Comparison of Physical Activity Patterns among Three Major Chronic Respiratory Diseases
Abstract
:1. Introduction
2. Materials and Methods
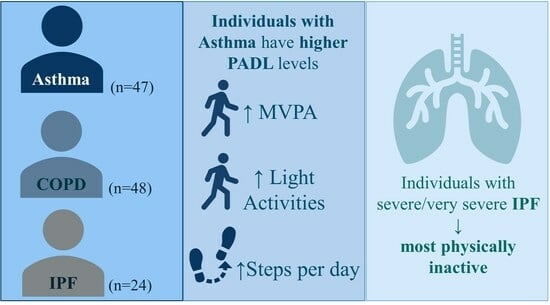
3. Results
3.1. Comparison of Physical Activity Levels among Different Chronic Respiratory Diseases
3.2. Comparison of Physical Activity Levels among Subjects with Different Severity of Chronic Respiratory Diseases
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saint-Maurice, P.F.; Troiano, R.P.; Bassett, D.R.; Graubard, B.I.; Carlson, S.A.; Shiroma, E.J.; Fulton, J.E.; Matthews, C.E. Association of Daily Step Count and Step Intensity with Mortality among US Adults. JAMA 2020, 323, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.P.; Wai, J.P.M.; Tsai, M.K.; Yang, Y.C.; Cheng, T.Y.D.; Lee, M.-C.; Chan, H.T.; Tsao, C.K.; Tsai, S.P.; Wu, X. Minimum amount of physical activity for reduced mortality and extended life expectancy: A prospective cohort study. Lancet 2011, 378, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Santos, E.; Frei, A.; Steurer-Stey, C.; de Batlle, J.; Rabinovich, R.A.; Raste, Y.; Hopkinson, N.S.; Polkey, M.I.; Van Remoortel, H.; Troosters, T.; et al. Determinants and outcomes of physical activity in patients with COPD: A systematic review. Thorax 2014, 69, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Waschki, B.; Kirsten, A.; Holz, O.; Muller, K.C.; Meyer, T.; Watz, H.; Magnussen, H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: A prospective cohort study. Chest 2011, 140, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, O.; Yamazaki, R.; Sano, H.; Iwanaga, T.; Higashimoto, Y.; Kume, H.; Tohda, Y. Physical activity in daily life in patients with idiopathic pulmonary fibrosis. Respir. Investig. 2018, 56, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Cordova-Rivera, L.; Gibson, P.G.; Gardiner, P.A.; McDonald, V.M. A Systematic Review of Associations of Physical Activity and Sedentary Time with Asthma Outcomes. J. Allergy Clin. Immunol. Pract. 2018, 6, 1968–1981.e2. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. 2023. Available online: http://goldcopd.org/ (accessed on 21 September 2023).
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2023. Available online: https://ginasthma.org/ (accessed on 21 September 2023).
