Gluteal Muscle Fatty Atrophy: An Independent Risk Factor for Surgical Treatment in Elderly Patients Diagnosed with Type-III Fragility Fractures of the Pelvis
Abstract
:1. Introduction
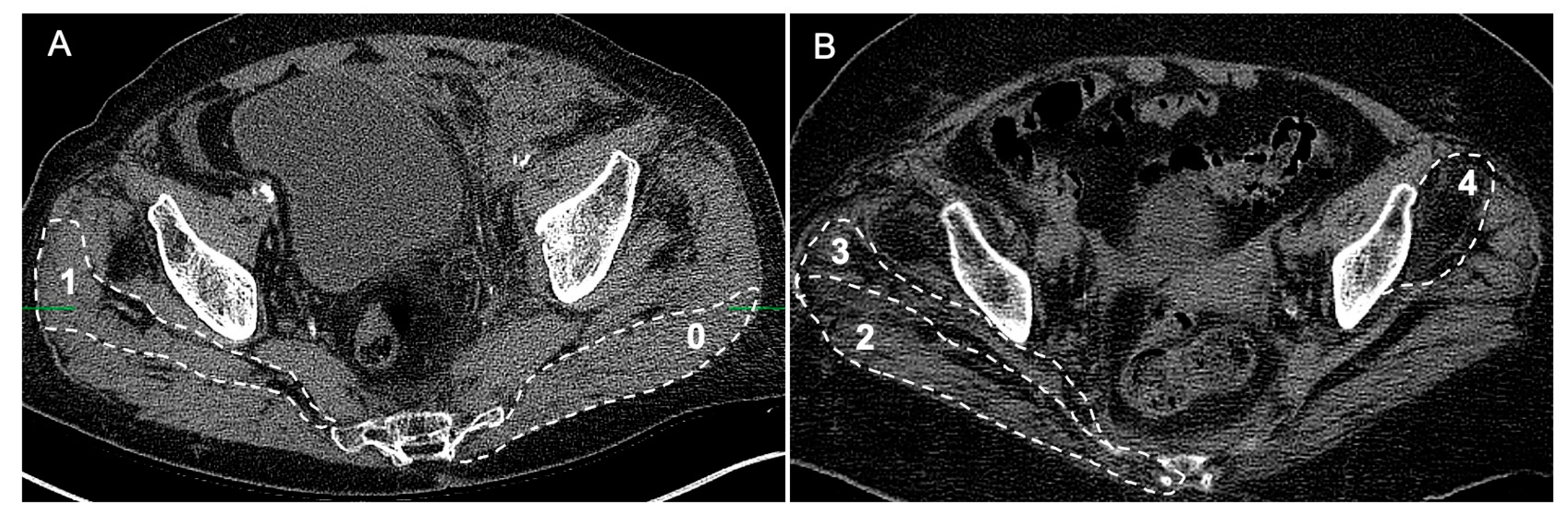
2. Methods and Materials
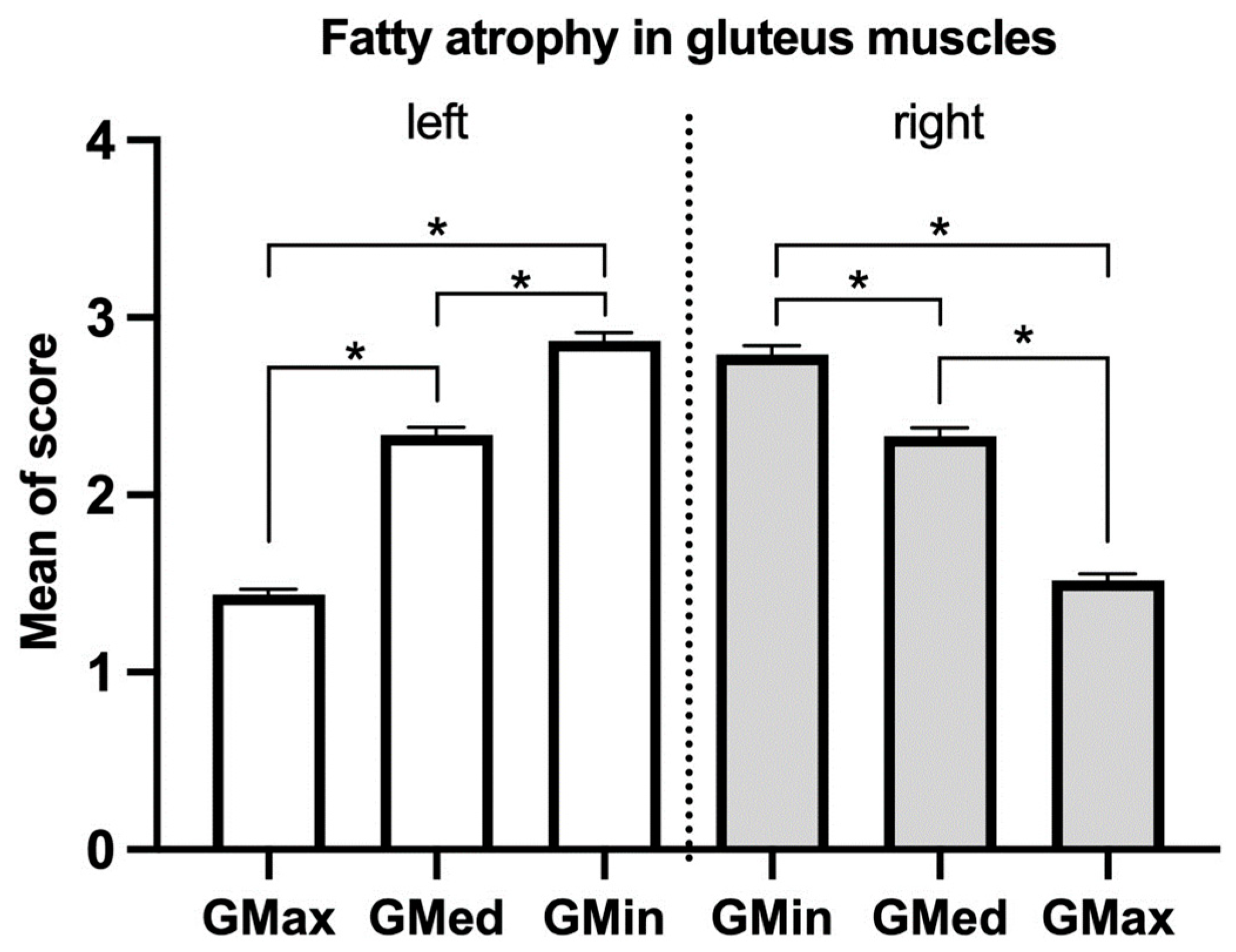
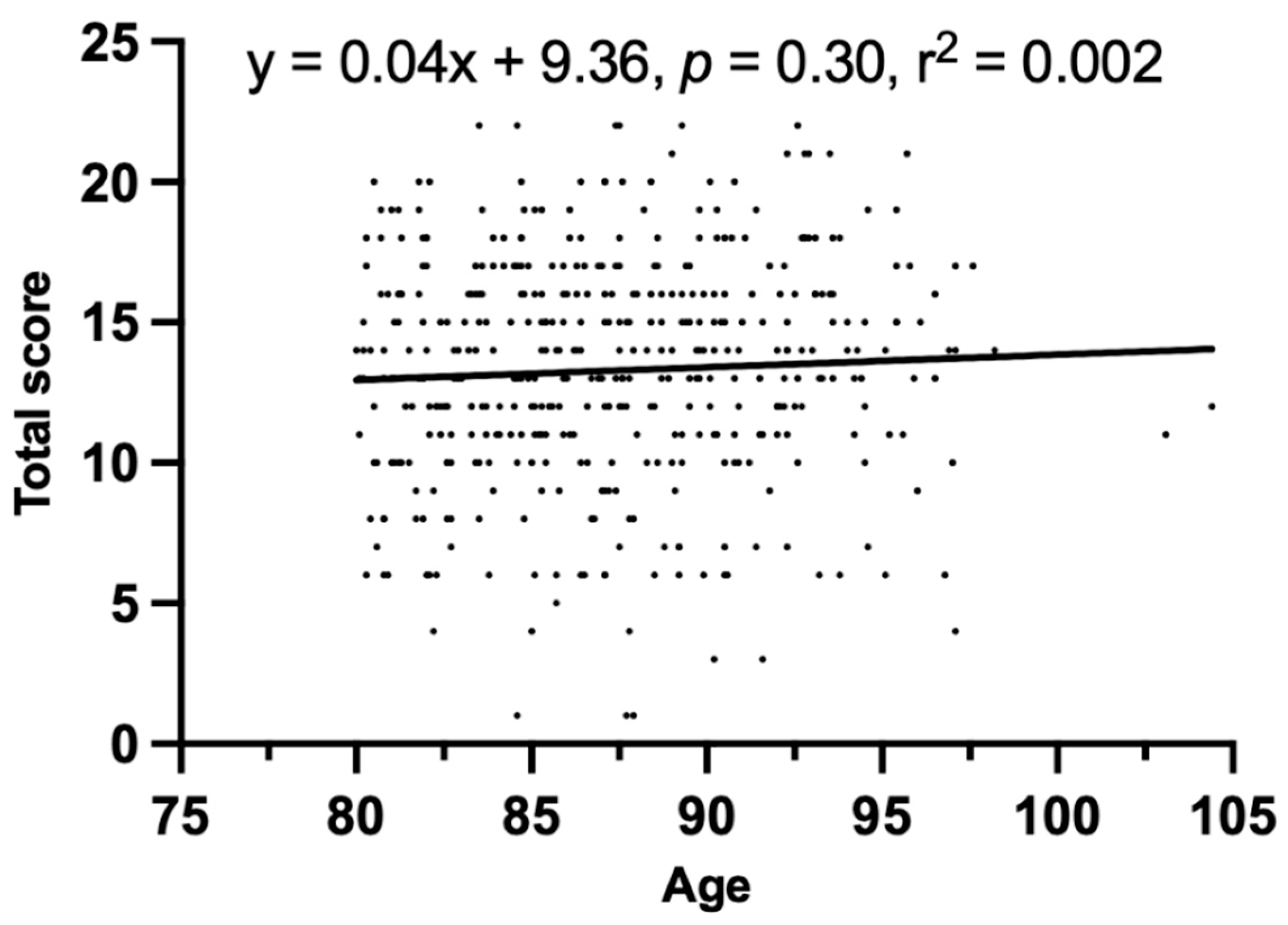
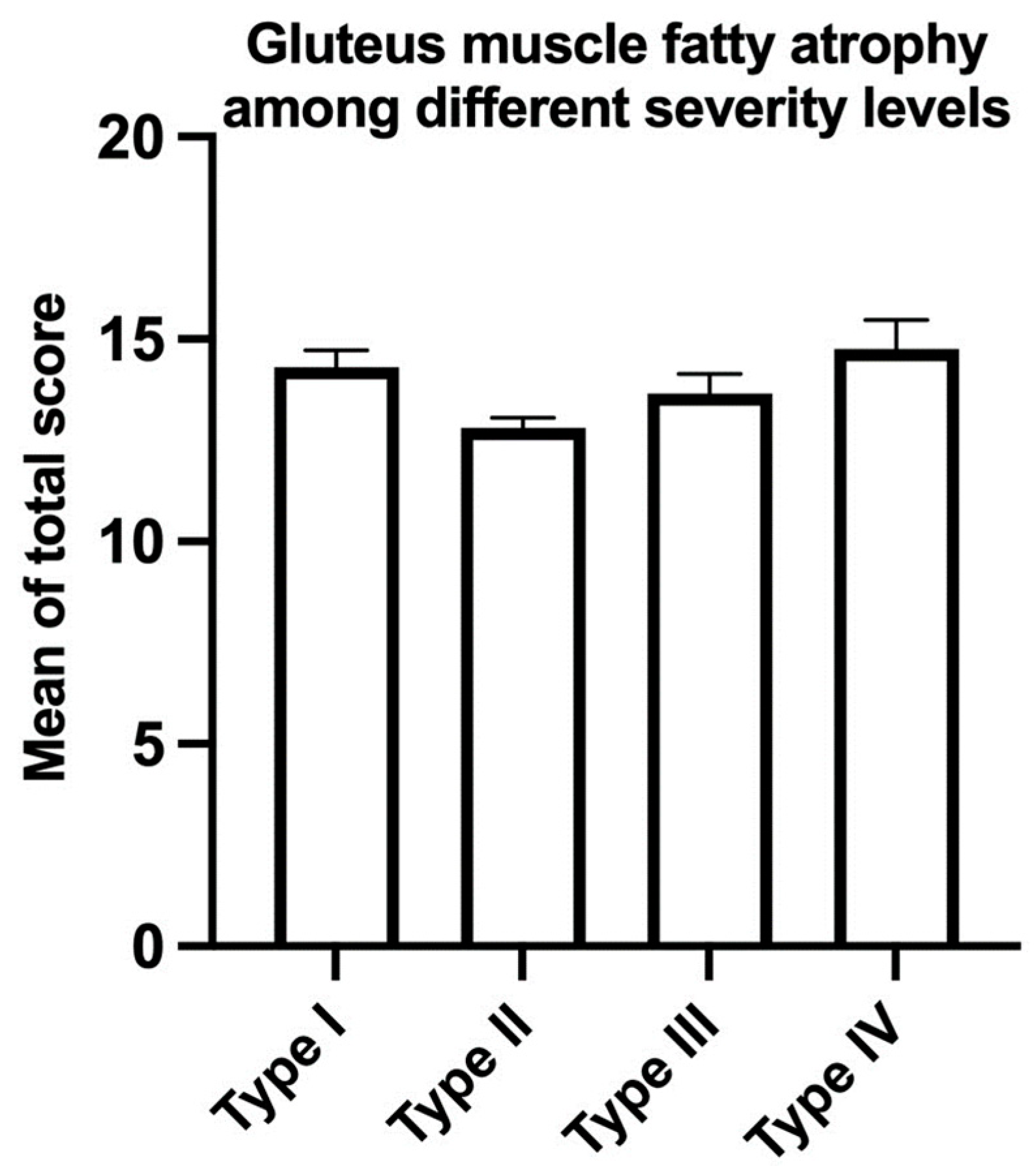
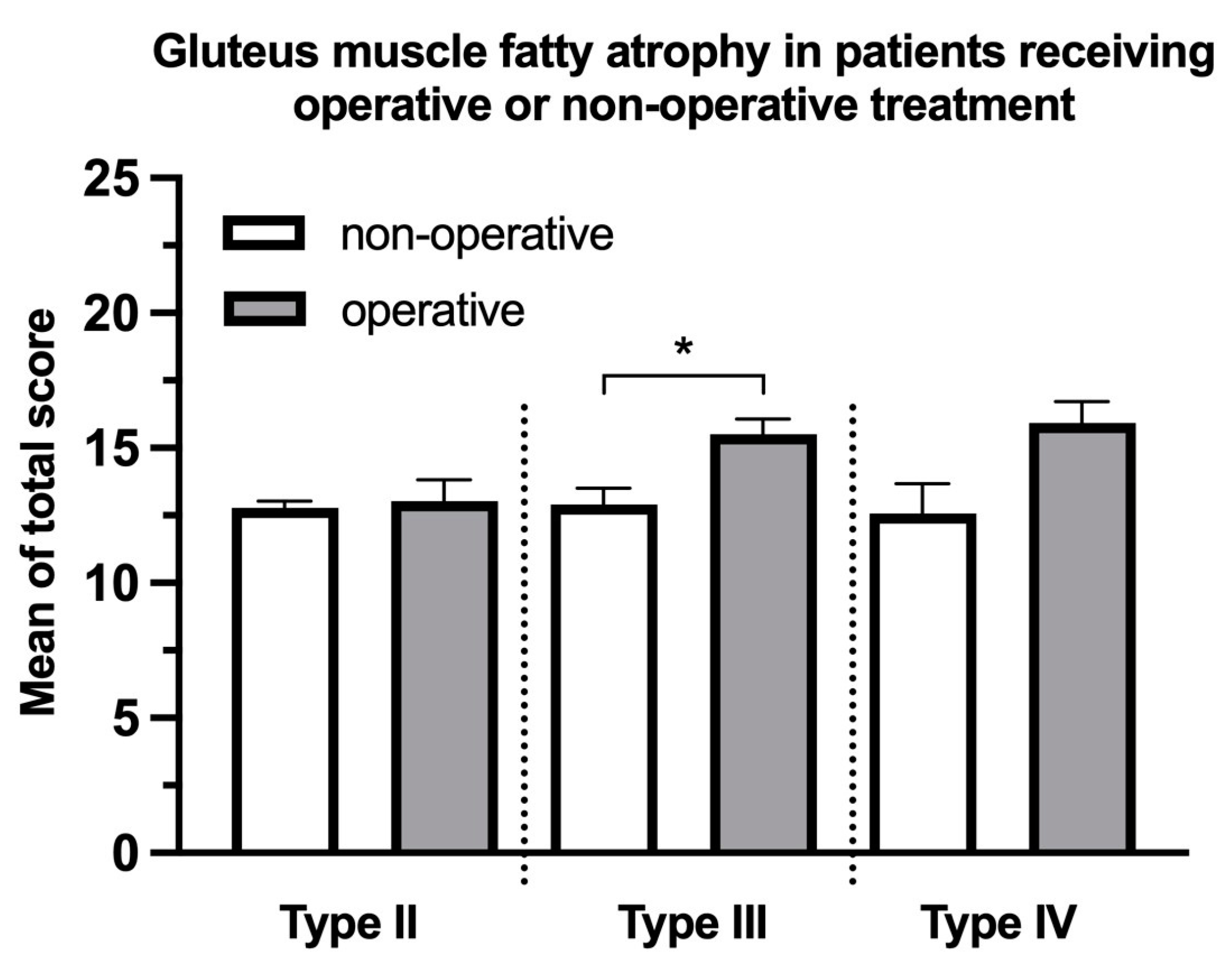
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kannus, P.; Parkkari, J.; Niemi, S.; Sievanen, H. Low-Trauma Pelvic Fractures in Elderly Finns in 1970–2013. Calcif. Tissue Int. 2015, 97, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Andrich, S.; Haastert, B.; Neuhaus, E.; Neidert, K.; Arend, W.; Ohmann, C.; Grebe, J.; Vogt, A.; Jungbluth, P.; Rosler, G.; et al. Epidemiology of Pelvic Fractures in Germany: Considerably High Incidence Rates among Older People. PLoS ONE 2015, 10, e0139078. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Alhambra, D.; Aviles, F.F.; Judge, A.; Van Staa, T.; Nogues, X.; Arden, N.K.; Diez-Perez, A.; Cooper, C.; Javaid, M.K. Burden of pelvis fracture: A population-based study of incidence, hospitalisation and mortality. Osteoporos. Int. 2012, 23, 2797–2803. [Google Scholar] [CrossRef] [PubMed]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef]
