Risk Factors for Severe Pain and Impairment of Daily Life Activities after Cesarean Section—A Prospective Multi-Center Study of 11,932 Patients
Abstract
:1. Introduction
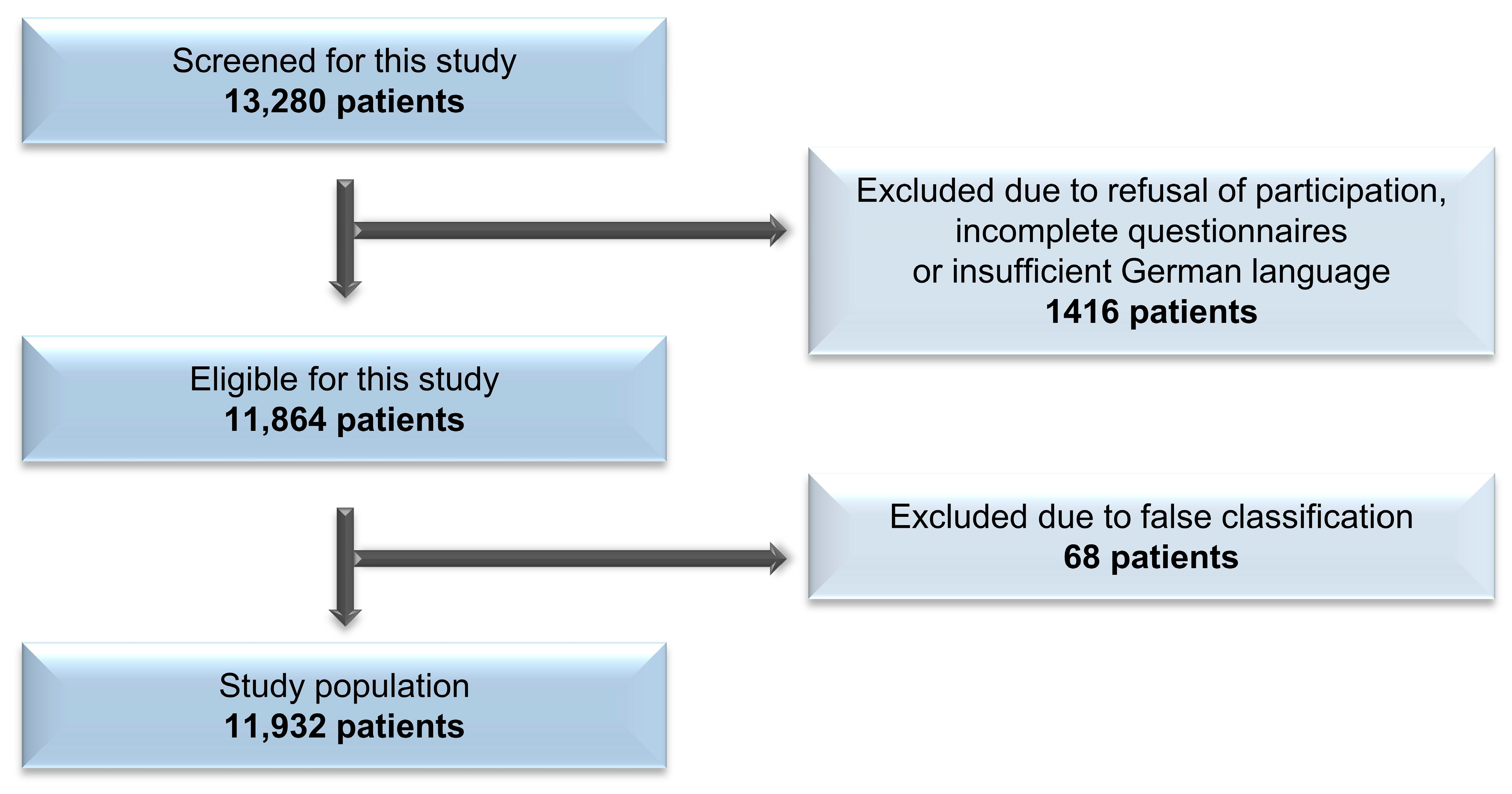
2. Methods
Statistical Analysis
3. Results
3.1. Postoperative Pain Intensity
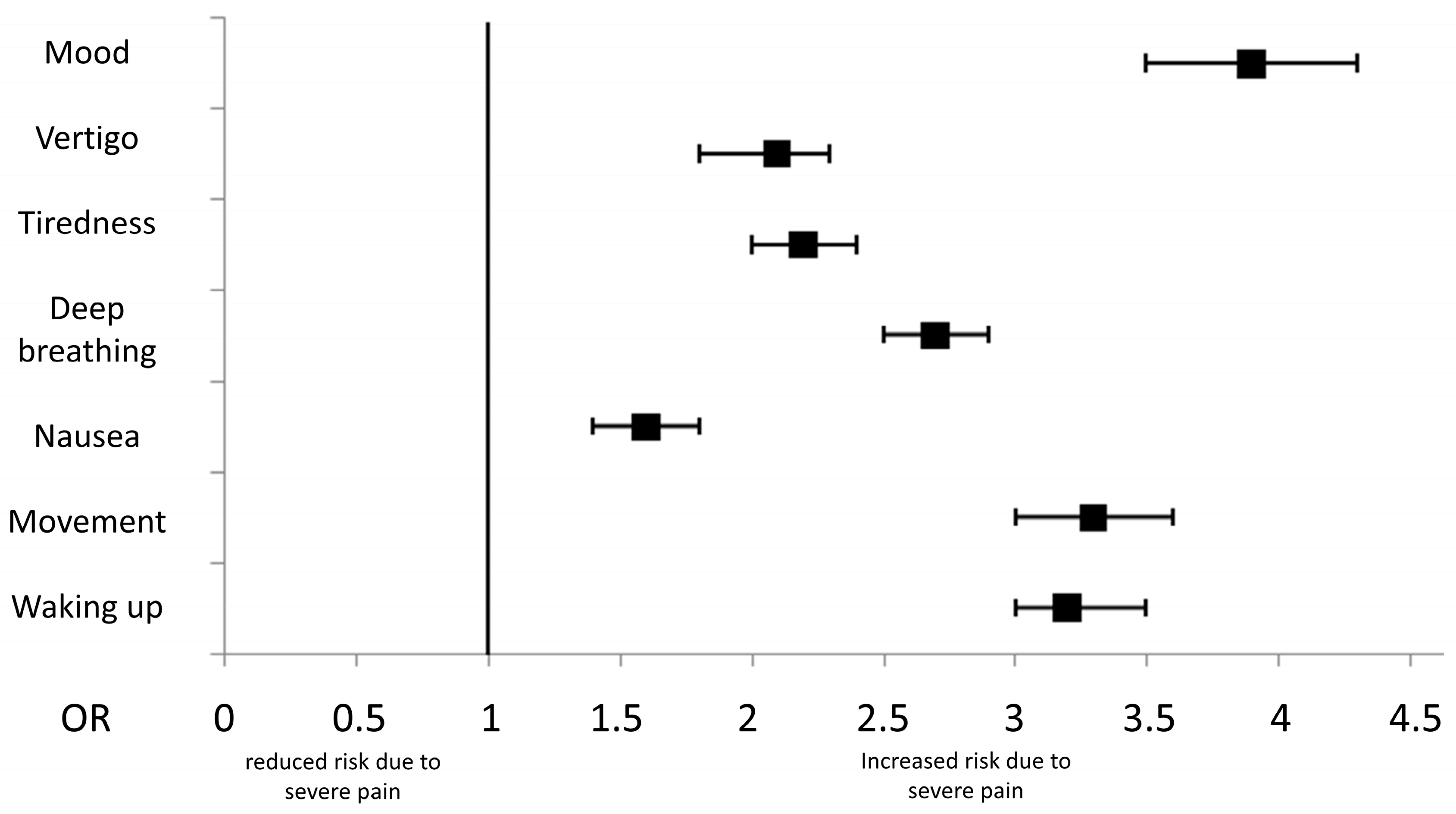
3.2. Impact of Severe Pain
3.3. Analgesic Therapy
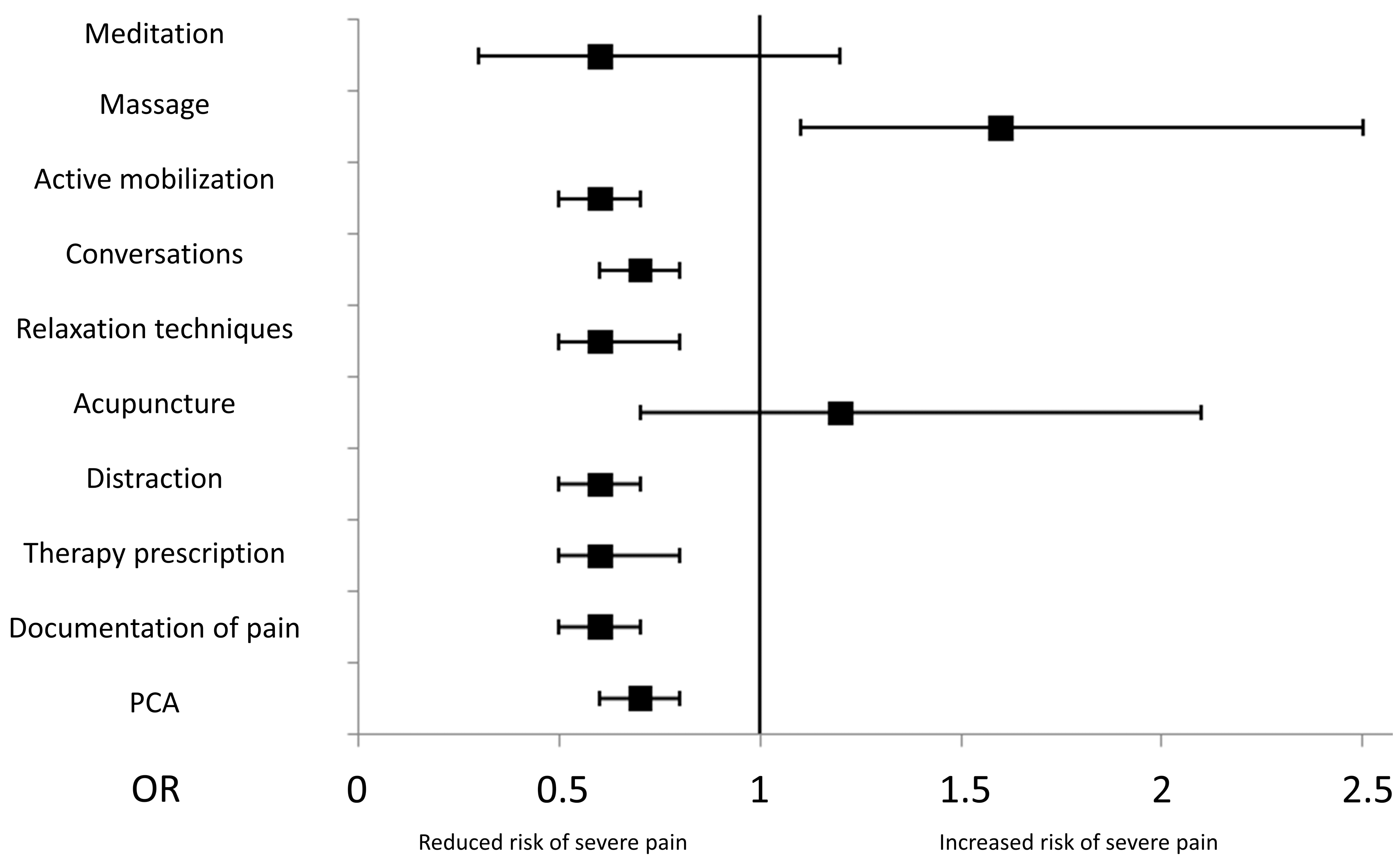
3.4. Non-Pharmacological Therapy
3.5. Primary vs. Urgent/Emergency Cesarean Section
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cesarean section | CS |
| Confidence interval | CI |
| Interquartile range | IQR |
| Numeric rating scale | NRS |
| Odds ratio | OR |
| Primary cesarean section | PCS |
| Quality improvement in postoperative pain management project | QUIPS |
| Standard deviation | SD |
| Urgent/emergency cesarean section | UCS |
References
- Cruz, J.J.; Kather, A.; Nicolaus, K.; Rengsberger, M.; Mothes, A.R.; Schleussner, E.; Meissner, W.; Runnebaum, I.B. Acute Postoperative Pain in 23 Procedures of Gynaecological Surgery Analysed in A Prospective Open Registry Study on Risk Factors and Consequences for the Patient. Sci. Rep. 2021, 11, 22148. [Google Scholar] [CrossRef]
- Gerbershagen, H.J.; Aduckathil, S.; Van Wijck, A.J.M.; Peelen, L.M.; Kalkman, C.J.; Meissner, W. Pain Intensity on the First Day after Surgery: A Prospective Cohort Study Comparing 179 Surgical Procedures. Anesthesiology 2013, 118, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Bjørnstad, J.; Ræder, J. Post-operative pain after caesarean section. Tidsskr Nor Laegeforen. 2020, 140. [Google Scholar] [CrossRef]
- Van Boekel, R.L.M.; Warlé, M.C.; Nielen, R.G.C.; Vissers, K.C.P.; Van Der Sande, R.; Bronkhorst, E.M.; Lerou, J.G.C.; Steegers, M.A.H. Relationship between Postoperative Pain and Overall 30-Day Complications in A Broad Surgical Population: An Observational Study. Ann. Surg. 2019, 269, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Gordon, D.B.; Leon-Casasola, O.A.d.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, E.H.; Degenhardt, E.; et al. Management of Postoperative Pain: A Clinical Practice Guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J. Pain 2016, 17, 131–157. [Google Scholar] [PubMed]
