Navigating Challenges in the Endovascular Treatment of Asymptomatic Aortoiliac Aneurysms: A 10-Year Comparative Analysis
Abstract
1. Introduction
2. Materials and Methods
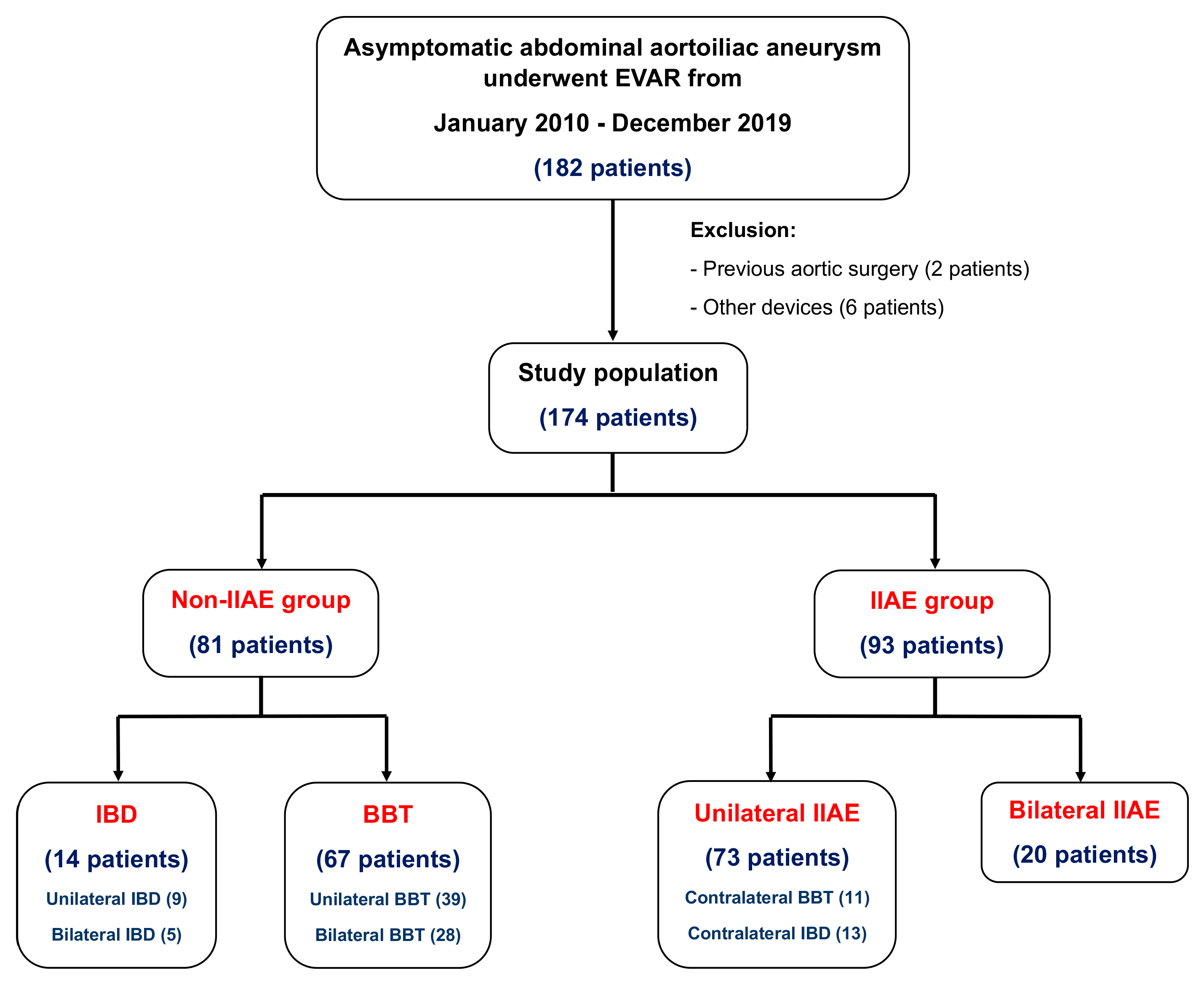
2.1. Patient Selection
- (1)
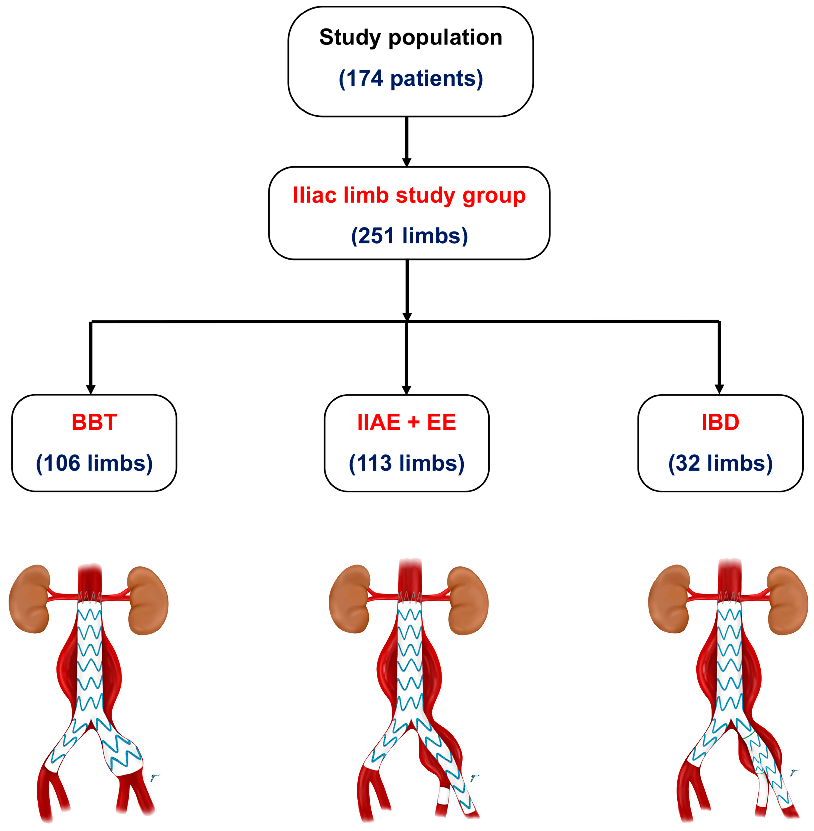
- Internal iliac artery embolization with a stent graft extension to the external iliac artery (IIAE + EE): this involved the use of coil embolization to block the hypogastric or internal iliac artery, followed by the deployment of a stent graft to the external iliac artery.
- (2)
- Bell-bottom technique (BBT): this technique used a flared-extension stent graft into the common iliac artery (a flared-extension iliac limb is defined as having a diameter ≥ 24 mm) without IIAE.
- (3)
- Iliac branch device (IBD): inn this approach, one or two iliac branch grafts were placed in the IIA to maintain blood flow.
2.2. Operative Techniques
2.3. Follow-Up Protocol
2.4. Outcomes
2.5. Statistical Analysis
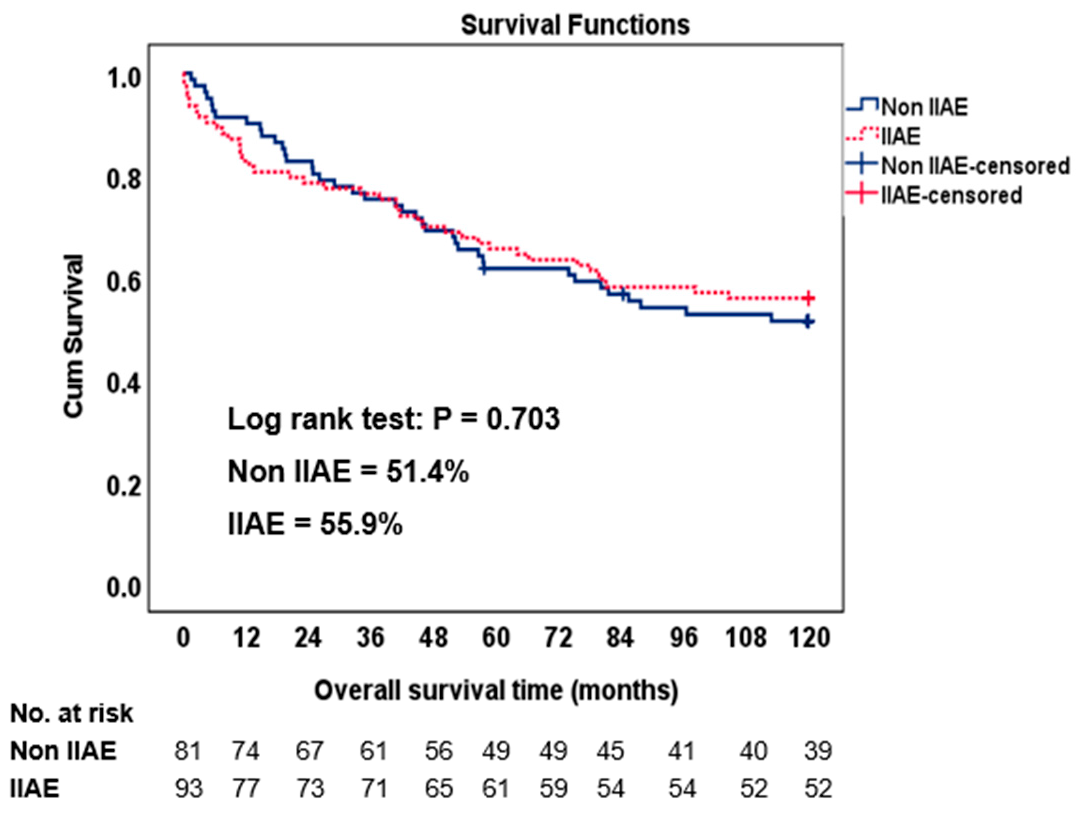
3. Results
Operative Details and Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Greenhalgh, R.M.; Brown, L.C.; Powell, J.T.; Thompson, S.G.; Epstein, D.; Sculpher, M.J. Endovascular versus open repair of abdominal aortic aneurysm. N. Engl. J. Med. 2010, 362, 1863–1871. [Google Scholar] [PubMed]
- Hatzl, J.; Peters, A.S.; Pfeiffer, S.; Meisenbacher, K.; Bischoff, M.S.; Böckler, D. Midterm single-center results after endovascular aneurysm sealing reveal a high rate of stent graft migration, secondary aneurysm ruptures, and device-related reinterventions. J. Vasc. Surg. 2021, 74, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.H.D.; Baptista-Silva, J.C.; Vasconcelos, V.; Flumignan, R.L.; Nakano, L.C. Internal iliac artery revascularisation versus internal iliac artery occlusion for endovascular treatment of aorto-iliac aneurysms. Cochrane Database Syst. Rev. 2020, 7, CD013168. [Google Scholar] [CrossRef] [PubMed]