- Rommens, P.M.; Arand, C.; Hofmann, A.; Wagner, D. When and How to Operate Fragility Fractures of the Pelvis? Indian J. Orthop. 2019, 53, 128–137. [Google Scholar] [CrossRef]
- Al Saedi, A.; Debruin, D.A.; Hayes, A.; Hamrick, M. Lipid metabolism in sarcopenia. Bone 2022, 164, 116539. [Google Scholar] [CrossRef]
- Eken, G.; Misir, A.; Tangay, C.; Atici, T.; Demirhan, N.; Sener, N. Effect of muscle atrophy and fatty infiltration on mid-term clinical, and functional outcomes after Achilles tendon repair. Foot Ankle Surg. 2021, 27, 730–735. [Google Scholar] [CrossRef]
- McCrum, E. MR Imaging of the Rotator Cuff. Magn. Reson. Imaging Clin. N. Am. 2020, 28, 165–179. [Google Scholar] [CrossRef]
- Sadler, S.; Cassidy, S.; Peterson, B.; Spink, M.; Chuter, V. Gluteus medius muscle function in people with and without low back pain: A systematic review. BMC Musculoskelet. Disord. 2019, 20, 463. [Google Scholar] [CrossRef]
- Semciw, A.I.; Green, R.A.; Murley, G.S.; Pizzari, T. Gluteus minimus: An intramuscular EMG investigation of anterior and posterior segments during gait. Gait Posture 2014, 39, 822–826. [Google Scholar] [CrossRef]