- Karlström, A.; Engström-Olofsson, R.; Norbergh, K.-G.; Sjöling, M.; Hildingsson, I. Postoperative Pain after Cesarean Birth Affects Breastfeeding and Infant Care. J. Obstet. Gynecol. Neonatal Nurs. Jognn 2007, 36, 430–440. [Google Scholar] [CrossRef]
- Eisenach, J.C.; Pan, P.H.; Smiley, R.; Lavand’homme, P.; Landau, R.; Houle, T.T. Severity of Acute Pain after Childbirth, But Not Type of Delivery, Predicts Persistent Pain and Postpartum Depression. Pain 2008, 140, 87–94. [Google Scholar] [CrossRef]
- Niklasson, B.; Georgsson Öhman, S.; Segerdahl, M.; Blanck, A. Risk Factors for Persistent Pain and Its Influence on Maternal Wellbeing after Cesarean Section. Acta Obstet. Gynecol. Scand. 2015, 94, 622–628. [Google Scholar] [CrossRef]
- Kehlet, H.; Jensen, T.S.; Woolf, C.J. Persistent Postsurgical Pain: Risk Factors and Prevention. Lancet 2006, 367, 1618–1625. [Google Scholar] [CrossRef]
- Blondal, K.; Halldorsdottir, S. The Challenge of Caring for Patients in Pain: From the Nurse’s Perspective. J. Clin. Nurs. 2009, 18, 2897–2906. [Google Scholar] [CrossRef]
- Rawal, N. Analgesia for Day-Case Surgery. Br. J. Anaesth. 2001, 87, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.Y.; Lui, J.C.Z. Postoperative Pain Management: Study of Patients’ Level of Pain and Satisfaction with Health Care Providers’ Responsiveness to Their Reports of Pain. Nurs. Health Sci. 2003, 5, 13–21. [Google Scholar] [CrossRef]
- Panda, S.; Begley, C.; Daly, D. Readmission Following Caesarean Section: Outcomes for Women in an Irish Maternity Hospital. Br. J. Midwifery 2016, 24, 322–328. [Google Scholar] [CrossRef]
