Time-Restricted Eating and Its Metabolic Benefits
Abstract
:1. Introduction
1.1. Intermittent Fasting Regimens
1.2. Cellular Responses to Fasting and Metabolic Switch
1.3. Effects of Time of Day on Obesity
1.4. Effects of TRE on Health
Obesity
2. Clinical Pearl: eTRE Carried Out to Match the Body’s Circadian Rhythm Is More Effective for Weight Loss
2.1. Diabetes
- Clinical Pearl: eTRE Helps Improve Insulin Resistance Independent of the Weight Loss Benefit
2.2. Nonalcoholic Fatty Liver Disease
- Clinical Pearl: TRE is a safe and efficacious method for improving fibrosis via weight-loss-dependent and weight-loss-independent mechanisms, including shifting metabolic processes away from hepatic lipogenesis, and for improving insulin resistance and metabolic syndrome.
2.3. Cardiovascular Diseases
- Clinical Pearl: TRE improved cardiometabolic risk factors by decreasing serum triglyceride, total cholesterol, and LDL cholesterol levels. TRE combined with calorie restriction is more efficacious for diastolic blood pressure control than calorie restriction.
2.4. Cancer
- Clinical Pearl: TRE has been shown to increase the activity of chemotherapeutic agents, such as tamoxifen and fulvestrant. Repeated fasting has been shown to reduce cell proliferation, cancer progression, and metastases by maintaining protein homeostasis and promoting mitochondrial biogenesis and autophagy.
2.5. Neurodegenerative Diseases
- Clinical Pearl: Changes in the metabolic pathway (from lipid synthesis and storage to the mobilization of fat through fatty acid oxidation and fatty acid-derived ketones) and weight loss were reported to improve mitochondrial function. They may help delay neurodegenerative disease and improve longevity.
2.6. Sleep
- Clinical Pearl: Most studies showed a negative or no impact on sleep with TRE.
2.7. Sarcopenia
- Clinical Pearl: TRE and resistance training appear to have a synergistic effect on preserving and improving lean muscle mass while also controlling blood glucose levels and reducing fat.
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADF | alternate-day fasting |
| eTRE | early TRE |
| HbA1c | hemoglobin A1c |
| MADF | modified alternate-day fasting |
| mTOR | mammalian target of rapamycin |
| NAFLD | nonalcoholic fatty liver disease |
| TRE | time-restricted eating |
| TREAT | Time-Restricted EAT |
References
- World Health Organization. World Obesity Day 2022–Accelerating Action to Stop Obesity. 2022. Available online: https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity (accessed on 19 June 2023).