- Flack, N.A.; Nicholson, H.D.; Woodley, S.J. A review of the anatomy of the hip abductor muscles, gluteus medius, gluteus minimus, and tensor fascia lata. Clin. Anat. 2012, 25, 697–708. [Google Scholar] [CrossRef]
- Daguet, E.; Jolivet, E.; Bousson, V.; Boutron, C.; Dahmen, N.; Bergot, C.; Vicaut, E.; Laredo, J.D. Fat content of hip muscles: An anteroposterior gradient. J. Bone Jt. Surg. 2011, 93, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.S.; Long, S.S.; Zoga, A.C.; Parker, L.; Morrison, W.B. Association of Gluteus Medius and Minimus Muscle Atrophy and Fall-Related Hip Fracture in Older Individuals Using Computed Tomography. J. Comput. Assist. Tomogr. 2016, 40, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Stasi, S.; Papathanasiou, G.; Chronopoulos, E.; Dontas, I.A.; Baltopoulos, I.P.; Papaioannou, N.A. The Effect of Intensive Abductor Strengthening on Postoperative Muscle Efficiency and Functional Ability of Hip-Fractured Patients: A Randomized Controlled Trial. Indian J. Orthop. 2019, 53, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Yerli, M.; Yuce, A.; Ayaz, M.B.; Bayraktar, T.O.; Erkurt, N.; Dedeoglu, S.S.; Imren, Y.; Gurbuz, H. Effect of psoas and gluteus medius muscles attenuation on hip fracture type. Hip Int. 2023, 33, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Howard, E.E.; Pasiakos, S.M.; Fussell, M.A.; Rodriguez, N.R. Skeletal Muscle Disuse Atrophy and the Rehabilitative Role of Protein in Recovery from Musculoskeletal Injury. Adv. Nutr. 2020, 11, 989–1001. [Google Scholar] [CrossRef]
- Rommens, P.M.; Hofmann, A. Comprehensive classification of fragility fractures of the pelvic ring: Recommendations for surgical treatment. Injury 2013, 44, 1733–1744. [Google Scholar] [CrossRef]
- Rommens, P.M.; Wagner, D.; Hofmann, A. Fragility Fractures of the Pelvis. JBJS Rev. 2017, 5, e3. [Google Scholar] [CrossRef]
- Lee, H.; Lee, E.; Jang, I.Y. Frailty and Comprehensive Geriatric Assessment. J. Korean Med. Sci. 2020, 35, e16. [Google Scholar] [CrossRef]
- Babayev, M.; Lachmann, E.; Nagler, W. The controversy surrounding sacral insufficiency fractures: To ambulate or not to ambulate? Am. J. Phys. Med. Rehabil. 2000, 79, 404–409. [Google Scholar] [CrossRef]
- Lee, D.G.; Bae, J.H. Fatty infiltration of the multifidus muscle independently increases osteoporotic vertebral compression fracture risk. BMC Musculoskelet. Disord. 2023, 24, 508. [Google Scholar] [CrossRef] [PubMed]
- Goutallier, D.; Postel, J.M.; Bernageau, J.; Lavau, L.; Voisin, M.C. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin. Orthop. Relat. Res. 1994, 304, 78–83. [Google Scholar] [CrossRef]
