Effect of Segmented Optical Axial Length on the Performance of New-Generation Intraocular Lens Power Calculation Formulas in Extremely Long Eyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Examinations
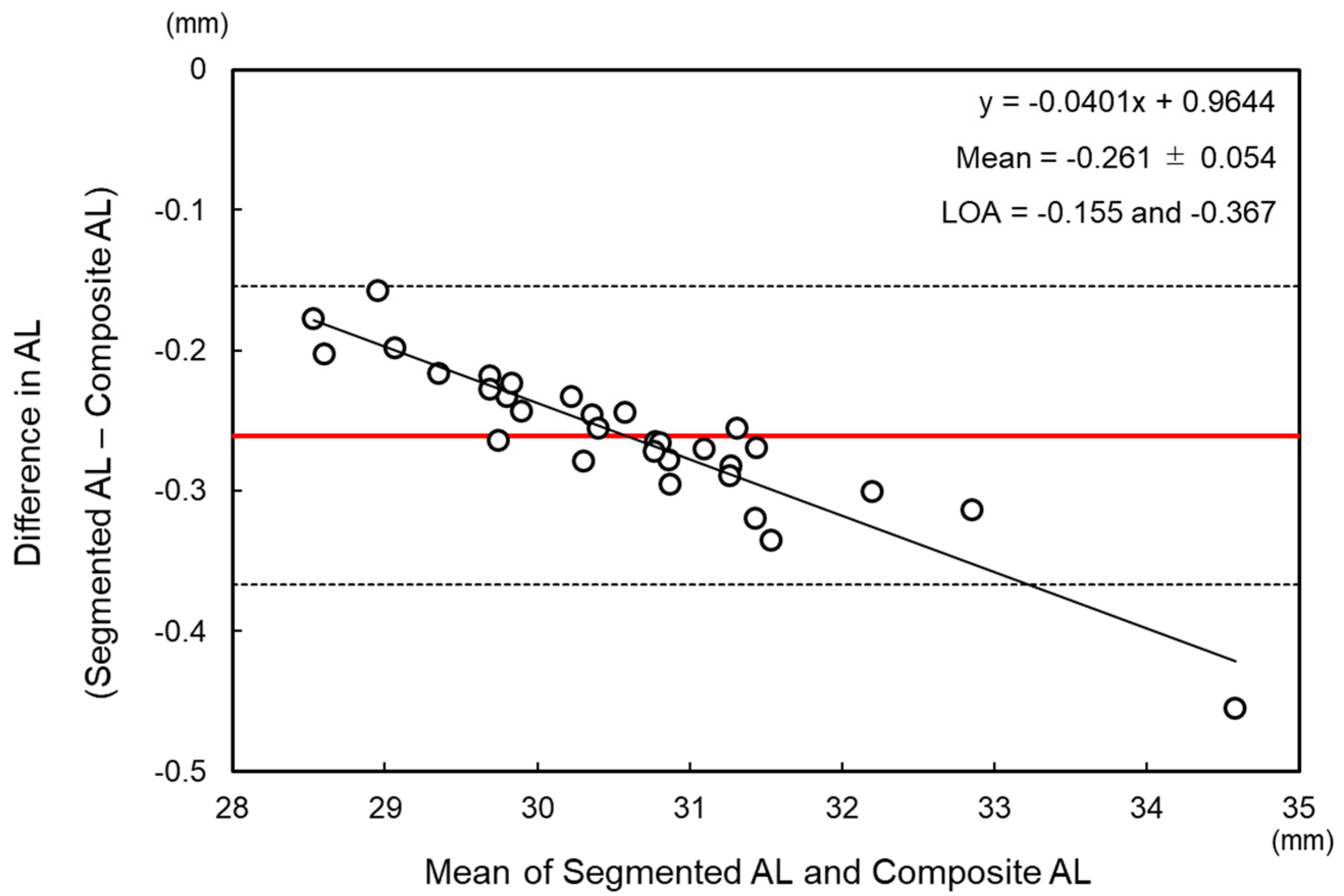
2.2. Calculation of Segmented Axial Length
ACD − 1.3837 × CCT/1000)/1.3394 + LT + [1.3496 × (0.9573 × Composite AL +
1.3304) − (1.3695 × ACD − 1.3837 × CCT/1000) − 1.4051 × LT − 1.3837 ×
CCT/1000]/1.3394 − 0.27
2.3. IOL Power Calculation and Data Collection
2.4. Statistical Analysis
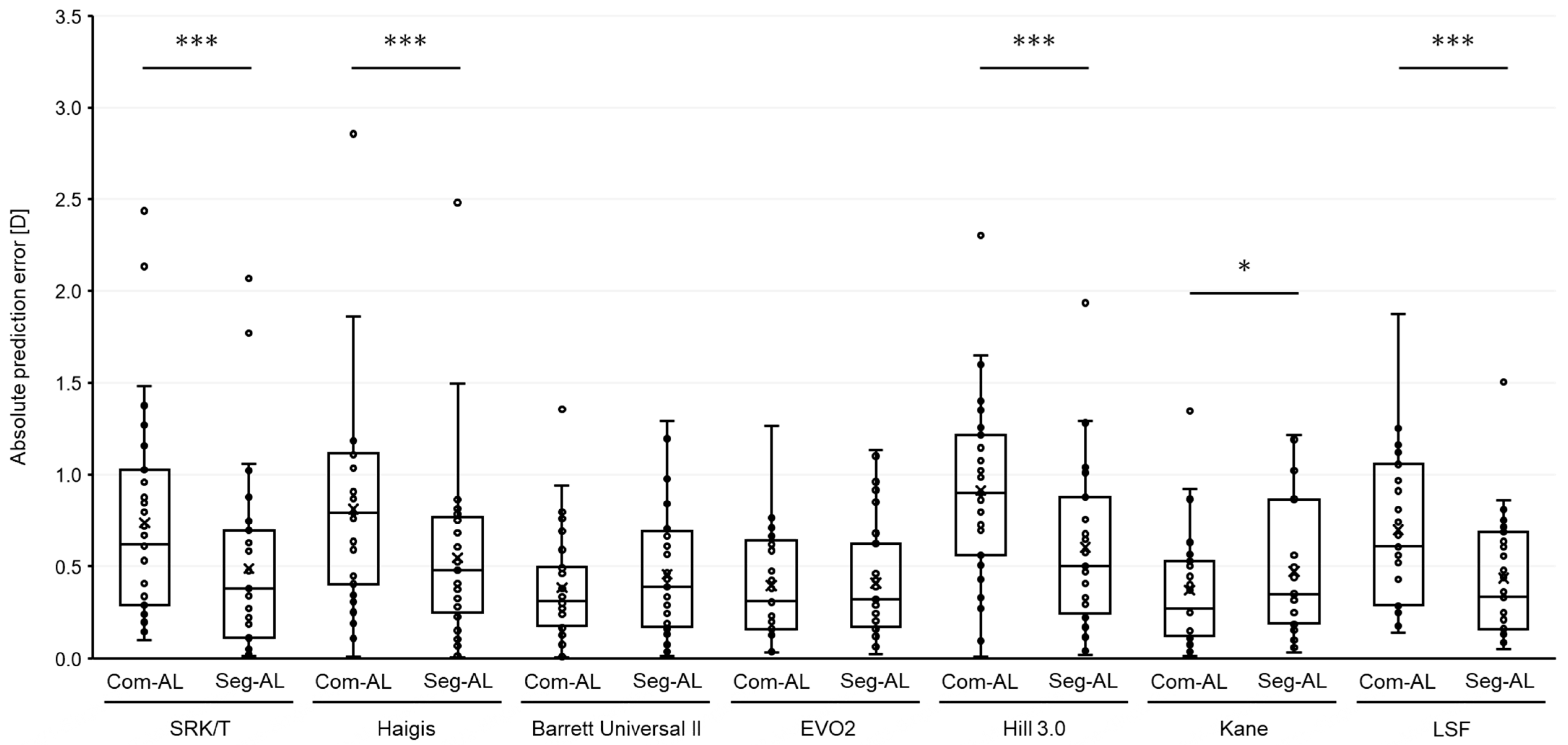
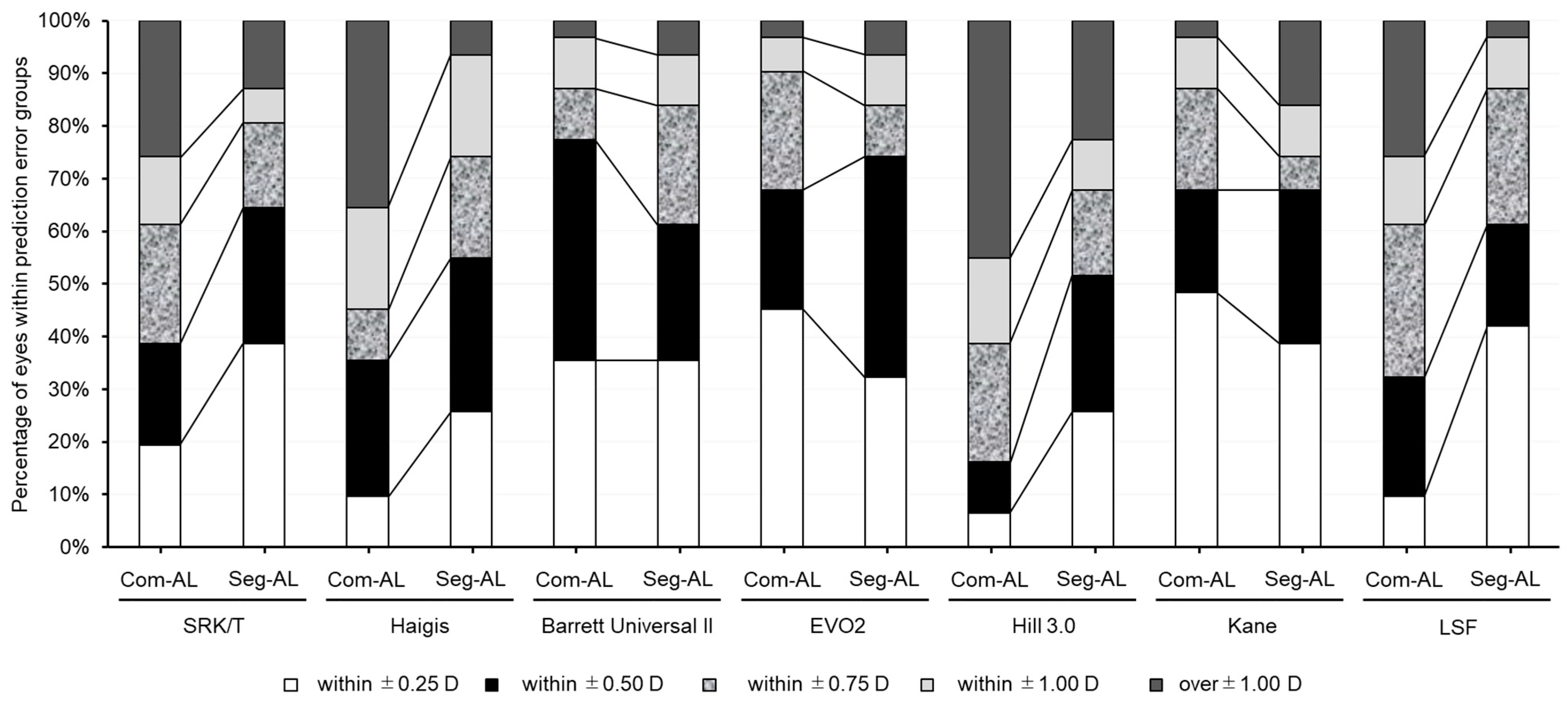
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.; Resnikoff, S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef]
- Flitcroft, D. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog. Retin. Eye Res. 2012, 31, 622–660. [Google Scholar] [CrossRef]