- Rynders, C.A.; Thomas, E.A.; Zaman, A.; Pan, Z.; Catenacci, V.A.; Melanson, E.L. Effectiveness of Intermittent Fasting and Time-Restricted Feeding Compared to Continuous Energy Restriction for Weight Loss. Nutrients 2019, 11, 2442. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Lee, S.A.; Donahoo, W.T.; McLaren, C.; Manini, T.; Leeuwenburgh, C.; Pahor, M. The Effects of Time Restricted Feeding on Overweight, Older Adults: A Pilot Study. Nutrients 2019, 11, 1500. [Google Scholar] [CrossRef]
- Parr, E.B.; Devlin, B.L.; Lim, K.H.C.; Moresi, L.N.Z.; Geils, C.; Brennan, L.; Hawley, J.A. Time-Restricted Eating as a Nutrition Strategy for Individuals with Type 2 Diabetes: A Feasibility Study. Nutrients 2020, 12, 3228. [Google Scholar] [CrossRef]
- Anton, S.; Ezzati, A.; Witt, D.; McLaren, C.; Vial, P. The effects of intermittent fasting regimens in middle-age and older adults: Current state of evidence. Exp. Gerontol. 2021, 156, 111617. [Google Scholar] [CrossRef]
- Elortegui Pascual, P.; Rolands, M.R.; Eldridge, A.L.; Kassis, A.; Mainardi, F.; Lê, K.A.; Karagounis, L.G.; Gut, P.; Varady, K.A. A meta-analysis comparing the effectiveness of alternate day fasting, the 5:2 diet, and time-restricted eating for weight loss. Obesity 2023, 31 (Suppl. 1), 9–21. [Google Scholar] [CrossRef]
- Eshghinia, S.; Mohammadzadeh, F. The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J. Diabetes Metab. Disord. 2013, 12, 4. [Google Scholar] [CrossRef]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G., 3rd; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- Denton, D.; Kumar, S. Autophagy-dependent cell death. Cell Death Differ. 2019, 26, 605–616. [Google Scholar] [CrossRef] [PubMed]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef] [PubMed]
- Bonham, M.P.; Bonnell, E.K.; Huggins, C.E. Energy intake of shift workers compared to fixed day workers: A systematic review and meta-analysis. Chronobiol. Int. 2016, 33, 1086–1100. [Google Scholar] [CrossRef]
- Manoogian, E.N.; Zadourian, A.; Lo, H.C.; Gutierrez, N.R.; Shoghi, A.; Rosander, A.; Pazargadi, A.; Ormiston, C.K.; Wang, X.; Sui, J.; et al. Feasibility of time-restricted eating and impacts on cardiometabolic health in 24-h shift workers: The Healthy Heroes randomized control trial. Cell Metab. 2022, 34, 1442–1456.e7. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378.e3. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499. [Google Scholar] [CrossRef]
- Welton, S.; Minty, R.; O’Driscoll, T.; Willms, H.; Poirier, D.; Madden, S.; Kelly, L. Intermittent fasting and weight loss: Systematic review. Can. Fam. Physician 2020, 66, 117–125. [Google Scholar]