- Jean-Baptiste, E.; Brizzi, S.; Bartoli, M.A.; Sadaghianloo, N.; Baqué, J.; Magnan, P.E.; Hassen-Khodja, R. Pelvic ischemia and quality of life scores after interventional occlusion of the hypogastric artery in patients undergoing endovascular aortic aneurysm repair. J. Vasc. Surg. 2014, 60, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Bratby, M.J.; Munneke, G.M.; Belli, A.M.; Loosemore, T.M.; Loftus, I.; Thompson, M.M.; Morgan, R.A. How safe is bilateral internal iliac artery embolization prior to EVAR? Cardiovasc. Intervent. Radiol. 2008, 31, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Massière, B.; Leão, R.; Vescovi, A.; Leal, D.; Vivas, P.; Vasconcelos, A.; von Ristow, A. Outcomes of bell-bottom technique compared to standard endovascular aneurysm repair. Vascular 2021, 29, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Kouvelos, G.N.; Katsargyris, A.; Antoniou, G.A.; Oikonomou, K.; Verhoeven, E.L. Outcome after Interruption or Preservation of Internal Iliac Artery Flow During Endovascular Repair of Abdominal Aorto-iliac Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 621–634. [Google Scholar] [CrossRef]
- Hobo, R.; Sybrandy, J.E.; Harris, P.L.; Buth, J. Endovascular repair of abdominal aortic aneurysms with concomitant common iliac artery aneurysm: Outcome analysis of the EUROSTAR Experience. J. Endovasc. Ther. 2008, 15, 12–22. [Google Scholar] [CrossRef]
- Torsello, G.; Schönefeld, E.; Osada, N.; Austermann, M.; Pennekamp, C.; Donas, K.P. Endovascular treatment of common iliac artery aneurysms using the bell-bottom technique: Long-term results. J. Endovasc. Ther. 2010, 17, 504–509. [Google Scholar] [CrossRef]