- Kanthan, G.L.; Mitchell, P.; Rochtchina, E.; Cumming, R.G.; Wang, J.J. Myopia and the long-term incidence of cataract and cataract surgery: The Blue Mountains Eye Study. Clin. Exp. Ophthalmol. 2014, 42, 347–353. [Google Scholar] [CrossRef]
- Wang, L.; Shirayama, M.; Ma, X.J.; Kohnen, T.; Koch, D.D. Optimizing intraocular lens power calculations in eyes with axial lengths above 25.0 mm. J. Cataract Refract. Surg. 2011, 37, 2018–2027. [Google Scholar] [CrossRef]
- Melles, R.B.; Holladay, J.T.; Chang, W.J. Accuracy of Intraocular Lens Calculation Formulas. Ophthalmology 2018, 125, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, Z.; Luo, Y.; Li, Z. Comparing the accuracy of the new-generation intraocular lens power calculation formulae in axial myopic eyes: A meta-analysis. Int. Ophthalmol. 2022, 43, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, L.; Kane, J.X.; Li, J.; Liu, L.; Wu, M. Accuracy of Artificial Intelligence Formulas and Axial Length Adjustments for Highly Myopic Eyes. Am. J. Ophthalmol. 2021, 223, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.L.; Cooke, T.L. A comparison of two methods to calculate axial length. J. Cataract Refract. Surg. 2019, 45, 284–292. [Google Scholar] [CrossRef]