- Kong, C.; Zhang, Y.; Wang, C.; Wang, P.; Li, X.; Wang, W.; Wang, Y.; Shen, J.; Ren, X.; Wang, T.; et al. Comprehensive geriatric assessment for older orthopedic patients and analysis of risk factors for postoperative complications. BMC Geriatr. 2022, 22, 644. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Guo, J.; Zhu, B.; Dong, Y.; Li, F. Epidemiology and burden of pelvic fractures: Results from the Global Burden of Disease Study 2019. Injury 2023, 54, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Rau, C.S.; Lin, T.S.; Wu, S.C.; Yang, J.C.; Hsu, S.Y.; Cho, T.Y.; Hsieh, C.H. Geriatric hospitalizations in fall-related injuries. Scand. J. Trauma Resusc. Emerg. Med. 2014, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Faust, L.M.; Lerchenberger, M.; Gleich, J.; Linhart, C.; Keppler, A.M.; Schmidmaier, R.; Bocker, W.; Neuerburg, C.; Zhang, Y. Predictive Value of Prognostic Nutritional Index for Early Postoperative Mobility in Elderly Patients with Pertrochanteric Fracture Treated with Intramedullary Nail Osteosynthesis. J. Clin. Med. 2023, 12, 1792. [Google Scholar] [CrossRef]
