Hyperthermic Intraperitoneal Chemotherapy (HIPEC): New Approaches and Controversies on the Treatment of Advanced Epithelial Ovarian Cancer—Systematic Review and Meta-Analysis
Abstract
:1. Introduction
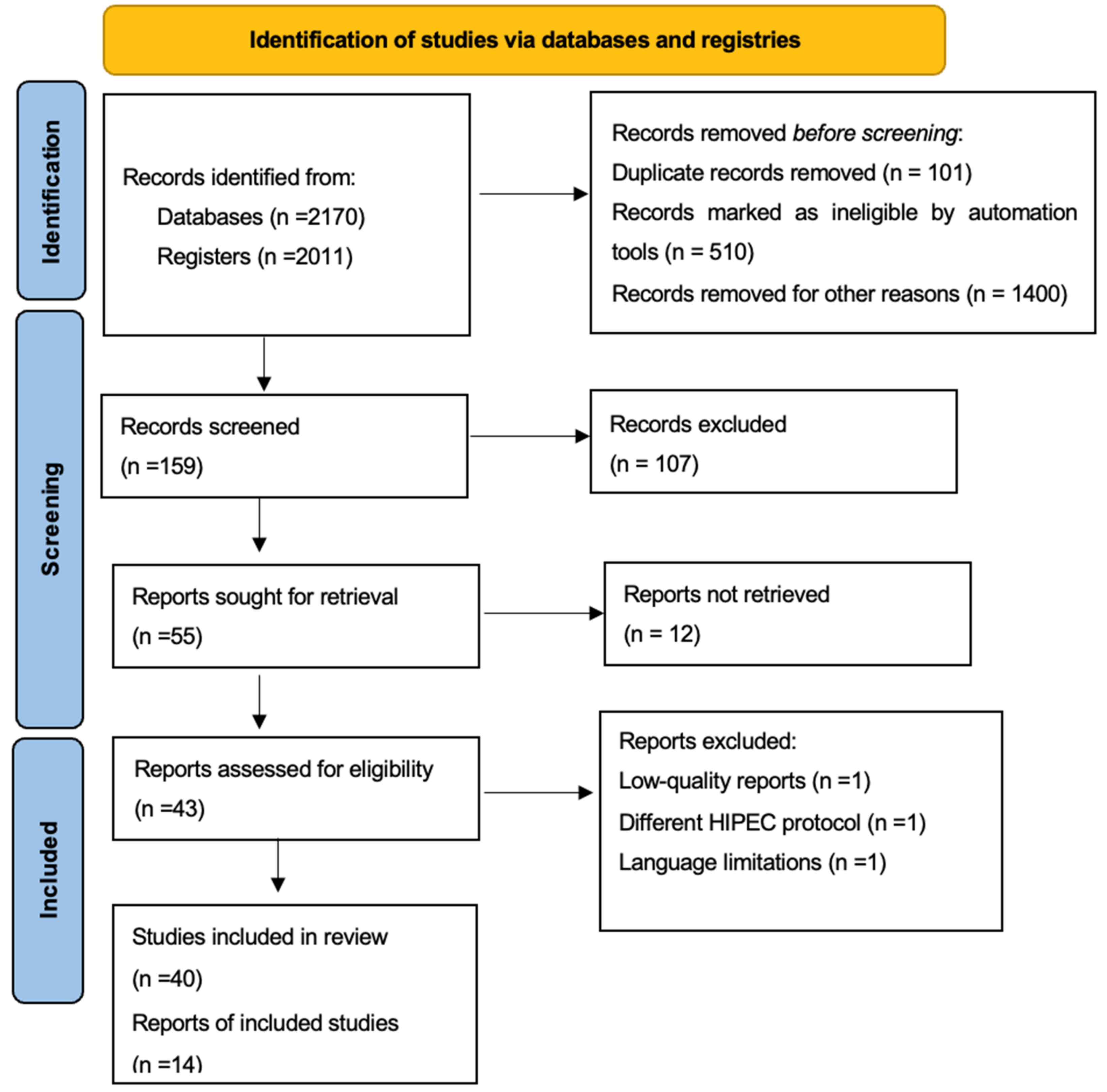
2. Materials and Methods
2.1. Search Method
2.2. Study Selection
2.3. Data Extraction
2.4. Outcomes
2.5. Statistical Analysis
2.6. Quality Assessment
3. Results
3.1. General Characteristics
3.2. Quality Assessment
3.3. Survival Outcomes
3.3.1. Primary Debulking Surgery
3.3.2. Interval Debulking Surgery
3.3.3. Recurrent Ovarian Cancer
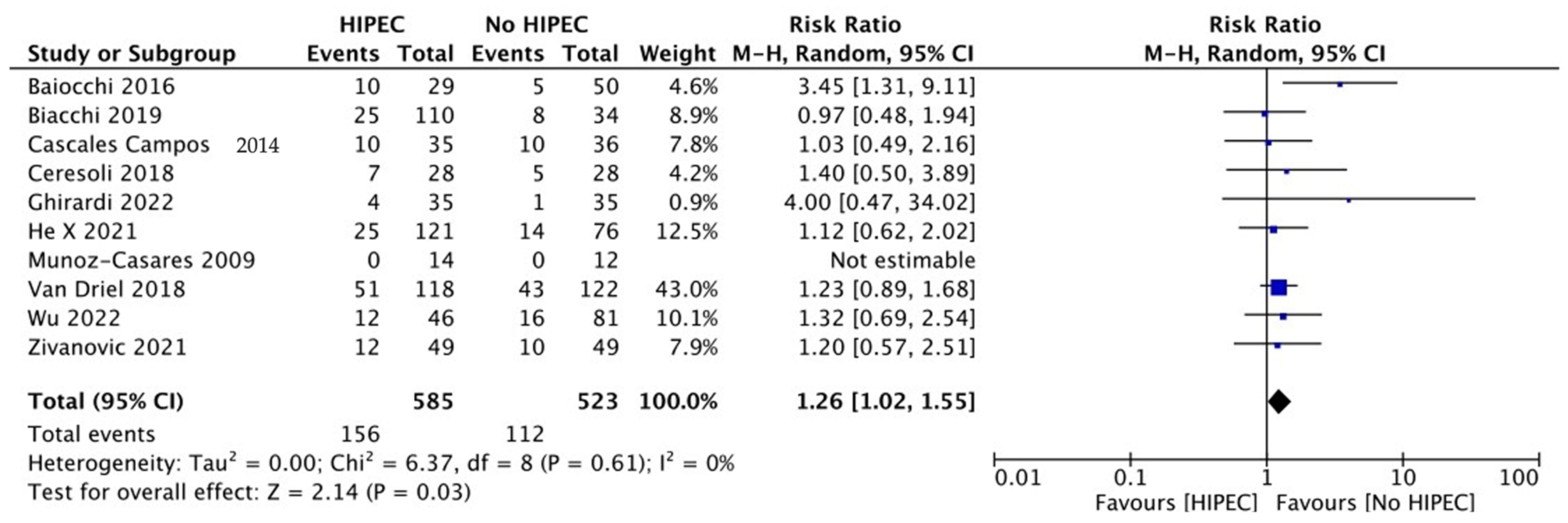
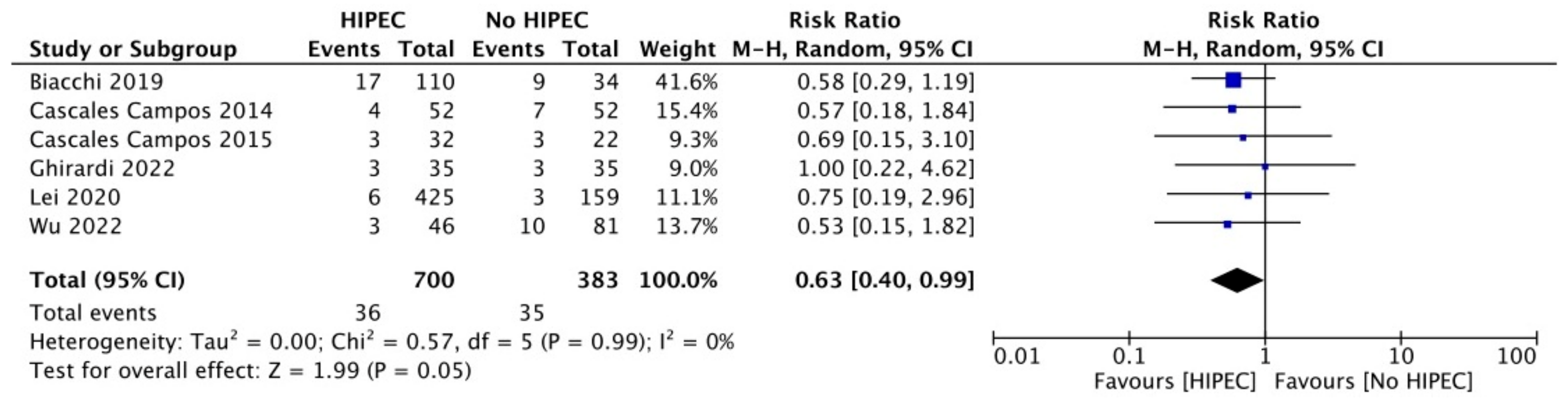
3.4. Surgical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://gco.iarc.fr/today/data/factsheets/cancers/25-Ovary-fact-sheet.pdf (accessed on 1 May 2023).
- Available online: https://seer.cancer.gov/statfacts/ (accessed on 1 May 2023).
- Zeppernick, F.; Meinhold-Heerlein, I. The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer. Arch. Gynecol. Obstet. 2014, 290, 839–842. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Baek, M.H.; Park, E.Y.; Ha, H.I.; Park, S.Y.; Lim, M.C.; Fotopoulou, C.; Bristow, R.E. Secondary Cytoreductive Surgery in Platinum-Sensitive Recurrent Ovarian Cancer: A Meta-Analysis. J. Clin. Oncol. 2022, 40, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Sehouli, J.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Mosgaard, B.J.; Selle, F.; Guyon, F.; et al. DESKTOP III Investigators. Randomized Trial of Cytoreductive Surgery for Relapsed Ovarian Cancer. N. Engl. J. Med. 2021, 385, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Zhu, J.; Feng, Y.; Tu, D.; Zhang, Y.; Zhang, P.; Jia, H.; Huang, X.; Cai, Y.; Yin, S.; et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): A multicentre, open-label, randomised, phase 3 trial. Lancet. Oncol. 2021, 22, 439–449. [Google Scholar] [CrossRef] [PubMed]
- De Bree, E.; Michelakis, D.; Anagnostopoulou, E. The current role of secondary cytoreductive surgery for recurrent ovarian cancer. Front. Oncol. 2022, 12, 1029976. [Google Scholar] [CrossRef] [PubMed]
- Benoit, L.; Koual, M.; Le Frère-Belda, M.A.; Zerbib, J.; Fournier, L.; Nguyen-Xuan, H.T.; Delanoy, N.; Bentivegna, E.; Bats, A.S.; Azaïs, H. Risks and benefits of systematic lymphadenectomy during interval debulking surgery for advanced high grade serous ovarian cancer. Eur. J. Surg. Oncol. 2022, 48, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.T.; Serra, A.; Llueca, M.; Llueca, A. Surgery in Recurrent Ovarian Cancer: A Meta-Analysis. Cancers 2023, 15, 3470. [Google Scholar] [CrossRef] [PubMed]
- Koole, S.; van Stein, R.; Sikorska, K.; Barton, D.; Perrin, L.; Brennan, D.; Zivanovic, O.; Mosgaard, B.J.; Fagotti, A.; Colombo, P.E.; et al. OVHIPEC-2 Steering Committee and the Dutch OVHIPEC group. Primary cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC) for FIGO stage III epithelial ovarian cancer: OVHIPEC-2, a phase III randomized clinical trial. Int. J. Gynecol. Cancer. 2020, 30, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; Van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Backes, F.J.; Bak-kum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.M.; Chitiyo, V.C.; Cristea, M.; et al. NCCN Guidelines® Insights: Ovarian Cancer, Version 3.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 972–980. [Google Scholar] [CrossRef]