- Lu, J.J.; Glousman, B.; Macsata, R.A.; Zettervall, S.L.; Lee, K.B.; Amdur, R.L.; Sidawy, A.N.; Nguyen, B.N. Preservation of pelvic perfusion with iliac branch devices does not decrease ischemic colitis compared with hypogastric embolization in endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 2020, 71, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Verzini, F.; Parlani, G.; Romano, L.; De Rango, P.; Panuccio, G.; Cao, P. Endovascular treatment of iliac aneurysm: Concurrent comparison of side branch endograft versus hypogastric exclusion. J. Vasc. Surg. 2009, 49, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Taudorf, M.; Grønvall, J.; Schroeder, T.V.; Lönn, L. Endovascular Aneurysm Repair Treatment of Aortoiliac Aneurysms: Can Iliac Branched Devices Prevent Gluteal Claudication? J. Vasc. Interv. Radiol. 2016, 27, 174–180. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L.; Blankensteijn, J.D.; Harris, P.L.; White, G.H.; Zarins, C.K.; Bernhard, V.M.; Matsumura, J.S.; May, J.; Veith, F.J.; Fillinger, M.F.; et al. Reporting standards for endovascular aortic aneurysm repair. J. Vasc. Surg. 2002, 35, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Yano, O.J.; Morrissey, N.; Eisen, L.; Faries, P.L.; Soundararajan, K.; Wan, S.; Teodorescu, V.; Kerstein, M.; Hollier, L.H.; Marin, M.L. Intentional internal iliac artery occlusion to facilitate endovascular repair of aortoiliac aneurysms. J. Vasc. Surg. 2001, 34, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Bannazadeh, M.; Jenkins, C.; Forsyth, A.; Kramer, J.; Aggarwal, A.; Somerset, A.E.; Bove, P.G.; Long, G.W. Outcomes for concomitant common iliac artery aneurysms after endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 2017, 66, 1390–1397. [Google Scholar] [CrossRef]
- Bosanquet, D.C.; Wilcox, C.; Whitehurst, L.; Cox, A.; Williams, I.M.; Twine, C.P. Systematic Review and Meta-analysis of the Effect of Internal Iliac Artery Exclusion for Patients Undergoing EVAR. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 534–548. [Google Scholar] [CrossRef]