- Jamshed, H.; Steger, F.L.; Bryan, D.R.; Richman, J.S.; Warriner, A.H.; Hanick, C.J.; Martin, C.K.; Salvy, S.-L.; Peterson, C.M. Effectiveness of Early Time-Restricted Eating for Weight Loss, Fat Loss, and Cardiometabolic Health in Adults With Obesity: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 953–962. [Google Scholar] [CrossRef]
- O’Connor, S.G.; Boyd, P.; Bailey, C.P.; Nebeling, L.; Reedy, J.; Czajkowski, S.M.; Shams-White, M.M. A qualitative exploration of facilitators and barriers of adherence to time-restricted eating. Appetite 2022, 178, 106266. [Google Scholar] [CrossRef]
- Liu, J.; Yi, P.; Liu, F. The Effect of Early Time-Restricted Eating vs Later Time-Restricted Eating on Weight Loss and Metabolic Health. J. Clin. Endocrinol. Metab. 2023, 108, 1824–1834. [Google Scholar] [CrossRef]
- Che, T.; Yan, C.; Tian, D.; Zhang, X.; Liu, X.; Wu, Z. Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: A randomised controlled trial. Nutr. Metab. 2021, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Obermayer, A.; Tripolt, N.J.; Pferschy, P.N.; Kojzar, H.; Jacan, A.; Schauer, M.; Aziz, F.; Oulhaj, A.; Aberer, F.; Sourij, C.; et al. INTERmittent FASTing in people with insulin-treated type 2 diabetes mellitus—The INTERFAST-2 study protocol. Diabet Med. 2022, 39, e14813. [Google Scholar] [CrossRef] [PubMed]
- Memel, Z.N.; Wang, J.; Corey, K.E. Intermittent Fasting as a Treatment for Nonalcoholic Fatty Liver Disease: What Is the Evidence? Clin. Liver Dis. 2022, 19, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Kang, J.; Kim, S.H.; Chung, H.S.; Kim, Y.J.; Yu, J.M.; Cho, S.T.; Oh, C.-M.; Kim, T. Beneficial Effects of Time-Restricted Eating on Metabolic Diseases: A Systemic Review and Meta-Analysis. Nutrients 2020, 12, 1267. [Google Scholar] [CrossRef]
- Jahrami, H.A.; Faris, M.E.; Janahi, A.I.; Janahi, M.I.; Abdelrahim, D.N.; Madkour, M.I.; Sater, M.S.; Hassan, A.B.; Bahammam, A.S. Does four-week consecutive, dawn-to-sunset intermittent fasting during Ramadan affect cardiometabolic risk factors in healthy adults? A systematic review, meta-analysis, and meta-regression. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2273–2301. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Caffa, I.; Spagnolo, V.; Vernieri, C.; Valdemarin, F.; Becherini, P.; Wei, M.; Brandhorst, S.; Zucal, C.; Driehuis, E.; Ferrando, L.; et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 2020, 583, 620–624. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, S.; Ye, Y.; Yin, S.; Fan, J.; Xia, M. Intermittent Fasting Improves Cardiometabolic Risk Factors and Alters Gut Microbiota in Metabolic Syndrome Patients. J. Clin. Endocrinol. Metab. 