- Wang, L.; Cao, D.; Weikert, M.P.; Koch, D.D. Calculation of axial length using a single group refractive index versus using different refractive indices for each ocular segment: Theoretical study and refractive outcomes. Ophthalmology 2018, 126, 663–670. [Google Scholar] [CrossRef]
- Goto, S.; Maeda, N.; Noda, T.; Ohnuma, K.; Koh, S.; Iehisa, I.; Nishida, K. Comparison of composite and segmental methods for acquiring optical axial length with swept-source optical coherence tomography. Sci. Rep. 2020, 10, 4474. [Google Scholar] [CrossRef]
- Drexler, W.; Findl, O.; Menapace, R.; Rainer, G.; Vass, C.; Hitzenberger, C.K.; Fercher, A.F. Partial coherence interferometry: A novel approach to biometry in cataract surgery. Am. J. Ophthalmol. 1998, 126, 524–534. [Google Scholar] [CrossRef]
- Olsen, T. Calculation of intraocular lens power: A review the statistical. Acta Ophthalmol. Scand. 2007, 85, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Norrby, S. Sources of error in intraocular lens power calculation. J. Cataract Refract. Surg. 2008, 34, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Miao, Y.; Savini, G.; McAlinden, C.; Chen, H.; Hu, Q.; Wang, Q. Precision of a new ocular biometer in eyes with cataract using swept source optical coherence tomography combined with Placido-disk corneal topography. Sci. Rep. 2017, 7, 13736. [Google Scholar] [CrossRef] [PubMed]
- Montés-Micó, R. Evaluation of six biometers based on different optical technologies. J. Cataract Refract. Surg. 2021, 48, 16. [Google Scholar] [CrossRef]
- Barrett, G.D. An improved universal theoretical formula for intraocular lens power prediction. J. Cataract Refract. Surg. 1993, 19, 713–720. [Google Scholar] [CrossRef]
- Warren, E.H. Hill-RBF Calculator Version 3.0. Available online: https://rbfcalculator.com/online/index.html (accessed on 30 December 2020).