- Naughton, P.A.; Park, M.S.; Kheirelseid, E.A.; O’Neill, S.M.; Rodriguez, H.E.; Morasch, M.D.; Madhavan, P.; Eskandari, M.K. A comparative study of the bell-bottom technique vs hypogastric exclusion for the treatment of aneurysmal extension to the iliac bifurcation. J. Vasc. Surg. 2012, 55, 956–962. [Google Scholar] [CrossRef][Green Version]
- Mehta, M.; Veith, F.J.; Ohki, T.; Cynamon, J.; Goldstein, K.; Suggs, W.D.; Wain, R.A.; Chang, D.W.; Friedman, S.G.; Scher, L.A.; et al. Unilateral and bilateral hypogastric artery interruption during aortoiliac aneurysm repair in 154 patients: A relatively innocuous procedure. J. Vasc. Surg. 2001, 33 (Suppl. S2), S27–S32. [Google Scholar] [CrossRef][Green Version]
- Kapetanios, D.; Banafsche, R.; Jerkku, T.; Spanos, K.; Hoffmann, U.; Fiorucci, B.; Rantner, B.; Tsilimparis, N. Current evidence on aortic remodeling after endovascular repair. J. Cardiovasc. Surg. 2019, 60, 186–190. [Google Scholar] [CrossRef] [PubMed]

| Non-IIAE Group | IIAE Group | p-Value | |
|---|---|---|---|
| (n = 81) | (n = 93) | ||
| Age (years) (mean ± SD) | 76.15 ± 7.23 | 74.91 ± 7.48 | 0.272 |
| Male (n (%)) | 70 (86.4%) | 74 (79.6%) | 0.233 |
| Comorbidities (n (%)) | |||
| Coronary artery disease | 21 (25.9%) | 30 (32.2%) | 0.360 |
| COPD | 10 (12.3%) | 13 (13.9%) | 0.751 |
| Hypertension | 65 (80.2%) | 69 (74.3%) | 0.344 |
| Dyslipidemia | 26 (32.1%) | 28 (30.1%) | 0.777 |
| Diabetes mellitus | 8 (9.9%) | 19 (20.4%) | 0.055 |
| Cerebrovascular disease | 14 (17.3%) | 9 (9.7%) | 0.139 |
| Chronic kidney disease | 10 (12.3%) | 14 (15.1%) | 0.605 |
| Current smoking | 6 (7.4%) | 2 (2.1%) | 0.612 |
| Hct (%) (median (min, max)) | 36.9 (24.8, 47.4) | 35.1 (21.7, 46.8) | 0.326 |
| Creatinine (mg/dL) (median (min, max)) | 1.28 (0.5, 14.9) | 1.18 (0.2, 6.5) | 0.091 |
