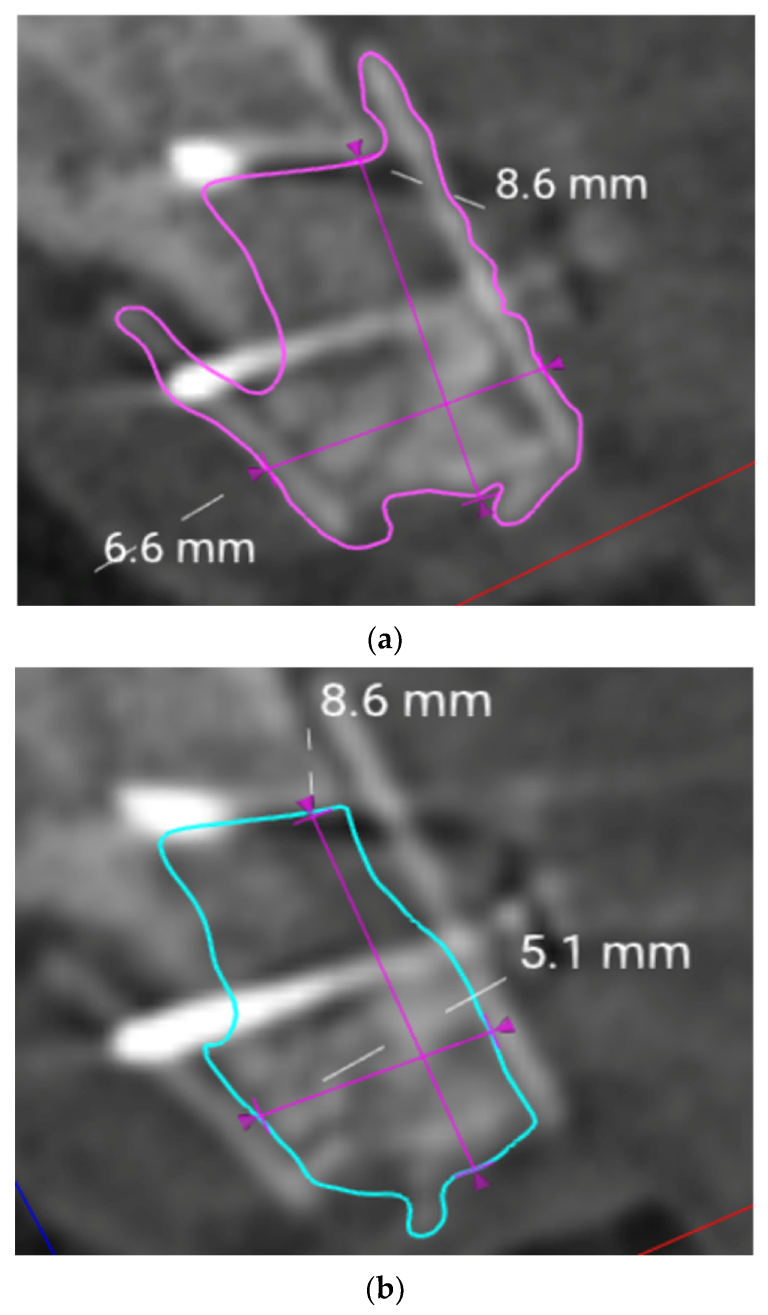
2.5. Evaluation of the Volumes
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| ASA | American Society of Anesthesiologists |
| CBCT | cone beam computed tomography |
| DICOM | digital imaging and communications in medicine |
| GBR | guided bone regeneration |
| SBB | split bone block technique |
| SD | standard deviation |
| STL | standard tessellation language |
References
- Fontana, F.; Maschera, E.; Rocchietta, I.; Simion, M. Clinical classification of complications in guided bone regeneration procedures by means of a nonresorbable membrane. Int. J. Periodontics Restor. Dent. 2011, 31, 265–273. [Google Scholar]
- Urban, I.A.; Montero, E.; Monje, A.; Sanz-Sanchez, I. Effectiveness of vertical ridge augmentation interventions: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 319–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Boyapati, L. “Pass” principles for predictable bone regeneration. Implant. Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Wikesjo, U.M.; Kean, C.J.; Zimmerman, G.J. Periodontal repair in dogs: Supraalveolar defect models for evaluation of safety and efficacy of periodontal reconstructive therapy. J. Periodontol. 1994, 65, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Monje, A.; Lozada, J.; Wang, H.L. Principles for vertical ridge augmentation in the atrophic posterior mandible: A technical review. Int. J. Periodontics Restor. Dent. 2017, 37, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Benic, G.I.; Hammerle, C.H. Horizontal bone augmentation by means of guided bone regeneration. Periodontol. 2000 2014, 66, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Fekry, Y.E.; Mahmoud, N.R. Vertical ridge augmentation of atrophic posterior mandible with corticocancellous onlay symphysis graft versus sandwich technique: Clinical and radiographic analysis. Odontology 2023, 111, 993–1002. [Google Scholar] [CrossRef]
- Spin-Neto, R.; Stavropoulos, A.; Coletti, F.L.; Faeda, R.S.; Pereira, L.A.; Marcantonio, E., Jr. Graft incorporation and implant osseointegration following the use of autologous and fresh-frozen allogeneic block bone grafts for lateral ridge augmentation. Clin. Oral Implant. Res. 2014, 25, 226–233. [Google Scholar] [CrossRef]
- Rocchietta, I.; Simion, M.; Hoffmann, M.; Trisciuoglio, D.; Benigni, M.; Dahlin, C. Vertical bone augmentation with an autogenous block or particles in combination with guided bone regeneration: A clinical and histological preliminary study in humans. Clin. Implant. Dent. Relat. Res. 2016, 18, 19–29. [Google Scholar] [CrossRef]
- Khoury, F.; Hanser, T. Three-dimensional vertical alveolar ridge augmentation in the posterior maxilla: A 10-year clinical study. Int. J. Oral Maxillofac. Implant. 2019, 34, 471–480. [Google Scholar] [CrossRef]