- Olza, I.; Leahy-Warren, P.; Benyamini, Y.; Kazmierczak, M.; Karlsdottir, S.I.; Spyridou, A.; Crespo-Mirasol, E.; Takács, L.; Hall, P.J.; Murphy, M.; et al. Women’s Psychological Experiences of Physiological Childbirth: A Meta-Synthesis. BMJ Open 2018, 8, E020347. [Google Scholar] [CrossRef] [PubMed]
- Hodnett, E.D. Pain and Women’s Satisfaction with the Experience of Childbirth: A Systematic Review. Am. J. Obstet. Gynecol. 2002, 186, S160–S172. [Google Scholar] [CrossRef] [PubMed]
- Aune, I.; Marit torvik, H.; Selboe, S.-T.; Skogås, A.-K.; Persen, J.; Dahlberg, U. Promoting a Normal Birth and A Positive Birth Experience—Norwegian Women’s Perspectives. Midwifery 2015, 31, 721–727. [Google Scholar] [CrossRef]
- Jochberger, S.; Ortner, C.; Klein, K.U. Schmerztherapie Während Der Geburt. Wien. Med. Wochenschr. 2017, 167, 368–373. [Google Scholar] [CrossRef]
- Tighe, P.J.; Riley, J.L.; Fillingim, R.B. Sex Differences in the Incidence of Severe Pain Events Following Surgery: A Review of 333,000 Pain Scores. Pain Med. (Malden Mass.) 2014, 15, 1390–1404. [Google Scholar] [CrossRef]
- Gerbershagen, H.J.; Dagtekin, O.; Rothe, T.; Heidenreich, A.; Gerbershagen, K.; Sabatowski, R.; Petzke, F.; Ozgür, E. Risk Factors for Acute and Chronic Postoperative Pain in Patients with Benign and Malignant Renal Disease after Nephrectomy. Eur. J. Pain 2009, 13, 853–860. [Google Scholar] [CrossRef]
- Musey, P.I.; Linnstaedt, S.D.; Platts-Mills, T.F.; Miner, J.R.; Bortsov, A.V.; Safdar, B.; Bijur, P.; Rosenau, A.; Tsze, D.S.; Chang, A.K.; et al. Gender Differences in Acute and Chronic Pain in The Emergency Department: Results of the 2014 Academic Emergency Medicine Consensus Conference Pain Section. Acad. Emerg. Med. off. J. Soc. Acad. Emerg. Med. 2014, 21, 1421–1430. [Google Scholar] [CrossRef]
- Schnabel, A.; Poepping, D.M.; Gerss, J.; Zahn, P.K.; Pogatzki-Zahn, E.M. Sex-Related Differences of Patient-Controlled Epidural Analgesia for Postoperative Pain. Pain 2012, 153, 238–244. [Google Scholar] [CrossRef]