| Albumin (mg/dL) (median (min, max)) | 3.8 (2.8, 4.9) | 3.8 (2.8, 4.7) | 0.770 |
| ASA classification (n (%)) | 0.758 | ||
| ASA II | 19 (23.4%) | 20 (21.5%) | |
| ASA III | 62 (76.5%) | 73 (78.5%) | |
| ASA IV | 0 (0%) | 0 (0%) | |
| Type of aneurysm | 1.000 | ||
| Fusiform | 80 (98.8%) | 91 (97.8%) | |
| Saccular | 1 (1.2%) | 2 (2.2%) | |
| AAA morphology | |||
| Aneurysm size (mm) (mean ± SD) | 61.43 ± 11.46 | 60.90 ± 13.35 | 0.779 |
| Aneurysm length (mm) (mean ± SD) | 121.36 ± 17.58 | 119.85 ± 23.64 | 0.638 |
| Neck diameter (mm) (mean ± SD) | 24.59 ± 5.09 | 24.50 ± 4.28 | 0.896 |
| Neck length (mm) (mean ± SD) | 29.16 ± 14.82 | 30.7 ± 14.32 | 0.484 |
| Endografts used * | 0.586 | ||
| Zenith | 39 (48.1%) | 42 (45.1%) | |
| Endurant | 36 (44.4%) | 38 (40.9%) | |
| Ovation | 2 (2.5%) | 4 (4.3%) | |
| AFX | 0 | 2 (2.1%) | |
| Treovance | 4 (4.9%) | 7 (7.5%) |
| BBT | IIAE + EE | IBD | p-Value | |
|---|---|---|---|---|
| (n = 106) | (n = 113) | (n = 32) | ||
| Side of CIAA | 0.452 | |||
| Right | 59 (55.7%) | 60 (53.1%) | 21 (65.6%) | |
| Left | 47 (44.3%) | 53 (46.9%) | 11 (34.4%) | |
| CIA diameter (mm) (mean ± SD) | 23.7 ± 6.3 | 35.0 ± 13.0 | 35.5 ± 12.1 | <0.001 |
| External iliac arteries > 6 mm | 99 (93.4%) | 109 (96.5%) | 32 (100%) | 0.234 |
| External iliac arteries tortuosity | 31 (29.2%) | 59 (52.2%) | 10 (31.3%) | 0.001 |
| External iliac arteries calcified | 6 (5.7%) | 1 (0.9%) | 2 (6.3%) | 0.113 |
| Non-IIAE Group | IIAE Group | p-Value | |
|---|---|---|---|
| (n = 81) | (n = 93) | ||
| EBL (mL), median (min–max) | 200, 30–2000 | 300, 50–3000 | 0.089 |
| Fluoroscope time (mins) (mean ± SD) | 40.07 ± 30.05 | 44.56 ± 27.88 | 0.732 |
| Contrast usage (mL) (mean ± SD) | 132.75 ± 90.74 | 125.91 ± 60.90 | 0.556 |
| Organ complications (n (%)) | 9 (11.1%) | 9 (9.7%) | 0.757 |
| Renal failure | 5 | 4 | |