- Urban, I.A.; Lozada, J.L.; Wessing, B.; Suarez-Lopez del Amo, F.; Wang, H.L. Vertical bone grafting and periosteal vertical mattress suture for the fixation of resorbable membranes and stabilization of particulate grafts in horizontal guided bone regeneration to achieve more predictable results: A technical report. Int. J. Periodontics Restor. Dent. 2016, 36, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Khoury, F.; Khoury, C. Mandibular bone block grafts: Diagnosis instrumentation, harvesting techniques and surgical procedures. In Bone Augmentation in Oral Implantology; Khoury, F., Antoun, H., Missika, P., Eds.; Quitessence Publishing Co, Ltd.: Chicago, IL, USA, 2007; pp. 115–212. [Google Scholar]
- Rinna, C.; Ungari, C.; Saltarel, A.; Cassoni, A.; Reale, G. Orbital floor restoration. J. Craniofac. Surg. 2005, 16, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Ozel, B.; Findikcioglu, K.; Sezgin, B.; Guney, K.; Barut, I.; Ozmen, S. A new option for the reconstruction of orbital floor defects with heterologous cortical bone. J. Craniomaxillofac. Surg. 2015, 43, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Rancitelli, D.; Poli, P.P.; Rasia Dal Polo, M.; Nannmark, U.; Maiorana, C. The use of a collagenated porcine cortical lamina in the reconstruction of alveolar ridge defects. A clinical and histological study. Minerva Stomatol. 2016, 65, 257–268. [Google Scholar]
- Wismeijer, D.; Joda, T.; Flügge, T.; Fokas, G.; Tahmaseb, A.; Bechelli, D.; Bohner, L.; Bornstein, M.; Burgoyne, A.; Caram, S.; et al. Group 5 iti consensus report: Digital technologies. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 436–442. [Google Scholar] [CrossRef]
- Gallucci, G.O.; Evans, C.; Tahmaseb, A.; Wismeijer, D.; Barter, S.; Donos, N. Iti Treatment Guide; Digital Workflows in Implant Dentistry; Quintessence Publishing: Warsaw, Poland, 2019; Volume 11. [Google Scholar]
- Charton, J.; Laurentjoye, M.; Kim, Y. 3D boolean operations in virtual surgical planning. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 1697–1709. [Google Scholar] [CrossRef]
- Mak, P.H.; Campbell, R.C.; Irwin, M.G. The asa physical status classification: Inter-observer consistency. American society of anesthesiologists. Anaesth. Intensive Care 2002, 30, 633–640. [Google Scholar] [CrossRef]
- von Arx, T.; Buser, D. Horizontal ridge augmentation using autogenous block grafts and the guided bone regeneration technique with collagen membranes: A clinical study with 42 patients. Clin. Oral Implant. Res. 2006, 17, 359–366. [Google Scholar] [CrossRef]
- Misch, C.M. Comparison of intraoral donor sites for onlay grafting prior to implant placement. Int. J. Oral Maxillofac. Implant. 1997, 12, 767–776. [Google Scholar]
- Happe, A. Use of a piezoelectric surgical device to harvest bone grafts from the mandibular ramus: Report of 40 cases. Int. J. Periodontics Restor. Dent. 2007, 27, 241–249. [Google Scholar]