- Spiliotis, J.; Halkia, E.; Lianos, E.; Kalantzi, N.; Grivas, A.; Efstathiou, E.; Giassas, S. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: A prospective randomized phase III study. Ann. Surg. Oncol. 2015, 22, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Reuss, A.; Sehouli, J.; Chiva, L.; du Bois, A. Brief Report About the Role of Hyperthermic Intraperitoneal Chemotherapy in a Prospective Randomized Phase 3 Study in Recurrent Ovarian Cancer from Spiliotis et al. Int. J. Gynecol. Cancer. 2017, 27, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Cho, J.; Lee, E.J.; Park, S.; Park, S.J.; Seol, A.; Lee, N.; Yim, G.W.; Lee, M.; Lim, W.; et al. Selection of patients with ovarian cancer who may show survival benefit from hyperthermic intraperitoneal chemotherapy: A systematic review and meta-analysis. Medicine 2019, 98, e18355. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Mendivil, A.A.; Rettenmaier, M.A.; Abaid, L.N.; Brown, J.V., 3rd; Mori, K.M.; Lopez, K.L.; Goldstein, B.H. Consolidation hyperthermic intraperitoneal chemotherapy for the treatment of advanced stage ovarian carcinoma: A 3 year experience. Cancer Chemother. Pharmacol. 2017, 80, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Zivanovic, O.; Chi, D.S.; Zhou, Q.; Iasonos, A.; Konner, J.A.; Makker, V.; Grisham, R.N.; Brown, A.K.; Nerenstone, S.; Diaz, J.P.; et al. Secondary Cytoreduction and Carboplatin Hyperthermic Intraperitoneal Chemotherapy for Platinum-Sensitive Recurrent Ovarian Cancer: An MSK Team Ovary Phase II Study. J. Clin. Oncol. 2021, 39, 2594–2604. [Google Scholar] [CrossRef] [PubMed]
- Cascales-Campos, P.A.; Gil, J.; Gil, E.; Feliciangeli, E.; González-Gil, A.; Parrilla, J.J.; Parrilla, P. Treatment of microscopic disease with hyperthermic intraoperative intraperitoneal chemotherapy after complete cytoreduction improves disease-free survival in patients with stage IIIC/IV ovarian cancer. Ann. Surg. Oncol. 2014, 21, 2383–2389. [Google Scholar] [CrossRef]
- Cascales-Campos, P.A.; Gil, J.; Feliciangeli, E.; Gil, E.; González-Gil, A.; López, V.; Ruiz-Pardo, J.; Nieto, A.; Parrilla, J.J.; Parrilla, P. The role of hyperthermic intraperitoneal chemotherapy using paclitaxel in platinum-sensitive recurrent epithelial ovarian cancer patients with microscopic residual disease after cytoreduction. Ann. Surg. Oncol. 2015, 22, 987–993. [Google Scholar] [CrossRef]
- Ghirardi, V.; De Felice, F.; D’Indinosante, M.; Bernardini, F.; Giudice, M.T.; Fagotti, A.; Scambia, G. Hyperthermic intraperitoneal chemotherapy (HIPEC) after primary debulking surgery in advanced epithelial ovarian cancer: Is BRCA mutational status making the difference? Cancer Treat. Res. Commun. 2022, 31, 100518. [Google Scholar] [CrossRef]
- Safra, T.; Grisaru, D.; Inbar, M.; Abu-Abeid, S.; Dayan, D.; Matceyevsky, D.; Weizman, A.; Klausner, J.M. Cytoreduction surgery with hyperthermic intraperitoneal chemotherapy in recurrent ovarian cancer improves progression-free survival, especially in BRCA-positive patients—A case-control study. J. Surg. Oncol. 2014, 110, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Biacchi, D.; Accarpio, F.; Ansaloni, L.; Macrì, A.; Ciardi, A.; Federici, O.; Spagnoli, A.; Cavaliere, D.; Vaira, M.; Sapienza, P.; et al. Upfront debulking surgery versus interval debulking surgery for advanced tubo-ovarian high-grade serous carcinoma and diffuse peritoneal metastases treated with peritonectomy procedures plus HIPEC. J. Surg. Oncol. 2019, 120, 1208–1219. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wei, L.; Li, R.; Jing, S.; Jia, L.; Li, Y.; Wang, Y.; Zhu, Y. Dense hyperthermic intraperitoneal chemotherapy with cisplatin in patients with stage III serous epithelial ovarian cancer: A retrospective study. BMC Cancer 2021, 21, 738. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Costantini, B.; Petrillo, M.; Vizzielli, G.; Fanfani, F.; Margariti, P.A.; Turco, L.C.; Piovano, E.; Scambia, G. Cytoreductive surgery plus HIPEC in platinum-sensitive recurrent ovarian cancer patients: A case-control study on survival in patients with two year follow-up. Gynecol. Oncol. 2012, 127, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.C.; Chang, S.J.; Park, B.; Yoo, H.J.; Yoo, C.W.; Nam, B.H.; Park, S.Y. HIPEC for Ovarian Cancer Collaborators. Survival After Hyperthermic Intraperitoneal Chemotherapy and Primary or Interval Cytoreductive Surgery in Ovarian Cancer: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wang, Y.; Wang, J.; Wang, K.; Tian, J.; Zhao, Y.; Chen, L.; Wang, J.; Luo, J.; Jia, M.; et al. Chinese Peritoneal Oncology Study Group (Gynecologic Oncology Study Group). Evaluation of Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Stage III Epithelial Ovarian Cancer. JAMA Netw. Open. 2020, 3, e2013940. [Google Scholar] [CrossRef] [PubMed]
- Ceresoli, M.; Verrengia, A.; Montori, G.; Busci, L.; Coccolini, F.; Ansaloni, L.; Frigerio, L. Effect of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on relapse pattern in primary epithelial ovarian cancer: A propensity score based case-control study. J. Gynecol. Oncol. 2018, 29, e53. [Google Scholar] [CrossRef]
- Batista, T.P.; Carneiro, V.C.G.; Tancredi, R.; Badiglian Filho, L.; Rangel, R.L.C.; Lopes, A.; Sarmento, B.J.Q.; Leão, C.S. A phase 2 trial of short-course Hyperthermic IntraPeritoneal Chemotherapy (HIPEC) at interval cytoreductive surgery (iCRS) for advanced ovarian cancer. Rev. Col. Bras. Cir. 2022, 49, e20223135. [Google Scholar] [CrossRef]
- Marrelli, D.; Petrioli, R.; Cassetti, D.; D’Ignazio, A.; Marsili, S.; Mazzei, M.A.; Lazzi, S.; Roviello, F. A novel treatment protocol with 6 cycles of neoadjuvant chemotherapy followed by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in stage III primary ovarian cancer. Surg. Oncol. 2021, 37, 101523. [Google Scholar] [CrossRef]
- Wu, M.F.; Liang, J.X.; Li, H.; Ye, Y.F.; Liang, W.F.; Wang, L.J.; Zhang, B.Z.; Chen, Q.; Lin, Z.Q.; Li, J. Effects of neoadjuvant hyperthermic intraperitoneal chemotherapy on chemotherapy response score and recurrence in high-grade serous ovarian cancer patients with advanced disease: A multicentre retrospective cohort study. BJOG 2022, 129 (Suppl. S2), 5–13. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, G.; Ferreira, F.O.; Mantoan, H.; da Costa, A.A.; Faloppa, C.C.; Kumagai, L.Y.; de Mello, C.A.; Takahashi, R.M.; Nakagawa, W.T.; Aguiar, S., Jr.; et al. Hyperthermic Intraperitoneal Chemotherapy after Secondary Cytoreduction in Epithelial Ovarian Cancer: A Single-center Comparative Analysis. Ann. Surg. Oncol. 2016, 23, 1294–1301. [Google Scholar] [CrossRef]