| Myocardial infarction | 3 | 3 | |
| Respiratory failure | 1 | 1 | |
| Liver failure | 0 | 1 | |
| Infective complications (n (%)) | 11 (13.6%) | 18 (19.3%) | 0.308 |
| UTI | 5 | 6 | |
| Pneumonia | 1 | 7 | |
| Arthritis | 2 | 1 | |
| Cholecystitis | 1 | 2 | |
| Colitis | 0 | 1 | |
| Pneumonia and UTI | 1 | 0 | |
| Wound infection | 1 | 0 | |
| Septicemia | 0 | 1 | |
| Length of stay (days), median (min–max) | 6, 3–120 | 5, 3–164 | 0.169 |
| In-hospital mortality (n (%)) | 1 (1.2%) | 4 (4.3%) | 0.374 |
| 30-day mortality (n (%)) | 0 | 2 (2.1%) | 0.500 |
| BBT | IIAE + EE | IBD | p-Value | |
|---|---|---|---|---|
| (n = 106) | (n = 113) | (n = 32) | ||
| Intraoperative limb complications | 8 (7.5%) | 4 (3.5%) | 1 (3.1%) | 0.349 |
| Endoleak type 1B | 8 | 3 | 0 | |
| EIA dissection | 0 | 1 | 0 | |
| EIA occlusion | 0 | 0 | 1 | |
| Adjunct limb procedure | 8 (7.5%) | 4 (3.5%) | 1 (3.1%) | 0.349 |
| Balloon inflation | 4 | 1 | 0 | |
| Iliac stent extension | 4 | 2 | 0 | |
| Embolectomy with stent/bypass | 0 | 1 | 1 |
| Bilateral IIAE + EE | Unilateral IIAE + EE | Non-IIAE | p-Value | |
|---|---|---|---|---|
| (n = 20) | (n = 73) | (n = 81) | ||
| Buttock claudication | 5 (25%) | 8 (11%) | 2 (2.5%) | 0.004 |
| Severe buttock ischemia | 3 (15%) | 0 (0.0%) | 0 (0.0%) | <0.001 |
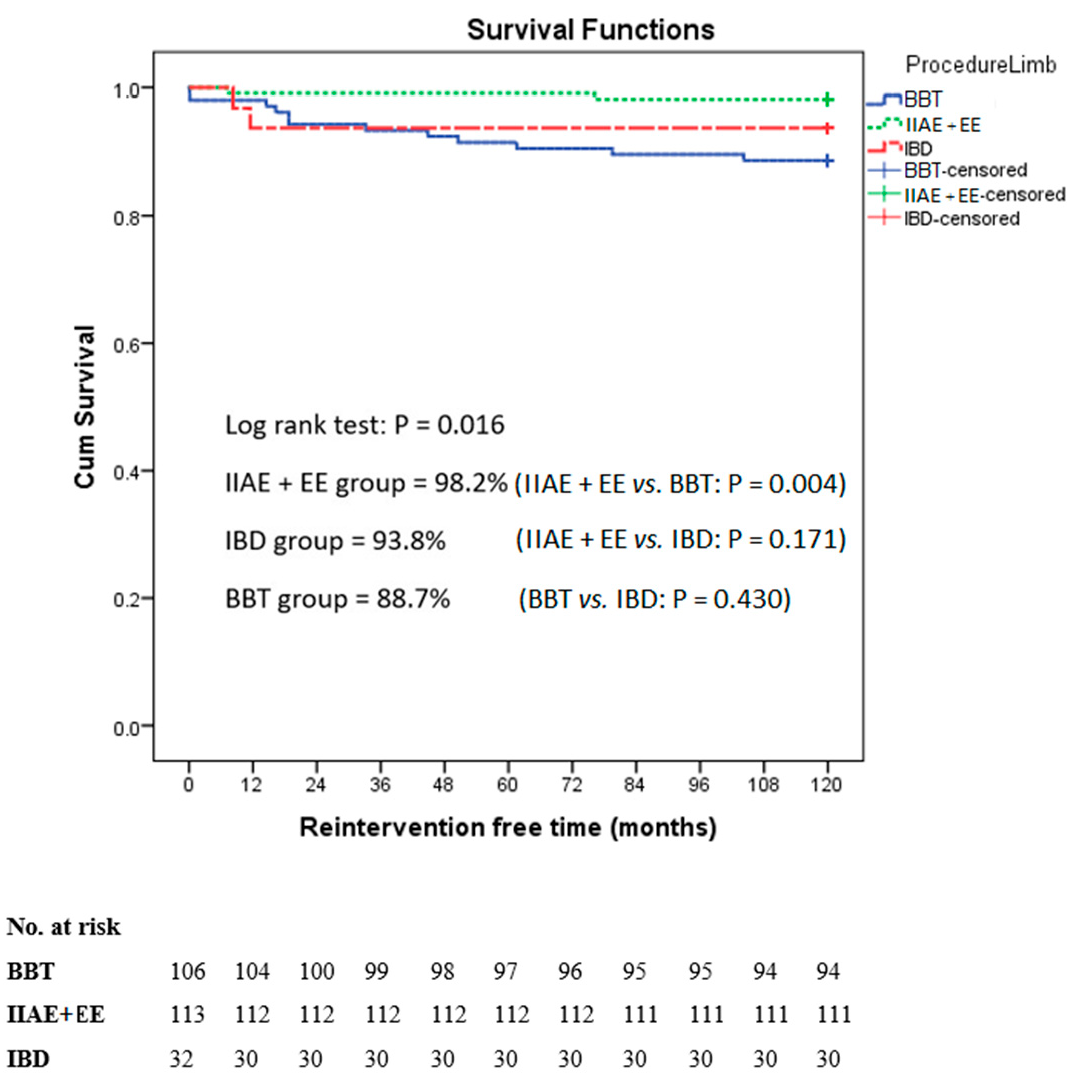
| Group | Complications | Reinterventions | Time to Reintervention | |
|---|---|---|---|---|
| BBT (106 limbs) | ||||
| 8 limbs | Endoleak type 1B | IIAE + EE | 5 days, 14 months, 16 months, 19 months (2), 3 years, 4 years, and 7 years | |
| 2 limbs | CIA enlargement | IIAE + EE | 5 years and 6 years | |
| 1 limb | Endoleak type 1B | IBD | 9 years | |
| 1 limb | Endoleak type 3 | Relining of the iliac stent | 4 days | |
| IIAE + EE (113 limbs) | ||||
| 1 limb | Endoleak type 1B from EIA | Relining of the iliac stent | 7 months | |