- Greco, A.J.; Vilella, R.C. Anatomy, Bony Pelvis and Lower Limb, Gluteus Minimus Muscle; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Shah, A.; Bordoni, B. Anatomy, Bony Pelvis and Lower Limb, Gluteus Medius Muscle; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Inacio, M.; Ryan, A.S.; Bair, W.N.; Prettyman, M.; Beamer, B.A.; Rogers, M.W. Gluteal muscle composition differentiates fallers from non-fallers in community dwelling older adults. BMC Geriatr. 2014, 14, 37. [Google Scholar] [CrossRef]
- Lassche, S.; Rietveld, A.; Heerschap, A.; van Hees, H.W.; Hopman, M.T.; Voermans, N.C.; Saris, C.G.; van Engelen, B.G.; Ottenheijm, C.A. Muscle fiber dysfunction contributes to weakness in inclusion body myositis. Neuromuscul. Disord. 2019, 29, 468–476. [Google Scholar] [CrossRef]
- Hamrick, M.W.; McGee-Lawrence, M.E.; Frechette, D.M. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front. Endocrinol. 2016, 7, 69. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]

| n = 429 | % | Operation, n | % | Age, Mean ± SD | |||
|---|---|---|---|---|---|---|---|
| Gender | Male | 91 | 21.21% | 14 | 15.38% | 86.85 ± 4.49 | |
| Female | 338 | 78.79% | 49 | 14.50% | 87.42 ± 4.54 | ||
| Classification | I | 55 | 1 | 1.82% | 87.60 ± 4.02 | ||
| Ia | 52 | 94.55% | 0 | 0.00% | 87.77 ± 4.00 | ||
| Ib | 3 | 5.45% | 1 | 33.33% | 84.70 ± 3.92 | ||