- Antonio, C.C.P.; Alida, G.G.; Elena, G.G.; Rocío, G.S.; Jerónimo, M.G.; Luis, A.R.J.; Aníbal, N.D.; Francisco, B.V.; Jesús, G.R.Á.; Pablo, R.R.; et al. Cytoreductive Surgery With or Without HIPEC After Neoadjuvant Chemotherapy in Ovarian Cancer: A Phase 3 Clinical Trial. Ann. Surg. Oncol. 2022, 29, 2617–2625. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Casares, F.C.; Rufián, S.; Rubio, M.J.; Díaz, C.J.; Díaz, R.; Casado, A.; Arjona, A.; Muñoz-Villanueva, M.C.; Muntané, J. The role of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) in the treatment of peritoneal carcinomatosis in recurrent ovarian cancer. Clin. Transl. Oncol. 2009, 11, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.C.; Chang, S.J.; Yoo, J.H.; Nam, B.H.; Bristow, R.; Park, S.Y. Randomized trial of hyperthermic intraperitoneal chemotherapy (HIPEC) in women with primary advanced peritoneal, ovarian, and tubal cancer. J. Clin. Oncol. 2017, 35 (Suppl. S15), 5520. [Google Scholar] [CrossRef]
- Angeloni, A.; De Vito, C.; Farina, A.; Terracciano, D.; Cennamo, M.; Passerini, R.; Bottari, F.; Schirinzi, A.; Vettori, R.; Steffan, A.; et al. New Analytical Approach for the Alignment of Different HE4 Automated Immunometric Systems: An Italian Multicentric Study. J. Clin. Med. 2022, 11, 1994. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.; Burgess, J.T.; O’Byrne, K.; Richard, D.J.; Bolderson, E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell. Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, A.; Trillsch, F.; Mahner, S.; Heitz, F.; Harter, P. Management of borderline ovarian tumors. Ann. Oncol. 2016, 27 (Suppl. S1), i20–i22. [Google Scholar] [CrossRef] [PubMed]
- Filis, P.; Mauri, D.; Markozannes, G.; Tolia, M.; Filis, N.; Tsilidis, K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: A systematic review and meta-analysis of randomized trials. ESMO Open. 2022, 7, 100586. [Google Scholar] [CrossRef]
- Ceelen, W.; Demuytere, J.; de Hingh, I. Hyperthermic Intraperitoneal Chemotherapy: A Critical Review. Cancers 2021, 13, 3114. [Google Scholar] [CrossRef]
- Classe, J.-M.; Meeus, P.; Leblanc, E.; Wernert, R.; Quenet, F.; Marchal, F.; Houvenaeghel, G.; Bats, S.-S.; Ferron, G.; Brigand, C.; et al. Hyperthermic intraperitoneal chemotherapy in platinum-sensitive relapsed epithelial ovarian cancer: The CHIPOR randomized phase III trial. J. Clin. Oncol. 2023, 41 (Suppl. S16), 5510. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Chan, C.Y.; Li, H.; Wu, M.F.; Liu, C.H.; Lu, H.W.; Lin, Z.Q.; Li, J. A Dose-Finding Trial for Hyperthermic Intraperitoneal Cisplatin in Gynecological Cancer Patients Receiving Hyperthermic Intraperitoneal Chemotherapy. Front. Oncol. 2021, 11, 616264. [Google Scholar] [CrossRef]
- Benoit, L.; Cheynel, N.; Ortega-Deballon, P.; Giacomo, G.D.; Chauffert, B.; Rat, P. Closed hyperthermic intraperitoneal chemotherapy with open abdomen: A novel technique to reduce exposure of the surgical team to chemotherapy drugs. Ann. Surg. Oncol. 2008, 15, 542–546. [Google Scholar] [CrossRef]
- Durán-Martínez, M.; Gómez-Dueñas, G.; Rodriguez-Ortíz, L.; Sanchez-Hidalgo, J.M.; Suárez, A.G.; Casado-Adam, Á.; Rufián-Peña, S.; Andujar, B.R.; Valenzuela-Molina, F.; Vázquez-Borrego, M.C.; et al. Learning curve for minimal invasive cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) procedures. Langenbecks Arch. Surg. 2023, 408, 146. [Google Scholar] [CrossRef]
- Aust, S.; Schwameis, R.; Gagic, T.; Müllauer, L.; Langthaler, E.; Prager, G.; Grech, C.; Reinthaller, A.; Krainer, M.; Pils, D.; et al. Precision Medicine Tumor Boards: Clinical Applicability of Personalized Treatment Concepts in Ovarian Cancer. Cancers 2020, 12, 548. [Google Scholar] [CrossRef] [PubMed]





| Author | Year | Median OS (Months) | Median PFS (Months) | ||
|---|---|---|---|---|---|
| HIPEC | Controls | HIPEC | Controls | ||
| Primary Debulking Surgery | |||||
| Lei [28] | 2020 | 49.8 | 34.0 | NR | NR |