- Faisal, A.; Alghamdi, B.J.; Ciavaglia, C.E.; Elbehairy, A.F.; Webb, K.A.; Ora, J.; Neder, J.A.; O'Donnell, D.E. Common Mechanisms of Dyspnea in Chronic Interstitial and Obstructive Lung Disorders. Am. J. Respir. Crit. Care Med. 2016, 193, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Watz, H.; Waschki, B.; Meyer, T.; Magnussen, H. Physical activity in patients with COPD. Eur. Respir. J. 2009, 33, 262–272. [Google Scholar] [CrossRef]
- Bahmer, T.; Kirsten, A.-M.; Waschki, B.; Rabe, K.F.; Magnussen, H.; Kirsten, D.; Gramm, M.; Hummler, S.; Brunnemer, E.; Kreuter, M.; et al. Clinical Correlates of Reduced Physical Activity in Idiopathic Pulmonary Fibrosis. Respiration 2016, 91, 497–502. [Google Scholar] [CrossRef]
- O'Donnell, D.E.; James, M.D.; Milne, K.M.; Neder, J.A. The Pathophysiology of Dyspnea and Exercise Intolerance in Chronic Obstructive Pulmonary Disease. Clin. Chest Med. 2019, 40, 343–366. [Google Scholar] [CrossRef]
- Mendes, P.; Wickerson, L.; Helm, D.; Janaudis-Ferreira, T.; Brooks, D.; Singer, L.G.; Mathur, S. Skeletal muscle atrophy in advanced interstitial lung disease. Respirology 2015, 20, 953–959. [Google Scholar] [CrossRef]
- Silva, H.; Mantoani, L.C.; Aguiar, W.F.; Goncalves, A.F.L.; da Silva, T.G.; Zamboti, C.L.; Ribeiro, M.; Probst, V.S.; Pitta, F.; Camillo, C.A. The impact of sleep duration on physical activity in daily life in patients with idiopathic pulmonary fibrosis. Physiother. Theory Pr. 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Spositon, T.; Cerci Neto, A.; Soares, F.M.C.; Pitta, F.; Furlanetto, K.C. Functional tests for adults with asthma: Validity, reliability, minimal detectable change, and feasibility. J. Asthma 2022, 59, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Spositon, T.; Rugila, D.F.; Pitta, F.; Furlanetto, K.C. Validity of the International Physical Activity Questionnaire (short form) in adults with asthma. PLoS ONE 2023, 18, e0282137. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.V.; Fontana, A.D.; Almeida, R.C.; Mantoani, L.C.; Camillo, C.A.; Furlanetto, K.C.; Rodrigues, F.; Cruz, J.; Marques, A.; Jácome, C.; et al. Cultural adaptation and validation of the Brazilian Portuguese version of the PROactive Physical Activity in COPD-clinical visit instrument for individuals with COPD. J. Bras. Pneumol. 2023, 49, e20220372. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, K.M.; Margaritopoulos, G.A.; Tomassetti, S.; Bonella, F.; Costabel, U.; Poletti, V. Interstitial lung disease. Eur. Respir. Rev. 2014, 23, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Pastre, J.; Barnett, S.; Ksovreli, I.; Taylor, J.; Brown, A.W.; Shlobin, O.A.; Ahmad, K.; Khangoora, V.; Aryal, S.; King, C.S.; et al. Idiopathic pulmonary fibrosis patients with severe physiologic impairment: Characteristics and outcomes. Respir. Res. 2021, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A.C.; Cordero, S.; Resende, A.C. Progressive fibrotic interstitial lung disease. J. Bras. Pneumol. 2023, 49, e20230098. [Google Scholar] [CrossRef]
- Van Remoortel, H.; Raste, Y.; Louvaris, Z.; Giavedoni, S.; Burtin, C.; Langer, D.; Wilson, F.; Rabinovich, R.; Vogiatzis, I.; Hopkinson, N.S.; et al. Validity of Six Activity Monitors in Chronic Obstructive Pulmonary Disease: A Comparison with Indirect Calorimetry. PLoS ONE 2012, 7, e39198. [Google Scholar] [CrossRef]
- Rabinovich, R.A.; Louvaris, Z.; Raste, Y.; Langer, D.; Van Remoortel, H.; Giavedoni, S.; Burtin, C.; Regueiro, E.M.; Vogiatzis, I.; Hopkinson, N.S.; et al. Validity of physical activity monitors during daily life in patients with COPD. Eur. Respir. J. 2013, 42, 1205–1215. [Google Scholar] [CrossRef]