| II | 286 | 29 | 10.14% | 87.16 ± 4.45 | |||
| IIa | 56 | 19.65% | 4 | 7.14% | 86.78 ± 5.68 | ||
| IIb | 37 | 12.98% | 6 | 16.22% | 86.64 ± 4.37 | ||
| IIc | 193 | 67.72% | 19 | 9.84% | 87.36 ± 4.25 | ||
| III | 68 | 20 | 29.41% | 88.26 ± 4.95 | |||
| IIIa | 14 | 19.72% | 1 | 7.14% | 89.85 ± 6.09 | ||
| IIIb | 22 | 30.99% | 8 | 36.36% | 88.24 ± 4.23 | ||
| IIIc | 32 | 45.07% | 11 | 34.38% | 87.57 ± 4.87 | ||
| IV | 20 | 13 | 65.00% | 85.78 ± 5.19 | |||
| IVa | 7 | 33.33% | 4 | 57.14% | 88.49 ± 6.22 | ||
| IVb | 6 | 28.57% | 5 | 83.33% | 83.41 ± 2.64 | ||
| IVc | 7 | 33.33% | 4 | 57.14% | 85.11 ± 5.00 |
| Factors | OR | 95% CI | p-Value |
|---|---|---|---|
| Sex (male) | 1.13 | 0.27–4.78 | 0.87 |
| Age | 0.93 | 0.83–1.05 | 0.26 |
| Total score | 1.22 | 1.01–1.47 | 0.04 * |
| HF or CAD | 0.63 | 0.12–3.31 | 0.39 |
| CKD | 1.90 | 0.42–8.53 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linhart, C.; Mehrens, D.; Gellert, L.M.; Ehrnthaller, C.; Gleich, J.; Lampert, C.; Lerchenberger, M.; Böcker, W.; Neuerburg, C.; Zhang, Y. Gluteal Muscle Fatty Atrophy: An Independent Risk Factor for Surgical Treatment in Elderly Patients Diagnosed with Type-III Fragility Fractures of the Pelvis. J. Clin. Med. 2023, 12, 6966. https://doi.org/10.3390/jcm12226966