| Lim [27] (PDS group) | 2021 | 71.3 | NR | 23.9 | 29.7 |
| Ghirardi [22] | 2022 | NR | NR | NR | NR |
| BRCA-wild type | NR | 62.0 | 26.6 | 19.4 | |
| BRCA-mutated | NR | NR | 26.7 | 38.0 | |
| Interval Debulking Surgery | |||||
| Mendivil [18] | 2017 | 33.8 | 33.6 | 25.1 | 20.0 |
| Ceresoli [29] | 2018 | NR | 32.5 | 14 | 13.2 |
| Van Driel [12] | 2018 | 45.7 | 33.9 | 14.2 | 10.7 |
| Cascales Campos [20] | 2014 | 52 | 45 | 18 | 12 |
| Batista [30] | 2021 | NR | NR | 18.1 | NR |
| Marrelli [31] | 2021 | 53 | 23 | 22 | NR |
| Lim [27] (IDS group) | 2022 | 61.8 | 48.2 | 17.4 | 15.4 |
| Wu [32] | 2022 | 51 | 44 | 22 | 16 |
| Recurrent Ovarian Cancer | |||||
| Spiliotis [14] | 2014 | NR | NR | NR | NR |
| Platinum sensitive disease | 26.8 | 15.2 | NR | NR | |
| Platinum resistance disease | 26.6 | 10.2 | NR | NR | |
| Cascales Campos [21] | 2015 | NR | NR | 21 | 22 |
| Baiocchi [33] | 2016 | 58.3 | 59.3 | 15.8 | 18.6 |
| Zivanovic [19] | 2021 | 52.5 | 59.7 | 12.3 | 15.7 |
| Author | Country | Year | Recruitment Period | EOC Stage (FIGO) | HIPEC Group, No. (%) | Control Group, No. (%) |
|---|---|---|---|---|---|---|
| Ghirardi [22] | Italy | 2022 | 2010–2015 | IIIB-IV | 35 (50%) | 35 (50%) |
| Lei [28] | China | 2020 | 2010–2017 | III | 425 (73.46%) | 159 (26.54%) |
| Lim [27] (subgroup PDS) | Republic of Korea | 2022 | 2010–2016 | III–IV | 58 (54.2%) | 49 (45.8%) |
| Cascales Campos [20] (subgroup PDS) | Spain | 2014 | 1998–2011 | IIIC-IV | 23 (44%) | 20 (57%) |
| Author | Year | HIPEC Protocol | Type of Tumor: Serous, No. (%) | Type of Tumor: Others, No. (%) | BRCA Mutations, No. (%) | No BRCA Mutations (HIPEC-No HIPEC) | No Tested BRCA Mutations (HIPEC-No HIPEC) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIPEC Drug | Temp (°C) | Duration (min) | HIPEC | Controls | HIPEC | Controls | HIPEC | Controls | ||||
| Ghirardi [22] | 2022 | Cisplatin 75 mg/m2 | 41.5 | 60 | NR | NR | NR | NR | 15/35 (42.9%) | 17/35 (48.6%) | 20/35 (57.1%)–18/35 (51.4%) | NR |
| Lei [28] | 2020 | Cisplatin 50 mg/m2 | 43 | 60 | 419/425 (98.6%) | 156/159 (98.1%) | 6/425 (1.4%) | 3/159 (1.8%) | NR | NR | NR | NR |
| Lim [27] (PDS subgroup) | 2022 | Cisplatin 75mg/m2 | 41.5 | 90 | 53/58 (91.4%) | 41/49 (83.7%) | 5/58 (8.6%) | 8/49 (16.3%) | NR | NR | NR | NR |
| Cascales Campos [20] (PDS subgroup) | 2014 | Cisplatin 75 mg/m2 | 42–43 | >60 | NR | NR | NR | NR | NR | NR | NR | NR |
| Author | Country | Year | Recruitment Period | EOC Stage (FIGO) | HIPEC Group, No. (%) | Control Group, No. (%) |
|---|---|---|---|---|---|---|
| Batista [30] | Portugal | 2021 | 2015−2019 | IIIB–IV | 15 | 0 |
| Cascales Campos [20] (IDS subgroup) | Spain | 2014 | 1998−2011 | IIIC–IV | 29 (56%) | 15 (45%) |
| Ceresoli [29] | Italy | 2018 | 2010−2016 | IIIC–IV | 28 (50%) | 28 (50%) |
| Lim [27] (IDS subgroup) | Republic of Korea | 2022 | 2010−2016 | III−IV | 34 (44%) | 43 (66%) |
| Marrelli [31] | Italy | 2021 | 2007−2014 | III | 46 (85%) | 8 (15%) |
| Mendivil [18] | Germany | 2017 | 2012−2015 | IIIA–IIIB–IIIC–IV | 69 (50%) | 69 (50%) |
| Wu [32] | China | 2022 | 2012−2020 | IIIC–IV | 46 (36.2%) | 81 (63.8%) |
| Van Driel [12] | The Netherlands | 2018 | 2007−2016 | III | 122 (49.8%) | 123 (50.2%) |
| Author | Year | HIPEC Protocol | Type of Tumor: Serous, No. (%) | Type of Tumor: Others No. (%) | BRCA Mutations, No. (%) | No BRCA Mutations (HIPEC-No HIPEC) | No Tested BRCA Mutations (HIPEC-No HIPEC) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIPEC Drug | Temp (°C) | Duration (min) | HIPEC | Controls | HIPEC | Controls | HIPEC | Controls | ||||