- Schopper, M.; Fleckenstein, J.; Irnich, D. Geschlechtsspezifische Aspekte Bei Akuten und Chronischen Schmerzen. Implikationen Für Diagnose und Therapie. Schmerz 2013, 27, 456–466. [Google Scholar] [CrossRef]
- Sato, H.; Droney, J.; Ross, J.; Olesen, A.E.; Staahl, C.; Andresen, T.; Branford, R.; Riley, J.; Arendt-Nielsen, L.; Drewes, A.M. Gender, Variation in Opioid Receptor Genes and Sensitivity to Experimental Pain. Mol. Pain 2013, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Niesters, M.; Dahan, A.; Kest, B.; Zacny, J.; Stijnen, T.; Aarts, L.; Sarton, E. Do Sex Differences Exist in Opioid Analgesia? A Systematic Review and Meta-Analysis of Human Experimental and Clinical Studies. Pain 2010, 151, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Louwen, F.; Wagner, U.; Abou-Dakn, M.; Dötsch, J.; Lawrenz, B.; Ehm, D.; Surbek, D.; Essig, A.; Greening, M.; Schäfers, R.; et al. Caesarean Section. Guideline of the DGGG, OEGGG and SGGG (S3-Level, AWMF Registry No. 015/084, June 2020). Geburtshilfe Frauenheilkd. 2021, 81, 896–921. [Google Scholar] [CrossRef] [PubMed]
- Betran, A.P.; Ye, J.; Moller, A.-B.; Souza, J.P.; Zhang, J. Trends and Projections of Caesarean Section Rates: Global and Regional Estimates. Bmj Glob. Health 2021, 6, e005671. [Google Scholar] [CrossRef]
- Rose, J.; Weiser, T.G.; Hider, P.; Wilson, L.; Gruen, R.L.; Bickler, S.W. Estimated Need for Surgery Worldwide Based on Prevalence of Diseases: A Modelling Strategy for The Who Global Health Estimate. Lancet. Glob. Health 2015, 3 (Suppl. S2), S13–S20. [Google Scholar] [CrossRef]
- Veef, E.; Van De Velde, M. Post-Cesarean Section Analgesia. Best Pract. Research. Clin. Anaesthesiol. 2022, 36, 83–88. [Google Scholar] [CrossRef]
- Meissner, W.; Mescha, S.; Rothaug, J.; Zwacka, S.; Goettermann, A.; Ulrich, K.; Schleppers, A. Quality Improvement in Postoperative Pain Management: Results from the Quips Project. Dtsch. Arztebl. Int. 2008, 105, 865–870. [Google Scholar] [CrossRef]