- Connell, B.J.; Kane, J.X. Comparison of the Kane formula with existing formulas for intraocular lens power selection. BMJ Open Ophthalmol. 2019, 4, e000251. [Google Scholar] [CrossRef]
- Ladas, J.G.; Siddiqui, A.A.; Devgan, U.; Jun, A.S. A 3-D super surface combining modern intraocular lens formulas to generate a super formula and maximize accuracy. JAMA Ophthalmol. 2015, 133, 1431–1436. [Google Scholar] [CrossRef]
- Wang, L.; Koch, D.D. Modified axial length adjustment formulas in long eyes. J. Cataract Refract. Surg. 2018, 44, 1396–1397. [Google Scholar] [CrossRef]
- Melles, R.B.; Kane, J.X.; Olsen, T.; Chang, W.J. Update on Intraocular Lens Calculation Formulas. Ophthalmology 2019, 126, 1334–1335. [Google Scholar] [CrossRef]
- Ma, Y.; Xiong, R.; Liu, Z.; Young, C.A.; Wu, Y.; Zheng, D.; Zhang, X.; Jin, G. Network Meta-analysis of IOL Power Calculation Formula Accuracy in 1016 Eyes with Long Axial Length. Am. J. Ophthalmol. 2023, 17, S0002-9394(23)00375-6. [Google Scholar]
- Moshirfar, M.; Durnford, K.M.; Jensen, J.L.; Beesley, D.P.; Peterson, T.S.; Darquea, I.M.; Ronquillo, Y.C.; Hoopes, P.C. Accuracy of Six Intraocular Lens Power Calculations in Eyes with Axial Lengths Greater than 28.0 mm. J. Clin. Med. 2022, 11, 5947. [Google Scholar] [CrossRef]
- Omoto, M.; Sugawara, K.; Torii, H.; Yotsukura, E.; Masui, S.; Shigeno, Y.; Nishi, Y.; Negishi, K. Investigating the Prediction Accuracy of Recently Updated Intraocular Lens Power Formulas with Artificial Intelligence for High Myopia. J. Clin. Med. 2022, 11, 4848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tan, X.; Wang, W.; Yang, G.; Xu, J.; Ruan, X.; Gu, X.; Luo, L. Effect of Axial Length Adjustment Methods on Intraocular Lens Power Calculation in Highly Myopic Eyes. Am. J. Ophthalmol. 2020, 214, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Koch, D.D.; Hill, W.; Abulafia, A. Pursuing perfection in intraocular lens calculations: III. Criteria for analyzing outcomes. J. Cataract Refract. Surg. 2017, 43, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, K.J.; Savini, G. Update on Intraocular Lens Power Calculation Study Protocols. Ophthalmology 2021, 128, e115–e120. [Google Scholar] [CrossRef] [PubMed]
- Holladay, J.T.M.; Wilcox, R.R.P.D.; Koch, D.D.; Wang, L. Review and recommendations for univariate statistical analysis of spherical equivalent prediction error for IOL power calculations. J. Cataract Refract. Surg. 2021, 47, 65–77. [Google Scholar] [CrossRef]
- Tsessler, M.; Cohen, S.; Wang, L.; Koch, D.D.; Zadok, D.; Abulafia, A. Evaluating the prediction accuracy of the Hill-RBF 3.0 formula using a heteroscedastic statistical method. J. Cataract Refract. Surg. 2022, 48, 37–43. [Google Scholar] [CrossRef]

| Mean ± SD | Min | Max | |
|---|---|---|---|
| Age (years old) | 69.6 ± 10.0 | 48 | 91 |
| Gender: female (%) | 45 | ||