2021, 106, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A. Cancer: Inflammation by remote control. Nature 2005, 435, 752–753. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Pacelli, F.Q.; Marcolin, G.; Bianco, A.; Paoli, A. Twelve Months of Time-restricted Eating and Resistance Training Improves Inflammatory Markers and Cardiometabolic Risk Factors. Med. Sci. Sports Exerc. 2021, 53, 2577–2585. [Google Scholar] [CrossRef]
- Roberts, N.T.; MacDonald, C.R.; Mohammadpour, H.; Antoch, M.P.; Repasky, E.A. Circadian Rhythm Disruption Increases Tumor Growth Rate and Accumulation of Myeloid-Derived Suppressor Cells. Adv. Biol. 2022, 6, e2200031. [Google Scholar] [CrossRef]
- Wegrzyn, L.R.; Tamimi, R.M.; Rosner, B.A.; Brown, S.B.; Stevens, R.G.; Eliassen, A.H.; Laden, F.; Willett, W.C.; Hankinson, S.E.; Schernhammer, E.S. Rotating Night-Shift Work and the Risk of Breast Cancer in the Nurses’ Health Studies. Am. J. Epidemiol. 2017, 186, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Kogevinas, M.; Espinosa, A.; Castelló, A.; Gómez-Acebo, I.; Guevara, M.; Martin, V.; Amiano, P.; Alguacil, J.; Peiro, R.; Moreno, V.; et al. Effect of mistimed eating patterns on breast and prostate cancer risk (MCC-Spain Study). Int. J. Cancer 2018, 143, 2380–2389. [Google Scholar] [CrossRef]
- Bartholomew, C.L.; Muhlestein, J.B.; Anderson, J.L.; May, H.T.; Knowlton, K.U.; Bair, T.L.; Le, V.T.; Bailey, B.W.; Horne, B.D. Association of periodic fasting lifestyles with survival and incident major adverse cardiovascular events in patients undergoing cardiac catheterization. Eur. J. Prev. Cardiol. 2022, 28, 1774–1781. [Google Scholar] [CrossRef]
- Ezzati, A.; Pak, V.M. The effects of time-restricted eating on sleep, cognitive decline, and Alzheimer’s disease. Exp. Gerontol. 2023, 171, 112033. [Google Scholar] [CrossRef] [PubMed]
- Zeb, F.; Wu, X.; Fatima, S.; Zaman, M.H.; Khan, S.A.; Safdar, M.; Alam, I.; Feng, Q. Time-restricted feeding regulates molecular mechanisms with involvement of circadian rhythm to prevent metabolic diseases. Nutrition 2021, 89, 111244. [Google Scholar] [CrossRef]
- He, M.; Wang, J.; Liang, Q.; Li, M.; Guo, H.; Wang, Y.; Deji, C.; Sui, J.; Wang, Y.W.; Liu, Y.; et al. Time-restricted eating with or without low-carbohydrate diet reduces visceral fat and improves metabolic syndrome: A randomized trial. Cell Rep. Med. 2022, 3, 100777. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, M.; Chen, W.; Liu, Z. The neuroprotective effects of intermittent fasting on brain aging and neurodegenerative diseases via regulating mitochondrial function. Free Radic. Biol. Med. 2022, 182, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Almeneessier, A.S.; BaHammam, A.S. How does diurnal intermittent fasting impact sleep, daytime sleepiness, and markers of the biological clock? Current insights. Nat. Sci. Sleep 2018, 10, 439–452. [Google Scholar] [CrossRef]