- Rothaug, J.; Weiss, T.; Meissner, W. Externe Validität Der Schmerzbedingten Funktionsbeeinträchtigung: Messen Wir, Was Wir Messen Wollen? Schmerz 2012, 26, 396–401. [Google Scholar] [CrossRef]
- Anderson, K.O. Role of Cutpoints: Why Grade Pain Intensity? Pain 2005, 113, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.M.; Zelman, D.C.; Smith, M.; Miaskowski, C. Categorizing the Severity of Cancer Pain: Further Exploration of the Establishment of Cutpoints. Pain 2005, 113, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Harris, K.; Hadi, S.; Chow, E. What Should Be the Optimal Cut Points for Mild, Moderate, and Severe Pain? J. Palliat. Med. 2007, 10, 1338–1346. [Google Scholar] [CrossRef]
- Gerbershagen, H.J.; Pogatzki-Zahn, E.; Aduckathil, S.; Peelen, L.M.; Kappen, T.H.; Van Wijck, A.J.M.; Kalkman, C.J.; Meissner, W. Procedure-Specific Risk Factor Analysis for the Development of Severe Postoperative Pain. Anesthesiology 2014, 120, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Marcus, H.; Gerbershagen, H.J.; Peelen, L.M.; Aduckathil, S.; Kappen, T.H.; Kalkman, C.J.; Meissner, W.; Stamer, U.M. Quality of Pain Treatment after Caesarean Section: Results of a Multicentre Cohort Study. Eur. J. Pain 2015, 19, 929–939. [Google Scholar] [CrossRef]
- Mccauley, M.; Actis Danna, V.; Mrema, D.; Van Den Broek, N. “We Know It’s Labour Pain, So We Don’t Do Anything”: Healthcare Provider’s Knowledge and Attitudes Regarding the Provision of Pain Relief during Labour and after Childbirth. BMC Pregnancy Childbirth 2018, 18, 444. [Google Scholar] [CrossRef]
- Carvalho, B.; Cohen, S.E.; Lipman, S.S.; Fuller, A.; Mathusamy, A.D.; Macario, A. Patient Preferences for Anesthesia Outcomes Associated with Cesarean Delivery. Anesth. Analg. 2005, 101, 1182–1187. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Caesarean Birth (NICE Guideline 192). 2023. Available online: https://www.nice.org.uk/guidance/ng192 (accessed on 25 September 2023).