Linhart C, Mehrens D, Gellert LM, Ehrnthaller C, Gleich J, Lampert C, Lerchenberger M, Böcker W, Neuerburg C, Zhang Y. Gluteal Muscle Fatty Atrophy: An Independent Risk Factor for Surgical Treatment in Elderly Patients Diagnosed with Type-III Fragility Fractures of the Pelvis. Journal of Clinical Medicine. 2023; 12(22):6966. https://doi.org/10.3390/jcm12226966
Chicago/Turabian StyleLinhart, Christoph, Dirk Mehrens, Luca Maximilian Gellert, Christian Ehrnthaller, Johannes Gleich, Christopher Lampert, Maximilian Lerchenberger, Wolfgang Böcker, Carl Neuerburg, and Yunjie Zhang. 2023. "Gluteal Muscle Fatty Atrophy: An Independent Risk Factor for Surgical Treatment in Elderly Patients Diagnosed with Type-III Fragility Fractures of the Pelvis" Journal of Clinical Medicine 12, no. 22: 6966. https://doi.org/10.3390/jcm12226966
APA StyleLinhart, C., Mehrens, D., Gellert, L. M., Ehrnthaller, C., Gleich, J., Lampert, C., Lerchenberger, M., Böcker, W., Neuerburg, C., & Zhang, Y. (2023). Gluteal Muscle Fatty Atrophy: An Independent Risk Factor for Surgical Treatment in Elderly Patients Diagnosed with Type-III Fragility Fractures of the Pelvis. Journal of Clinical Medicine, 12(22), 6966. https://doi.org/10.3390/jcm12226966