| Batista [30] | 2021 | Cisplatin (25 mg/L)—cisplatin + doxorubicin (15 mg/L) | 41–43 | 30 | 12/15 (80%) | 0 | 3/15 (20%) | 0 | 4/15 (26%) | 0 | NR | NR |
| Cascales Campos [20] (IDS subgroup) | 2014 | Cisplatin 75 mg/m2 | 42–43 | >60 | NR | NR | NR | NR | NR | NR | NR | NR |
| Ceresoli [29] | 2018 | Cisplatin 100 mg/m2—paclitaxel 175 mg/m2 | 41.5 | 90 | 25/28 (89.3%) | 27/28 (96.4%) | 3/28 (10.7%) | 1/28 (3.6%) | NR | NR | NR | NR |
| Lim (IDS subgroup) [27] | 2022 | Cisplatin 75 mg/m2 | 41.5 | 90 | 32/34 (94.1%) | 38/43 (88.4%) | 2/34 (5.9%) | 7/43 (11,6%) | NR | NR | NR | NR |
| Marrelli [31] | 2021 | Mitomycin C 25 mg/m2—cisplatin 100 mg/m | 41–42 | 60 | 37/46 (80%) | NR | 9/46 (20%) | NR | NR | NR | NR | NR |
| Mendivil [18] | 2017 | Carboplatin AUC10 | 41.5 | 90 | 48/69 (69.6%) | 45/69 (65.2%) | 21/69 (30.4%) | 24/69 (34.8%) | NR | NR | NR | NR |
| Wu [32] | 2022 | Cisplatin 70–80 mg/m2 | 43 | 60 | 46/46 (100%) | 81/81 (100%) | 0/46 (0%) | 0/81 (0%) | 8/46 (17.4%) | 17/81 (21%) | 19/46 (41.3%)–45/81 (55.6%) | 19/46 (41.3%)–19/81 (23.5%) |
| Van Driel [12] | 2018 | Cisplatin 100 mg/m2 | 40 | 90 | 116/122 (95%) | 109/123 (88.6%) | 6/122 (5%) | 14/123 (11.4%) | NR | NR | NR | NR |
| Author | Country | Year | Recruitment Period | EOC Stage (FIGO) | HIPEC Group, No. (%) | Control Group, No. (%) |
|---|---|---|---|---|---|---|
| Spiliotis [14] | ||||||
| Platinum sensitive disease | Greece | 2014 | 2006–2013 | IIIC–IV | 38 (51%) | 36 (49%) |
| Platinum resistant disease | Greece | 2014 | 2006–2013 | IIIC–IV | 22 (47.8%) | 24 (52.2%) |
| Cascales Campos [21] | Spain | 2015 | 2001–2012 | I–IV | 32 (59%) | 22 (41%) |
| Baiocchi [33] | Brazil | 2016 | 2000–2014 | I–IV | 29 (36.7%) | 50 (63.3%) |
| Zivanovic [19] | Germany | 2021 | 2014–2019 | I–IV | 49 (50%) | 49 (50%) |
| Author | Year | HIPEC Protocol | Type of Tumor: Serous, No. (%) | Type of Tumor: Others No. (%) | ||||
|---|---|---|---|---|---|---|---|---|
| HIPEC Drug | Temp (°C) | Duration (min) | HIPEC | Controls | HIPEC | Controls | ||
| Spiliotis [14] | ||||||||
| Platinum sensitive disease | 2014 | Cisplatin 100 mg/m2 + paclitaxel 175 mg/m2 | 42.5 | 60 | NR | NR | NR | NR |
| Platinum resistant disease | 2014 | Doxorubicin 35 mg/m2 + paclitaxel 175 mg/m2 | 42.5 | 60 | NR | NR | NR | NR |
| Cascales Campos [21] | 2015 | Paclitaxel 60 mg/m2 | 42 | NR | NR | NR | NR | NR |
| Baiocchi [33] | 2016 | Mitomycin C 10 mg/m2—Cisplatin 50 mg/m2—Doxorubicin | 41–42 | 90 | 18/29 (62%) | 38/50 (76%) | 11/29 (38%) | 12/50 (24%) |
| Zivanovic [19] | 2021 | Carboplatin 800 mg/m2 | 41–43 | 90 | 47/49 (96%) | NR | 48/49 (98%) | NR |
| Author | Year | No. (%) No BRCA Mutations (HIPEC-No HIPEC) | No. (%) BRCA Mutations (HIPEC-No HIPEC) |
|---|---|---|---|
| Cascales Campos [20] | 2014 | NR | NR |
| Spiliotis [14] | 2015 | NR | NR |
| Platinum sensitive disease | 2014 | NR | NR |
| Platinum resistant disease | 2014 | NR | NR |
| Cascales Campos [21] | 2015 | NR | NR |
| Baiocchi [33] | 2016 | NR | NR |
| Zivanovic [19] | 2021 | 39/49 (80%)–38/49 (78%) | 10/49 (20%)–11/49 (22%) |
| Author | Year | ≥Grade 3 Complications, No. (%) | Restricted Performance Status (ECOG ≥ 1), No. (%) | ||
|---|---|---|---|---|---|
| HIPEC | Controls | HIPEC | Controls | ||
| Primary Debulking Surgery | |||||
| Lei [28] | 2020 | NR | NR | NR | NR |
| Lim (PDS group) [27] | 2021 | NR | NR | NR | NR |