- Roofthooft, E.; Joshi, G.P.; Rawal, N.; Van De Velde, M. Prospect Guideline for Elective Caesarean Section: An Update. Anaesthesia 2023, 78, 1176–1177. [Google Scholar] [CrossRef]
- Bremerich, D.H.; Greve, S. Die neue S1-Leitlinie „Geburtshilfliche Analgesie und Anästhesie“— Vorstellung und Kommentar [The new S1 guidelines "Obstetric analgesia and anesthesia"-Presentation and comments]. Anaesthesist 2021, 70, 229–236. [Google Scholar] [CrossRef]
- Pogatzki-Zahn, E.; Meißner, W. 001-025l S3-LeitlinieBehandlung Akuter Perioperativer Posttraumatischer Schmerzen, Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin e. V. (DGAI), Berlin, Germany. 2022. Available online: https://register.awmf.org/de/leitlinien/detail/001-025 (accessed on 25 September 2023).
- Sutton, C.D.; Carvalho, B. Optimal Pain Management after Cesarean Delivery. Anesthesiol. Clin. 2017, 35, 107–124. [Google Scholar] [CrossRef]
- Practice Guidelines for Obstetric Anesthesia: An Updated Report by the American Society of Anesthesiologists Task force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology 2016, 124, 270–300. [CrossRef]
- La Via, L.; Minardi, C.; Brancati, S.; Valenti, S.; Messina, S.; Garofalo, E.; Bruni, A.; Murabito, P.; Bernardini, R.; Sanfilippo, F. Fentanyl vs Morphine as Adjuvant to Spinal Anesthesia for Caesarean Section: An Observational Study. Euromediterranean Biomed. J. 2023, 18, 79–83. [Google Scholar]
- Botea, M.O.; Lungeanu, D.; Petrica, A.; Sandor, M.I.; Huniadi, A.C.; Barsac, C.; Marza, A.M.; Moisa, R.C.; Maghiar, L.; Botea, R.M.; et al. Perioperative Analgesia and Patients’ Satisfaction in Spinal Anesthesia for Cesarean Section: Fentanyl Versus Morphine. J. Clin. Med. 2023, 12, 6346. [Google Scholar] [CrossRef]
- La Via, L.; Santonocito, C.; Bartolotta, N.; Lanzafame, B.; Morgana, A.; Continella, C.; Cirica, G.; Astuto, M.; Sanfilippo, F. A-2 Agonists vs. Fentanyl as Adjuvants for Spinal Anesthesia in Elective Cesarean Section: A Meta-Analysis. Minerva Anestesiol. 2023, 89, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, H.; Ak, M.; Gökahmetoğlu, G.; Aksoy, Ü. The Comparison of Intraincisional Bupivacaine Infiltration and Intravenous Paracetamol Administration for Pain Alleviation after Cesarean Section: A Double-Blinded Randomized Placebo Controlled Clinical Trial. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3467–3474. [Google Scholar] [PubMed]
- Dagasan Cetin, G.; Dostbil, A.; Aksoy, M.; Kasali, K.; Ince, R.; Kahramanlar, A.A.; Atalay, C.; topdagi Yilmaz, E.P.; Ince, I.; Ozkal, M.S. Intraperitoneal Instillation Versus Wound Infiltration for Postoperative Pain Relief after Cesarean Delivery: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. J. Obstet. Gynaecol. Res. 2023, 49, 209–219. [Google Scholar] [CrossRef]
- Hussein, A.; Torky, H.; Aly, R.; Abdel-Rasheed, M.; El-Baz, A.; Mahmoud, H.; Sileem, S.; Badawy, M.; Sayd, Z.; Dief, O.; et al. Lidocaine vs. Tramadol vs. Placebo Wound Infiltration for Post-Cesarean Section Pain Relief: A Randomized Controlled Trial. J. Perinat. Med. 2022, 50, 1073–1077. [Google Scholar] [CrossRef]
- Kayman-Kose, S.; Arioz, D.T.; Toktas, H.; Koken, G.; Kanat-Pektas, M.; Kose, M.; Yilmazer, M. Transcutaneous Electrical Nerve Stimulation (Tens) for Pain Control after Vaginal Delivery and Cesarean Section. J. Matern. -Fetal Neonatal Med. off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2014, 27, 1572–1575. [Google Scholar] [CrossRef]