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Donahoo, W.T. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 2016, 24, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Sears, D.D. Sears, Metabolic Effects of Intermittent Fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawi, N.; Madkour, M.; Jahrami, H.; Salahat, D.; Alhasan, F.; BaHammam, A.; Faris, M.A.-I. Effect of diurnal intermittent fasting during Ramadan on ghrelin, leptin, melatonin, and cortisol levels among overweight and obese subjects: A prospective observational study. PLoS ONE 2020, 15, e0237922. [Google Scholar] [CrossRef]
- Steger, F.L.; Jamshed, H.; Bryan, D.R.; Richman, J.S.; Warriner, A.H.; Hanick, C.J.; Martin, C.K.; Salvy, S.; Peterson, C.M. Early time-restricted eating affects weight, metabolic health, mood, and sleep in adherent completers: A secondary analysis. Obesity 2023, 31, 96–107. [Google Scholar] [CrossRef]
- Zhao, D.; Guallar, E.; Woolf, T.B.; Martin, L.; Lehmann, H.; Coughlin, J.; Holzhauer, K.; Goheer, A.A.; McTigue, K.M.; Lent, M.R.; et al. Association of Eating and Sleeping Intervals With Weight Change Over Time: The Daily24 Cohort. J. Am. Heart Assoc. 2023, 12, e026484. [Google Scholar] [CrossRef]
- Kotarsky, C.J.; Johnson, N.R.; Mahoney, S.J.; Mitchell, S.L.; Schimek, R.L.; Stastny, S.N.; Hackney, K.J. Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiol. Rep. 2021, 9, e14868. [Google Scholar] [CrossRef]
- Dote-Montero, M.; Sanchez-Delgado, G.; Ravussin, E. Effects of Intermittent Fasting on Cardiometabolic Health: An Energy Metabolism Perspective. Nutrients 2022, 14, 489. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Traylor, D.A.; Gorissen, S.H.M.; Phillips, S.M. Perspective: Protein Requirements and Optimal Intakes in Aging: Are We Ready to Recommend More Than the Recommended Daily Allowance? Adv. Nutr. 2018, 9, 171–182. [Google Scholar] [CrossRef] [PubMed]
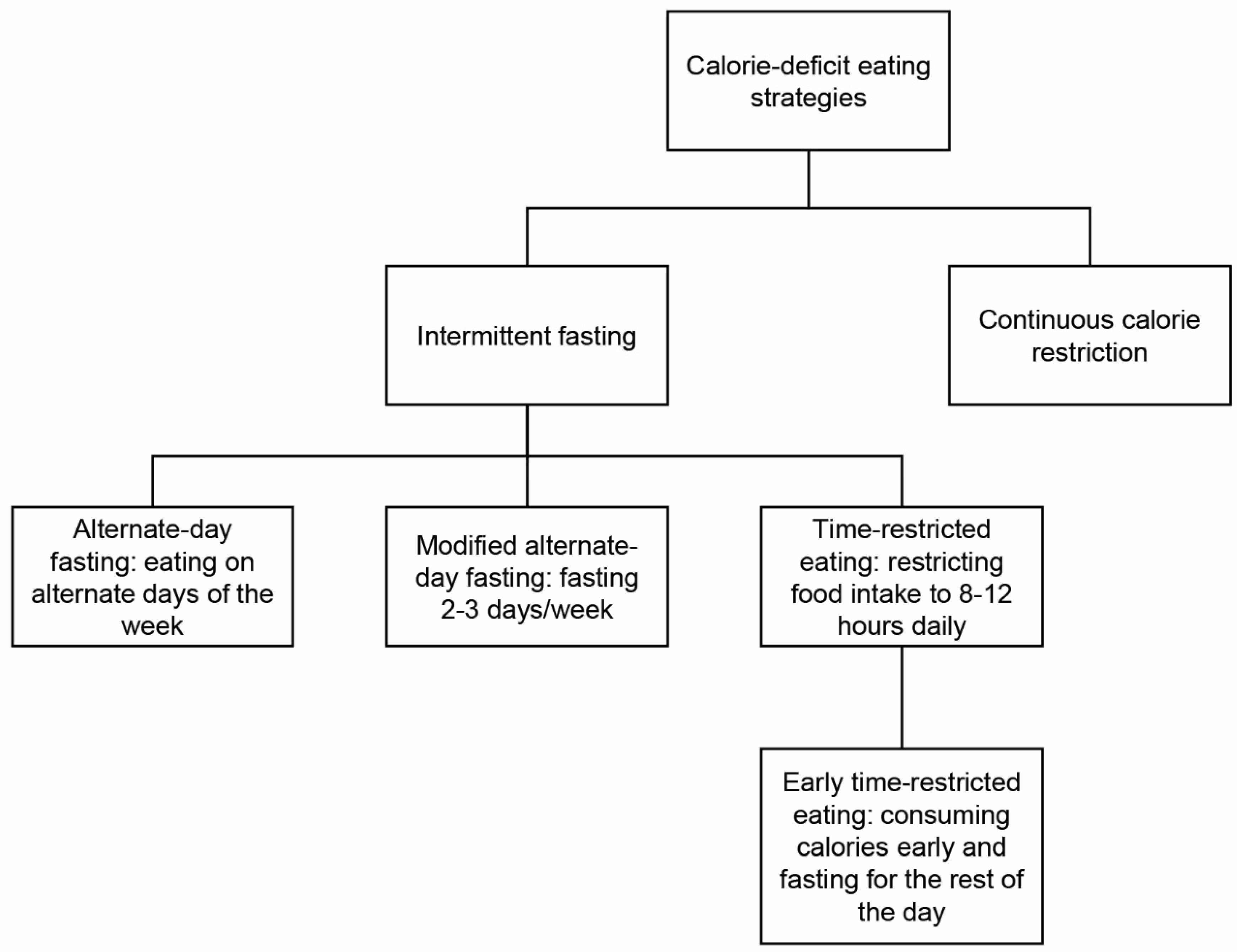
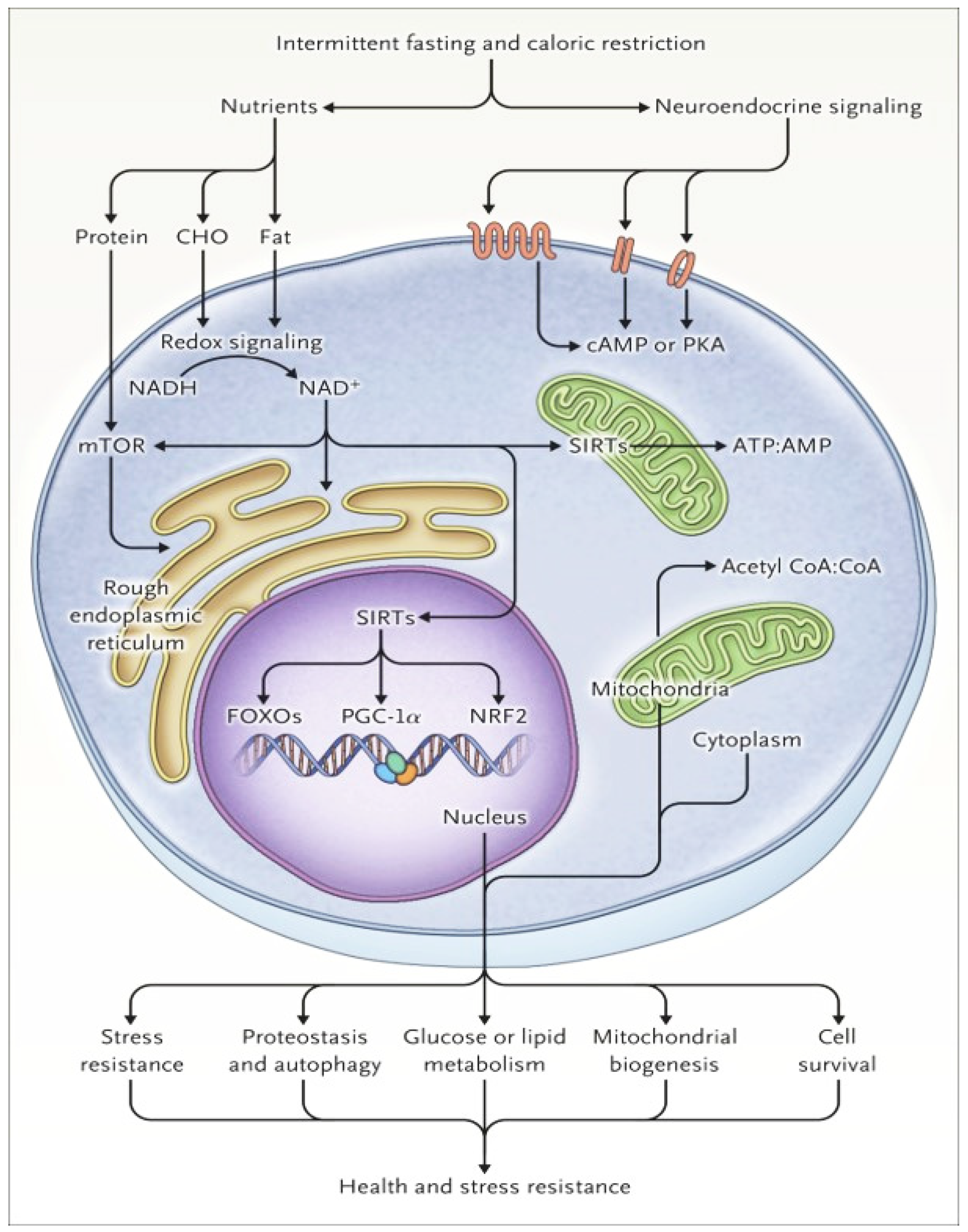
| Metabolic Condition | TRE Effects | Comments |
|---|---|---|
| Weight Loss | Promotes weight loss through reduced calorie intake within the restricted eating window. | The article mentions variable outcomes in different studies, suggesting the need for further investigation. |