- Demeyer, H.; Burtin, C.; Van Remoortel, H.; Hornikx, M.; Langer, D.; Decramer, M.; Gosselink, R.; Janssens, W.; Troosters, T. Standardizing the analysis of physical activity in patients with COPD following a pulmonary rehabilitation program. Chest 2014, 146, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Geidl, W.; Carl, J.; Cassar, S.; Lehbert, N.; Mino, E.; Wittmann, M.; Wagner, R.; Schultz, K.; Pfeifer, K. Physical Activity and Sedentary Behaviour Patterns in 326 Persons with COPD before Starting a Pulmonary Rehabilitation: A Cluster Analysis. J. Clin. Med. 2019, 8, 1346. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Crapo, R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. General considerations for lung function testing. Eur. Respir. J. 2005, 26, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A.; Sato, T.; Rodrigues, S.C. New reference values for forced spirometry in white adults in Brazil. J. Bras. Pneumol. 2007, 33, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Britto, R.R.; Probst, V.S.; de Andrade, A.F.; Samora, G.A.; Hernandes, N.A.; Marinho, P.E.; Karsten, M.; Pitta, F.; Parreira, V.F. Reference equations for the six-minute walk distance based on a Brazilian multicenter study. Braz. J. Phys. Ther. 2013, 17, 556–563. [Google Scholar] [CrossRef]
- Hopkinson, N.S.; Tennant, R.C.; Dayer, M.J.; Swallow, E.B.; Hansel, T.T.; Moxham, J.; Polkey, M.I. A prospective study of decline in fat free mass and skeletal muscle strength in chronic obstructive pulmonary disease. Respir. Res. 2007, 8, 25. [Google Scholar] [CrossRef]
- Cordova-Rivera, L.; Gibson, P.G.; Gardiner, P.A.; McDonald, V.M. Physical activity associates with disease characteristics of severe asthma, bronchiectasis and COPD. Respirology 2019, 24, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Breuls, S.; Pereira de Araujo, C.; Blondeel, A.; Yserbyt, J.; Janssens, W.; Wuyts, W.; Troosters, T.; Demeyer, H. Physical activity pattern of patients with interstitial lung disease compared to patients with COPD: A propensity-matched study. PLoS ONE 2022, 17, e0277973. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Janson, C.; Emtner, M. Accuracy of three activity monitors in patients with chronic obstructive pulmonary disease: A comparison with video recordings. COPD 2014, 11, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzis, I.; Zakynthinos, G.; Andrianopoulos, V. Mechanisms of physical activity limitation in chronic lung diseases. Pulm. Med. 2012, 2012, 634761. [Google Scholar] [CrossRef]
- Freitas, P.D.; Silva, A.G.; Ferreira, P.G.; Da Silva, A.; Salge, J.M.; Carvalho-Pinto, R.M.; Cukier, A.; Brito, C.M.; Mancini, M.C.; Carvalho, C.R.F. Exercise Improves Physical Activity and Comorbidities in Obese Adults with Asthma. Med. Sci. Sports Exerc. 2018, 50, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Rochester, C.L.; Alison, J.A.; Carlin, B.; Jenkins, A.R.; Cox, N.S.; Bauldoff, G.; Bhatt, S.P.; Bourbeau, J.; Burtin, C.; Camp, P.G.; et al. Pulmonary Rehabilitation for Adults with Chronic Respiratory Disease: An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2023, 208, e7–e26. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; Zuwallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An official american thoracic society/european respiratory society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- Mendoza, L.; Horta, P.; Espinoza, J.; Aguilera, M.; Balmaceda, N.; Castro, A.; Ruiz, M.; Diaz, O.; Hopkinson, N.S. Pedometers to enhance physical activity in COPD: A randomised controlled trial. Eur. Respir. J. 2015, 45, 347–354. [Google Scholar] [CrossRef]
- WHO Guidelines on Physical Activity and Sedentary Behaviour. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 21 September 2023).