- Chen, W.; Liu, C.; Yang, Y.; Tian, L. The Effect of Transcutaneous Electrical Nerve Stimulation (Tens) on Pain Control and Phenylethanolamine-N-Methyltransferase (Pnmt) Gene Expression after Cesarean Section. Cell. Mol. Biol. 2021, 67, 153–157. [Google Scholar] [CrossRef]
- Navarro Nuñez, C.; Pacheco Carrasco, M. Estimulación Eléctrica Transcutánea (Eet) Para Reducir El Dolor Después de la Cesárea. Ginecol. Obstet. Mex. 2000, 68, 60–63. [Google Scholar]
- Kasapoğlu, I.; Kasapoğlu Aksoy, M.; Çetinkaya Demir, B.; Altan, L. The Efficacy of Transcutaneous Electrical Nerve Stimulation Therapy in Pain Control after Cesarean Section Delivery Associated with Uterine Contractions and Abdominal Incision. Turk. J. Phys. Med. Rehabil. 2020, 66, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Beiranvand, S.; Noparast, M.; Eslamizade, N.; Saeedikia, S. The Effects of Religion and Spirituality on Postoperative Pain, Hemodynamic Functioning and Anxiety after Cesarean Section. Acta Medica Iran. 2014, 52, 909–915. [Google Scholar]
- Wu, M.-S.; Chen, K.-H.; Chen, I.-F.; Huang, S.K.; Tzeng, P.-C.; Yeh, M.-L.; Lee, F.-P.; Lin, J.-G.; Chen, C. The Efficacy of Acupuncture in Post-Operative Pain Management: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, E0150367. [Google Scholar] [CrossRef] [PubMed]
- Kırca, A.Ş.; Gül, D.K. Effect of Acupressure Applied after Cesarean Section Under Spinal Anesthesia Postpone the Duration of Taking Analgesics and on The Gastrointestinal System: A Randomized Controlled Trial. Explore 2023, 19, 58–64. [Google Scholar] [CrossRef]
- Stamenkovic, D.; Baumbach, P.; Radovanovic, D.; Novovic, M.; Ladjevic, N.; Dubljanin Raspopovic, E.; Palibrk, I.; Unic-Stojanovic, D.; Jukic, A.; Jankovic, R.; et al. The Perioperative Pain Management Bundle Is Feasible: Findings from the Pain Out Registry. Clin. J. Pain 2023, 39, 537–545. [Google Scholar] [CrossRef]
- Gerbershagen, K.; Gerbershagen, H.J.; Lutz, J.; Cooper-Mahkorn, D.; Wappler, F.; Limmroth, V.; Gerbershagen, M. Pain Prevalence and Risk Distribution Among Inpatients in A German Teaching Hospital. Clin. J. Pain 2009, 25, 431–437. [Google Scholar] [CrossRef]
- Neville, A.; Lee, L.; Antonescu, I.; Mayo, N.E.; Vassiliou, M.C.; Fried, G.M.; Feldman, L.S. Systematic Review of Outcomes Used to Evaluate Enhanced Recovery after Surgery. Br. J. Surg. 2014, 101, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Schwenkglenks, M.; Gerbershagen, H.J.; Taylor, R.S.; Pogatzki-Zahn, E.; Komann, M.; Rothaug, J.; Volk, T.; Yahiaoui-Doktor, M.; Zaslansky, R.; Brill, S.; et al. Correlates of Satisfaction with Pain Treatment in the Acute Postoperative Period: Results from the International Pain Out Registry. Pain 2014, 155, 1401–1411. [Google Scholar] [CrossRef]
- Gerbershagen, H.J.; Rothaug, J.; Kalkman, C.J.; Meissner, W. Determination of Moderate-to-Severe Postoperative Pain on the Numeric Rating Scale: A Cut-off Point Analysis Applying Four Different Methods. Br. J. Anaesth. 2011, 107, 619–626. [Google Scholar] [CrossRef]
- Zalon, M.L. Mild, Moderate, and Severe Pain in Patients Recovering from Major Abdominal Surgery. Pain Manag. Nurs. off. J. Am. Soc. Pain Manag. Nurses 2014, 15, E1–E12. [Google Scholar] [CrossRef]
- Shaffer, E.E.; Pham, A.; Woldman, R.L.; Spiegelman, A.; Strassels, S.A.; Wan, G.J.; Zimmerman, T. Estimating the Effect of Intravenous Acetaminophen for Postoperative Pain Management on Length of Stay and Inpatient Hospital Costs. Adv. Ther. 2017, 33, 2211–2228. [Google Scholar] [CrossRef] [PubMed]