| Central corneal thickness (mm) | 524 ± 38 | 445 | 577 |
| Anterior chamber depth (mm) | 3.57 ± 0.36 | 2.51 | 4.2 |
| Lens thickness (mm) | 4.62 ± 0.43 | 3.71 | 5.65 |
| Composite axial length (mm) | 30.71 ± 1.28 | 28.62 | 34.81 |
| Segmented axial length (mm) | 30.45 ± 1.23 | 28.44 | 34.36 |
| Implanted IOL power (diopter) | 2.0 ± 1.8 | −3.0 | 5.0 |
| MPE | SD | MAE | MedAE | Maximum | ±0.5 (%) | ±1.0 (%) | |
|---|---|---|---|---|---|---|---|
| SRK/T-Com AL | 0.698 | 0.613 | 0.737 * | 0.619 | 2.44 | 38.7 | 74.2 * |
| SRK/T-Seg AL | 0.329 | 0.610 | 0.489 | 0.379 | 2.067 | 64.5 | 87.1 * |
| Haigis-Com AL | 0.768 | 0.620 | 0.810 | 0.791 | 2.86 | 35.5 * | 64.5 * |
| Haigis-Seg AL | 0.385 | 0.625 | 0.546 | 0.481 | 2.48 | 54.8 * | 93.5 * |
| BUII-Com AL | 0.076 | 0.472 | 0.371 | 0.300 | 1.355 | 77.4 | 96.8 |
| BUII-Seg AL | −0.319 | 0.475 | 0.457 | 0.390 | 0.975 | 61.3 | 93.5 |
| EVO2-Com AL | 0.160 | 0.469 | 0.396 | 0.310 | 1.265 | 67.7 | 96.8 |
| EVO2-Seg AL | −0.193 | 0.480 | 0.409 | 0.320 | 0.915 | 74.2 | 93.5 |
| Hill3-Com AL | 0.881 | 0.473 | 0.911 | 0.900 | 2.305 | 16.1 * | 58.1 * |
| Hill3-Seg AL | 0.559 | 0.491 | 0.603 | 0.500 | 1.935 | 51.6 * | 77.4 * |
| Kane-Com AL | −0.018 | 0.492 | 0.370 | 0.270 | 1.35 | 67.7 | 96.8 |
| Kane-Seg AL | −0.330 | 0.503 | 0.471 | 0.350 | 1.03 | 67.7 | 83.9 |
| LSF-Com AL | 0.447 | 0.762 | 0.699 | 0.610 | 1.875 | 32.3 | 74.2 * |
| LSF-Seg AL | 0.298 | 0.461 | 0.437 | 0.335 | 1.505 | 61.3 | 96.8 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goto, S.; Maeda, N.; Uehara, K.; Ogawa, K.; Matsumaru, M.; Sugiyama, S.; Ohnuma, K.; Lawu, T.; Noda, T. Effect of Segmented Optical Axial Length on the Performance of New-Generation Intraocular Lens Power Calculation Formulas in Extremely Long Eyes. J. Clin. Med. 2023, 12, 6959. https://doi.org/10.3390/jcm12226959
Goto S, Maeda N, Uehara K, Ogawa K, Matsumaru M, Sugiyama S, Ohnuma K, Lawu T, Noda T. Effect of Segmented Optical Axial Length on the Performance of New-Generation Intraocular Lens Power Calculation Formulas in Extremely Long Eyes. Journal of Clinical Medicine. 2023; 12(22):6959. https://doi.org/10.3390/jcm12226959
Chicago/Turabian StyleGoto, So, Naoyuki Maeda, Kota Uehara, Keiko Ogawa, Maki Matsumaru, Saori Sugiyama, Kazuhiko Ohnuma, Tjundewo Lawu, and Toru Noda. 2023. "Effect of Segmented Optical Axial Length on the Performance of New-Generation Intraocular Lens Power Calculation Formulas in Extremely Long Eyes" Journal of Clinical Medicine 12, no. 22: 6959. https://doi.org/10.3390/jcm12226959
APA StyleGoto, S., Maeda, N., Uehara, K., Ogawa, K., Matsumaru, M., Sugiyama, S., Ohnuma, K., Lawu, T., & Noda, T. (2023). Effect of Segmented Optical Axial Length on the Performance of New-Generation Intraocular Lens Power Calculation Formulas in Extremely Long Eyes. Journal of Clinical Medicine, 12(22), 6959. https://doi.org/10.3390/jcm12226959