- Esposito, M.; Grusovin, M.G.; Coulthard, P.; Worthington, H.V. The efficacy of various bone augmentation procedures for dental implants: A cochrane systematic review of randomized controlled clinical trials. Int. J. Oral Maxillofac. Implant. 2006, 21, 696–710. [Google Scholar]
- Herford, A.S.; Nguyen, K. Complex bone augmentation in alveolar ridge defects. Oral Maxillofac. Surg. Clin. N. Am. 2015, 27, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Bahat, O.; Fontanessi, R.V. Implant placement in three-dimensional grafts in the anterior jaw. Int. J. Periodontics Restor. Dent. 2001, 21, 357–365. [Google Scholar]
- Khoury, F.; Antoun, H.; Missika, P. Bone Augmentation in Oral Implantology; Quintessence: Chicago, IL, USA, 2007. [Google Scholar]
- Cunha, G.; Carvalho, P.H.A.; Quirino, L.C.; Torres, L.H.S.; Filho, V.A.P.; Gabrielli, M.F.R.; Gabrielli, M.A.C. Titanium mesh exposure after bone grafting: Treatment approaches-a systematic review. Craniomaxillofac Trauma. Reconstr. 2022, 15, 397–405. [Google Scholar] [CrossRef]
- Chiapasco, M.; Zaniboni, M.; Boisco, M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin. Oral Implant. Res. 2006, 17 (Suppl. S2), 136–159. [Google Scholar] [CrossRef]
- Aghaloo, T.L.; Moy, P.K. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int. J. Oral Maxillofac. Implant. 2007, 22, 49–70. [Google Scholar]
- Naenni, N.; Lim, H.C.; Papageorgiou, S.N.; Hammerle, C.H.F. Efficacy of lateral bone augmentation prior to implant placement: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 287–306. [Google Scholar] [CrossRef]
- Urban, I.A.; Saleh, M.H.A.; Ravida, A.; Forster, A.; Wang, H.L.; Barath, Z. Vertical bone augmentation utilizing a titanium-reinforced ptfe mesh: A multi-variate analysis of influencing factors. Clin. Oral Implant. Res. 2021, 32, 828–839. [Google Scholar] [CrossRef]
- Troeltzsch, M.; Troeltzsch, M.; Kauffmann, P.; Gruber, R.; Brockmeyer, P.; Moser, N.; Rau, A.; Schliephake, H. Clinical efficacy of grafting materials in alveolar ridge augmentation: A systematic review. J. Craniomaxillofac. Surg. 2016, 44, 1618–1629. [Google Scholar] [CrossRef]
- Ritter, L.; Reiz, S.D.; Rothamel, D.; Dreiseidler, T.; Karapetian, V.; Scheer, M.; Zoller, J.E. Registration accuracy of three-dimensional surface and cone beam computed tomography data for virtual implant planning. Clin. Oral Implant. Res. 2012, 23, 447–452. [Google Scholar] [CrossRef]
- Maiorana, C.; Beretta, M.; Salina, S.; Santoro, F. Reduction of autogenous bone graft resorption by means of bio-oss coverage: A prospective study. Int. J. Periodontics Restor. Dent. 2005, 25, 19–25. [Google Scholar]
- Wachtel, H.; Fickl, S.; Hinze, M.; Bolz, W.; Thalmair, T. The bone lamina technique: A novel approach for lateral ridge augmentation—A case series. Int. J. Periodontics Restor. Dent. 2013, 33, 491–497. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Merli, M.; Migani, M.; Esposito, M. Vertical ridge augmentation with autogenous bone grafts: Resorbable barriers supported by ostheosynthesis plates versus titanium-reinforced barriers. A preliminary report of a blinded, randomized controlled clinical trial. Int. J. Oral Maxillofac. Implant. 2007, 22, 373–382. [Google Scholar]