- Borges, N.D.C.; Pereira, L.V.; Moura, L.A.d.; Silva, T.C.; Pedroso, C.F. Predictors for Moderate to Severe Acute Postoperative Pain after Cesarean Section. Pain Res. Manag. 2016, 2016, 5783817. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.S.; Ahmed, K.; Blakeway, E.; Skapinakis, P.; Nihoyannopoulos, L.; Macleod, K.; Sevdalis, N.; Ashrafian, H.; Platt, M.; Darzi, A.; et al. Catastrophizing: A Predictive Factor for Postoperative Pain. Am. J. Surg. 2011, 201, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Gravani, S.; Matiatou, M.; Nikolaidis, P.T.; Menenakos, E.; Zografos, C.G.; Zografos, G.; Albanopoulos, K. Anxiety and Depression Affect Early Postoperative Pain Dimensions after Bariatric Surgery. J. Clin. Med. 2020, 10, 53. [Google Scholar] [CrossRef]
| CS n = 11,932 | PCS n = 7686 | UCS n = 4246 | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Age (years) | |||
| 18–20 | 244 (2.1) | 146 (1.9) | 98 (2.3) |
| 21–30 | 4546 (38.3) | 2786 (36.2) | 1760 (41.5) |
| 31–40 | 6432 (54.9) | 4225 (55.0) | 2207 (52.0) |
| ≥41 | 478 (4.0) | 332 (4.3) | 146 (3.4) |
| Missing data | 232 (1.9) | 197 (2.6) | 35 (0.8) |
| Preoperative chronic pain | |||
| No pain | 11,466 (96.9) | 7360 (95.8) | 4106 (96.7) |
| Pain | 363 (3.1) | 244 (3.2) | 119 (2.8) |
| Missing data | 103 (0.9) | 82 (1.1) | 21 (0.5) |
| Type of anesthesia | |||
| General | 996 (8.3) | 494 (6.4) | 502 (11.8) |
| Regional | 8580 (71.9) | 5434 (70.7) | 3146 (74.1) |
| Missing data | 2356 (19.7) | 1758 (22.9) | 598 (14.1) |
| Duration of surgery (min) | Mean 39.8 SD 20.5 | Mean 40.2 SD 19.6 | Mean 39.0 SD 21.7 |
| PCS | UCS | OR (CI) | |
|---|---|---|---|
| n (%) | n (%) | ||
| Opioid | 2484 (40.9) | 1920 (46.1) | 1.24 (1.14–1.34) |
| Oxycodone | 1528 (25.1) | 767 (18.4) | 0.67 (0.61–0.74) |
| Morphine | 122 (2.0) | 135 (3.2) | 1.64 (1.28–2.10) |
| Pethidine | 65 (1.1) | 94 (2.3) | 2.14 (1.55–2.94) |
| Piritramide | 955 (15.7) | 988 (23.7) | 1.67 (1.51–1.84) |
| Sufentanil | 48 (0.8) | 53 (1.3) | 1.62 (1.09–2.40) |
| Tramadol | 215 (3.5) | 228 (5.5) | 1.58 (1.31–1.91) |
| Missing information about indication for surgery 1692 (14.2) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emrich, N.L.A.; Tascón Padrón, L.; Komann, M.; Arnold, C.; Dreiling, J.; Meißner, W.; Strizek, B.; Gembruch, U.; Jiménez Cruz, J. Risk Factors for Severe Pain and Impairment of Daily Life Activities after Cesarean Section—A Prospective Multi-Center Study of 11,932 Patients. J. Clin. Med. 2023, 12, 6999. https://doi.org/10.3390/jcm12226999
Emrich NLA, Tascón Padrón L, Komann M, Arnold C, Dreiling J, Meißner W, Strizek B, Gembruch U, Jiménez Cruz J. Risk Factors for Severe Pain and Impairment of Daily Life Activities after Cesarean Section—A Prospective Multi-Center Study of 11,932 Patients. Journal of Clinical Medicine. 2023; 12(22):6999. https://doi.org/10.3390/jcm12226999
Chicago/Turabian StyleEmrich, Norah L. A., Laura Tascón Padrón, Marcus Komann, Christin Arnold, Johannes Dreiling, Winfried Meißner, Brigitte Strizek, Ulrich Gembruch, and Jorge Jiménez Cruz. 2023. "Risk Factors for Severe Pain and Impairment of Daily Life Activities after Cesarean Section—A Prospective Multi-Center Study of 11,932 Patients" Journal of Clinical Medicine 12, no. 22: 6999. https://doi.org/10.3390/jcm12226999
APA StyleEmrich, N. L. A., Tascón Padrón, L., Komann, M., Arnold, C., Dreiling, J., Meißner, W., Strizek, B., Gembruch, U., & Jiménez Cruz, J. (2023). Risk Factors for Severe Pain and Impairment of Daily Life Activities after Cesarean Section—A Prospective Multi-Center Study of 11,932 Patients. Journal of Clinical Medicine, 12(22), 6999. https://doi.org/10.3390/jcm12226999