| Diabetes (Prediabetes and Type 2) | Improves insulin resistance independent of weight loss. Enhances fatty acid mobilization, β oxidation, and ketone body production. Induces autophagy. | TRE shows promise in improving insulin sensitivity and glycemic control in prediabetes and type 2 diabetes patients. |
| NAFLD (Nonalcoholic Fatty Liver Disease) | Shifts metabolic processes away from hepatic lipogenesis. Improves insulin resistance and metabolic syndrome. | While human studies on TRE and NAFLD are limited, TRE appears to benefit liver health, potentially independent of weight loss. |
| Cardiovascular Health | May lower blood pressure and improve lipid profiles. Possible benefits for cardiometabolic risk factors. | The evidence suggests potential positive effects on cardiovascular health, but further research is required for definitive conclusions. |
| Cancer | Increases anticancer activity of certain chemotherapeutic agents. May inhibit tumor growth. | TRE, in combination with chemotherapy, has shown potential benefits in enhancing the efficacy of cancer treatment and inhibiting tumor growth. |
| Neurodegenerative Diseases | May improve cognitive function through circadian rhythm regulation and reduced neuroinflammation. | The relationship between TRE and neurodegenerative diseases is still evolving, necessitating more comprehensive research. |
| Sleep | Mixed results in studies, with some suggesting improvements in sleep quality. | The impact of TRE on sleep quality varies in different studies, requiring further investigation for a conclusive assessment. |
| Sarcopenia | May preserve lean muscle mass and improve body composition when combined with resistance training. | Combining TRE with resistance training shows potential for preserving lean muscle mass, especially in overweight and obese adults. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, S.; Persons, P.A.; Lorenzo, A.M.; Chaliki, S.S.; Bersoux, S. Time-Restricted Eating and Its Metabolic Benefits. J. Clin. Med. 2023, 12, 7007. https://doi.org/10.3390/jcm12227007
Mishra S, Persons PA, Lorenzo AM, Chaliki SS, Bersoux S. Time-Restricted Eating and Its Metabolic Benefits. Journal of Clinical Medicine. 2023; 12(22):7007. https://doi.org/10.3390/jcm12227007
Chicago/Turabian StyleMishra, Sneha, Patress A. Persons, Andrea M. Lorenzo, Swarna S. Chaliki, and Sophie Bersoux. 2023. "Time-Restricted Eating and Its Metabolic Benefits" Journal of Clinical Medicine 12, no. 22: 7007. https://doi.org/10.3390/jcm12227007
APA StyleMishra, S., Persons, P. A., Lorenzo, A. M., Chaliki, S. S., & Bersoux, S. (2023). Time-Restricted Eating and Its Metabolic Benefits. Journal of Clinical Medicine, 12(22), 7007. https://doi.org/10.3390/jcm12227007