| Ghirardi [17] | 2022 | 4/35 (11.42%) | 1/35 (2.8%) | 3/35 (8.6%) | 3/35 (8.6%) |
| BRCA-wild type | NR | NR | NR | NR | |
| BRCA-mutated | NR | NR | NR | NR | |
| Interval Debulking Surgery | |||||
| Mendivil [18] | 2017 | 0/69 (0%) | NR | NR | NR |
| Ceresoli [29] | 2018 | 7/28 (25%) | 5/28 (18%) | NR | NR |
| Van Driel [12] | 2018 | 51/118 (43%) | 43/122 (35%) | NR | NR |
| Cascales Campos [20] | 2014 | NR | NR | NR | NR |
| Batista [30] | 2021 | 3/15 (20%) | 0 | 12/15 (80%) | 0 |
| Marrelli [31] | 2021 | 13/46 (28%) | NR | NR | NR |
| Lim (group ICS) [27] | 2022 | NR | NR | NR | NR |
| Wu [32] | 2022 | 12/46 (26%) | 16/81 (20%) | 3/46 (6.5%) | 10/81 (12%) |
| Recurrent Ovarian Cancer | |||||
| Spiliotis [14] | 2014 | NR | NR | NR | NR |
| Platinum sensitive disease | NR | NR | NR | NR | |
| Platinum resistant disease | NR | NR | NR | NR | |
| Cascales Campos [21] | 2015 | NR | NR | NR | NR |
| Baiocchi [33] | 2016 | 10/29 (34.5%) | 5/50 (10.6%) | NR | NR |
| Zivanovic [19] | 2021 | 12/49 (24%) | 10/49 (20%) | NR | NR |
| Studies | Participants | RR (95% CI) | I2 | |
|---|---|---|---|---|
| Anemia | 5 [12,13,27,28,34] | 1100 | 1.02 (0.98 to 1.05) | 5% |
| Bowel related complications | 5 [12,21,24,25,28] | 665 | 1.14 (0.66 to 1.99) | 70% |
| Dyspnea | 5 [12,21,27,28,34] | 1111 | 1.01 (0.67 to 1.51) | 0% |
| Renal complications | 3 [27,28,34] | 802 | 1.28 (1.03 to 1.59) | 0% |
| Haemorrhage | 5 [19,21,27,28,34] | 1052 | 0.62 (0.18 to 2.08) | 31% |
| Author | Year | Bowel related Complications (HIPEC-No HIPEC) | Anemia (HIPEC-No HIPEC) | Dyspnea (HIPEC-No HIPEC) | Hemorrhage (HIPEC-No HIPEC) | Renal Complications (HIPEC-No HIPEC) | Cardiac Complications (HIPEC-No HIPEC |
|---|---|---|---|---|---|---|---|
| Primary Debulking Surgery | |||||||
| Lei [28] | 2020 | 2/425 (0.4%)–6/159(3.7%) | 400/425 (94%)–142/159(89%) | 3/425 (0.6%)–4/159 (2.5%) | 2/425 (0.4%)–5/159(3.1%) | 35/425 (8.2%)–7/159 (4.4%) | 2/425 (0.4%)–3/159 (1.9%) |
| Lim [27] | 2021 | 68/92 (73%)–82/92 (89%) | 92/92 (100%)–92/92 (100%) | 63/92 (68%)–69/92(75%) | 3/92 (3%)–9/92(10%) | 44/92 (47%)–63/92 (68%) | 46/92 (50%)–48/92 (52%) |
| Ghirardi [22] | 2022 | NR | NR | NR | NR | NR | NR |
| Cascales Campos [20] | 2014 | NR | NR | NR | NR | NR | NR |
| Interval Debulking Surgery | |||||||
| Mendivil [18] | 2017 | NR | 22/69 (32%) | NR | 10/69 (14%) | NR | NR |
| Ceresoli [29] | 2018 | NR | NR | NR | NR | NR | NR |
| Van Driel [12] | 2018 | 52/122 (42%)–51/118 (43%) | 7/122 (6%)–5/118 (4%) | 13/122 (11%)–8/118 (7%) | 4/122 (3%)–2/118 (2%) | NR | 6/122 (5%)–8/118 (7%) |
| Cascales Campos [20] | 2014 | NR | NR | NR | NR | NR | NR |
| Batista [30] | 2021 | 2/15 (13%) | 5/15 (33%) | NR | 1/15 (6%) | NR | NR |
| Marrelli [31] | 2021 | NR | 10/46 (22%) | 6/46 (13%) | 2/46 (4%) | 2/46 (4%) | 1/46 (2%) |
| Lim [27] | 2022 | 68/92 (73%)–82/92 (89%) | 92/92 (100%)–92/92 (100%) | 63/92 (68%)–69/92 (75%) | 3/92 (3%)–9/92 (10%) | 44/92 (47%)–63/92 (68%) | 46/92 (50%)–48/92 (52%) |
| Wu [32] | 2022 | NR | NR | NR | NR | NR | NR |
| Recurrent Ovarian Cancer | |||||||
| Spiliotis [14] | 2014 | NR | NR | NR | NR | NR | NR |
| Platinum sensitive disease | NR | NR | NR | NR | NR | NR | |
| Platinum resistant disease | NR | NR | NR | NR | NR | NR | |
| Cascales Campos [21] | 2015 | 4/32 (12%)–2/22 (9%) | NR | 1/32 (3%)–0 | 1/32 (3%)–1/22 (5%) | NR | NR |
| Baiocchi [28] | 2016 | NR | NR | NR | NR | NR | NR |
| Zivanovic [13] | 2021 | 18/49 (37%)–32/49 (65%) | 7/49 (14.3%)–8/49 (16.3%) | NR | 1/49 (2%)–0 | NR | NR |