- Demeyer, H.; Louvaris, Z.; Frei, A.; Rabinovich, R.A.; de Jong, C.; Gimeno-Santos, E.; Loeckx, M.; Buttery, S.C.; Rubio, N.; Van der Molen, T.; et al. Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: A multicentre randomised controlled trial. Thorax 2017, 72, 415–423. [Google Scholar] [CrossRef]
- Litrownik, D.; Gilliam, E.A.; Wayne, P.M.; Richardson, C.R.; Kadri, R.; Rist, P.M.; Moy, M.L.; Yeh, G.Y. Development of a Novel Intervention (Mindful Steps) to Promote Long-Term. Walking Behavior in Chronic Cardiopulmonary Disease: Protocol for a Randomized Controlled Trial. JMIR Res. Protoc. 2021, 10, e27826. [Google Scholar] [CrossRef]
- Shah Gupta, R.; Koteci, A.; Morgan, A.; George, P.M.; Quint, J.K. Incidence and prevalence of interstitial lung diseases worldwide: A systematic literature review. BMJ Open Respir. Res. 2023, 10, e001291. [Google Scholar] [CrossRef]


| Variables | Asthma (n = 47) | COPD (n = 48) | IPF (n = 24) |
|---|---|---|---|
| Age, years | 48 ± 15 a,b | 66 ± 9 | 63 ± 9 |
| Sex, M/F | 16/31 | 26/22 | 15/9 |
| BMI, kg/m2 | 28 ± 6 | 29 ± 5 | 28 ± 5 |
| FVC, %pred | 86 ± 16 b | 80 ± 19 | 67 ± 19 a |
| FEV1, %pred | 73 ± 17 a | 51 ± 18 | 70 ± 19 a |
| FEV1/FVC | 70 ± 11 a,b | 51 ± 11 | 84 ± 6 a |
| 6MWT, meters | 551 ± 95 a,b | 443 ± 92 | 440 ± 101 |
| 6MWT, %pred | 98 ± 13 a,b | 84 ± 17 | 77 ± 24 |
| QF_MVC, Kgf | 21 ± 12 b | 24 ± 10 + | 36 ± 14 a |
| Variables | Asthma (n = 32) | COPD (n = 24) | IPF (n = 6) | p-Ancova |
|---|---|---|---|---|
| Sedentary, min/day | 383 (354–412) | 367 (333–400) | 437 (372–502) | 0.152 |
| Light, min/day | 269 (244–294) b | 232 (203–260) | 148 (92–203) a | 0.001 |
| MVPA, min/day | 18 (13–24) a | 7 (1–13) | 2 (−10–15) * | 0.027 |
| Steps/day, n/day | 5643 (4750–6536) b | 4273 (3248–5298) | 2324 (338–4309) | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantoani, L.C.; Furlanetto, K.C.; Camillo, C.A.; de Oliveira, J.M.; Polastri, C.; Schneider, L.P.; Zamboti, C.L.; Hernandes, N.A.; Pitta, F. Comparison of Physical Activity Patterns among Three Major Chronic Respiratory Diseases. J. Clin. Med. 2023, 12, 6832. https://doi.org/10.3390/jcm12216832
Mantoani LC, Furlanetto KC, Camillo CA, de Oliveira JM, Polastri C, Schneider LP, Zamboti CL, Hernandes NA, Pitta F. Comparison of Physical Activity Patterns among Three Major Chronic Respiratory Diseases. Journal of Clinical Medicine. 2023; 12(21):6832. https://doi.org/10.3390/jcm12216832
Chicago/Turabian StyleMantoani, Leandro Cruz, Karina Couto Furlanetto, Carlos Augusto Camillo, Joice Mara de Oliveira, Cláudia Polastri, Lorena Paltanin Schneider, Camile Ludovico Zamboti, Nidia Aparecida Hernandes, and Fabio Pitta. 2023. "Comparison of Physical Activity Patterns among Three Major Chronic Respiratory Diseases" Journal of Clinical Medicine 12, no. 21: 6832. https://doi.org/10.3390/jcm12216832
APA StyleMantoani, L. C., Furlanetto, K. C., Camillo, C. A., de Oliveira, J. M., Polastri, C., Schneider, L. P., Zamboti, C. L., Hernandes, N. A., & Pitta, F. (2023). Comparison of Physical Activity Patterns among Three Major Chronic Respiratory Diseases. Journal of Clinical Medicine, 12(21), 6832. https://doi.org/10.3390/jcm12216832