| 1 limb | Endoleak type 2 from inadequate IIAE | IIAE + EE | 6 years | |
| IBD (32 limbs) | ||||
| 1 limb | Endoleak type 3 | Relining of the iliac stent | 12 months | |
| 1 limb | Stent kinking | Relining of the iliac stent | 8 months | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinsakchai, K.; Ketklin, N.; Hongku, K.; Wongwanit, C.; Puangpunngam, N.; Hahtapornsawan, S.; Thongsai, S.; Prapassaro, T.; Sermsathanasawadi, N.; Ruangsetakit, C.; et al. Navigating Challenges in the Endovascular Treatment of Asymptomatic Aortoiliac Aneurysms: A 10-Year Comparative Analysis. J. Clin. Med. 2023, 12, 7000. https://doi.org/10.3390/jcm12227000
Chinsakchai K, Ketklin N, Hongku K, Wongwanit C, Puangpunngam N, Hahtapornsawan S, Thongsai S, Prapassaro T, Sermsathanasawadi N, Ruangsetakit C, et al. Navigating Challenges in the Endovascular Treatment of Asymptomatic Aortoiliac Aneurysms: A 10-Year Comparative Analysis. Journal of Clinical Medicine. 2023; 12(22):7000. https://doi.org/10.3390/jcm12227000
Chicago/Turabian StyleChinsakchai, Khamin, Natcha Ketklin, Kiattisak Hongku, Chumpol Wongwanit, Nattawut Puangpunngam, Suteekhanit Hahtapornsawan, Sasima Thongsai, Tossapol Prapassaro, Nuttawut Sermsathanasawadi, Chanean Ruangsetakit, and et al. 2023. "Navigating Challenges in the Endovascular Treatment of Asymptomatic Aortoiliac Aneurysms: A 10-Year Comparative Analysis" Journal of Clinical Medicine 12, no. 22: 7000. https://doi.org/10.3390/jcm12227000
APA StyleChinsakchai, K., Ketklin, N., Hongku, K., Wongwanit, C., Puangpunngam, N., Hahtapornsawan, S., Thongsai, S., Prapassaro, T., Sermsathanasawadi, N., Ruangsetakit, C., & Mutirangura, P. (2023). Navigating Challenges in the Endovascular Treatment of Asymptomatic Aortoiliac Aneurysms: A 10-Year Comparative Analysis. Journal of Clinical Medicine, 12(22), 7000. https://doi.org/10.3390/jcm12227000