- Velázquez, Ó.I.; Tresguerres, F.G.F.; Berrocal, I.L.; Tresguerres, I.F.; López-Pintor, R.M.; Carballido, J.; López-Quiles, J.; Torres, J. Split bone block technique: 4-month results of a randomised clinical trial comparing clinical and radiographic outcomes between autogenous and xenogeneic cortical plates. Int. J. Oral Implant. 2021, 14, 41–52. [Google Scholar]
- Merli, M.; Moscatelli, M.; Mazzoni, A.; Mazzoni, S.; Pagliaro, U.; Breschi, L.; Motroni, A.; Nieri, M. Fence technique: Guided bone regeneration for extensive three-dimensional augmentation. Int. J. Periodontics Restor. Dent. 2013, 33, 129–136. [Google Scholar] [CrossRef]
- Schnutenhaus, S.; Martin, T.; Dreyhaupt, J.; Rudolph, H.; Luthardt, R.G. Dimensional changes of the soft tissue after alveolar ridge preservation with a collagen material. A clinical randomized trial. Open Dent. J. 2018, 12, 389–399. [Google Scholar] [CrossRef]
- Vannier, M.W. Evaluation of 3D imaging. Crit. Rev. Diagn. Imaging 2000, 41, 315–378. [Google Scholar] [CrossRef]


| Patient No. | Total Augmented Volume (Graft Volume) (mm3) | Augmented Volume without Lamina (No Graft Volume) (mm3) |
|---|---|---|
| 1 | 310.26 | 219.12 |
| 2 | 405.06 | 246.24 |
| 3 | 301.68 | 173.68 |
| 4 | 460.53 | 334.14 |
| 5 | 468.91 | 262.15 |
| 6 | 349.09 | 269.72 |
| Mean | 382.59 | 250.84 |
| Standard Deviation | 73.39 | 53.67 |
| Patient No. | Maximum Vertical Gain (mm) |
|---|---|
| 1 | 7.4 |
| 2 | 8.6 |
| 3 | 7 |
| 4 | 11.3 |
| 5 | 8.9 |
| 6 | 10.6 |
| Mean | 8.97 |
| Standard Deviation | 1.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Happe, A.; Blender, S.M.; Luthardt, R.G.; Rudolph, H.; Kuhn, K. Digital Evaluation of Vertical Ridge Augmentation with the Modified Shell Technique Using a Xenogeneic Bone Lamina: A Case Series. J. Clin. Med. 2023, 12, 7013. https://doi.org/10.3390/jcm12227013
Happe A, Blender SM, Luthardt RG, Rudolph H, Kuhn K. Digital Evaluation of Vertical Ridge Augmentation with the Modified Shell Technique Using a Xenogeneic Bone Lamina: A Case Series. Journal of Clinical Medicine. 2023; 12(22):7013. https://doi.org/10.3390/jcm12227013
Chicago/Turabian StyleHappe, Arndt, Sarah M. Blender, Ralph G. Luthardt, Heike Rudolph, and Katharina Kuhn. 2023. "Digital Evaluation of Vertical Ridge Augmentation with the Modified Shell Technique Using a Xenogeneic Bone Lamina: A Case Series" Journal of Clinical Medicine 12, no. 22: 7013. https://doi.org/10.3390/jcm12227013
APA StyleHappe, A., Blender, S. M., Luthardt, R. G., Rudolph, H., & Kuhn, K. (2023). Digital Evaluation of Vertical Ridge Augmentation with the Modified Shell Technique Using a Xenogeneic Bone Lamina: A Case Series. Journal of Clinical Medicine, 12(22), 7013. https://doi.org/10.3390/jcm12227013