| Author | Year | Infections (HIPEC-No HIPEC) | Performance Status (ECOG) 0 (HIPEC-No HIPEC) | Performance Status (ECOG) 1 (HIPEC-No HIPEC) | Performance Status (ECOG) 2 (HIPEC-No HIPEC) | Performance Status (ECOG) 3 (HIPEC-No HIPEC) |
|---|---|---|---|---|---|---|
| Primary Debulking Surgery | ||||||
| Lei [28] | 2020 | 56/425 (13%)–27/159 (17%) | NR | 419/425 (98.6%)–156/159 (98.1%) | NR | 6/425 (1.4%)–3/159 (1.9%) |
| Lim [27] (Total group) | 2021 | 43/92 (47%)–47/92 (51%) | NR | NR | NR | NR |
| Ghirardi [22] | 2022 | NR | 32/35 (91.4%)–32/35 (91.4%) | 3/35 (8.6%)–3/35 (8.6%) | NR | NR |
| Cascales Campos [20] (Total group) | 2014 | NR | NR | 48/52 (92%)–28/35 (80%) | 4/52 (8%)–7/35 (20%) | NR |
| Interval Debulking Surgery | ||||||
| Mendivil [18] | 2017 | NR | NR | NR | NR | NR |
| Ceresoli [29] | 2018 | NR | NR | NR | NR | NR |
| Van Driel [12] | 2018 | 21/118 (18%)–14/122 (11%) | NR | NR | NR | NR |
| Cascales Campos [20] (Total group) | 2014 | NR | NR | 48/52 (92%)–28/35 (80%) | 4/52 (8%)–7/35 (20%) | NR |
| Batista [30] | 2021 | 3/15 (20%) | 3/15 (20%) | 9/15 (60%) | 3/15 (20%) | NR |
| Marrelli [31] | 2021 | NR | NR | NR | NR | NR |
| Lim [27] (Total group) | 2022 | 43/92 (47%)–47/92 (51%) | NR | NR | NR | NR |
| Wu [32] | 2022 | NR | NR | 3/46 (6.5%)–10/81 (12.4%) | 43/46 (93.5%)–71/81 (87.7%) | NR |
| Recurrent Ovarian Cancer | ||||||
| Spiliotis [14] | 2014 | NR | NR | NR | NR | NR |
| Platinum sensitive disease | NR | NR | NR | NR | NR | |
| Platinum resistant disease | NR | NR | NR | NR | NR | |
| Cascales Campos [21] | 2015 | 3/32 (9%)–1/22 (5%) | NR | 29/32 (91%)–19/22 (86%) | 3/32 (9%)–3/22 (14%) | NR |
| Baiocchi [33] | 2016 | NR | NR | NR | NR | NR |
| Zivanovic [19] | 2021 | 7/49 (14%)–3/49 (6%) | NR | NR | NR | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Corte, L.; Conte, C.; Palumbo, M.; Guerra, S.; Colacurci, D.; Riemma, G.; De Franciscis, P.; Giampaolino, P.; Fagotti, A.; Bifulco, G.; et al. Hyperthermic Intraperitoneal Chemotherapy (HIPEC): New Approaches and Controversies on the Treatment of Advanced Epithelial Ovarian Cancer—Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 7012. https://doi.org/10.3390/jcm12227012
Della Corte L, Conte C, Palumbo M, Guerra S, Colacurci D, Riemma G, De Franciscis P, Giampaolino P, Fagotti A, Bifulco G, et al. Hyperthermic Intraperitoneal Chemotherapy (HIPEC): New Approaches and Controversies on the Treatment of Advanced Epithelial Ovarian Cancer—Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(22):7012. https://doi.org/10.3390/jcm12227012
Chicago/Turabian StyleDella Corte, Luigi, Carmine Conte, Mario Palumbo, Serena Guerra, Dario Colacurci, Gaetano Riemma, Pasquale De Franciscis, Pierluigi Giampaolino, Anna Fagotti, Giuseppe Bifulco, and et al. 2023. "Hyperthermic Intraperitoneal Chemotherapy (HIPEC): New Approaches and Controversies on the Treatment of Advanced Epithelial Ovarian Cancer—Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 22: 7012. https://doi.org/10.3390/jcm12227012
APA StyleDella Corte, L., Conte, C., Palumbo, M., Guerra, S., Colacurci, D., Riemma, G., De Franciscis, P., Giampaolino, P., Fagotti, A., Bifulco, G., & Scambia, G. (2023). Hyperthermic Intraperitoneal Chemotherapy (HIPEC): New Approaches and Controversies on the Treatment of Advanced Epithelial Ovarian Cancer—Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(22), 7012. https://doi.org/10.3390/jcm12227012










