T-Cell Engaging Antibodies in Diffuse Large B Cell Lymphoma—An Update
Abstract
:1. Introduction
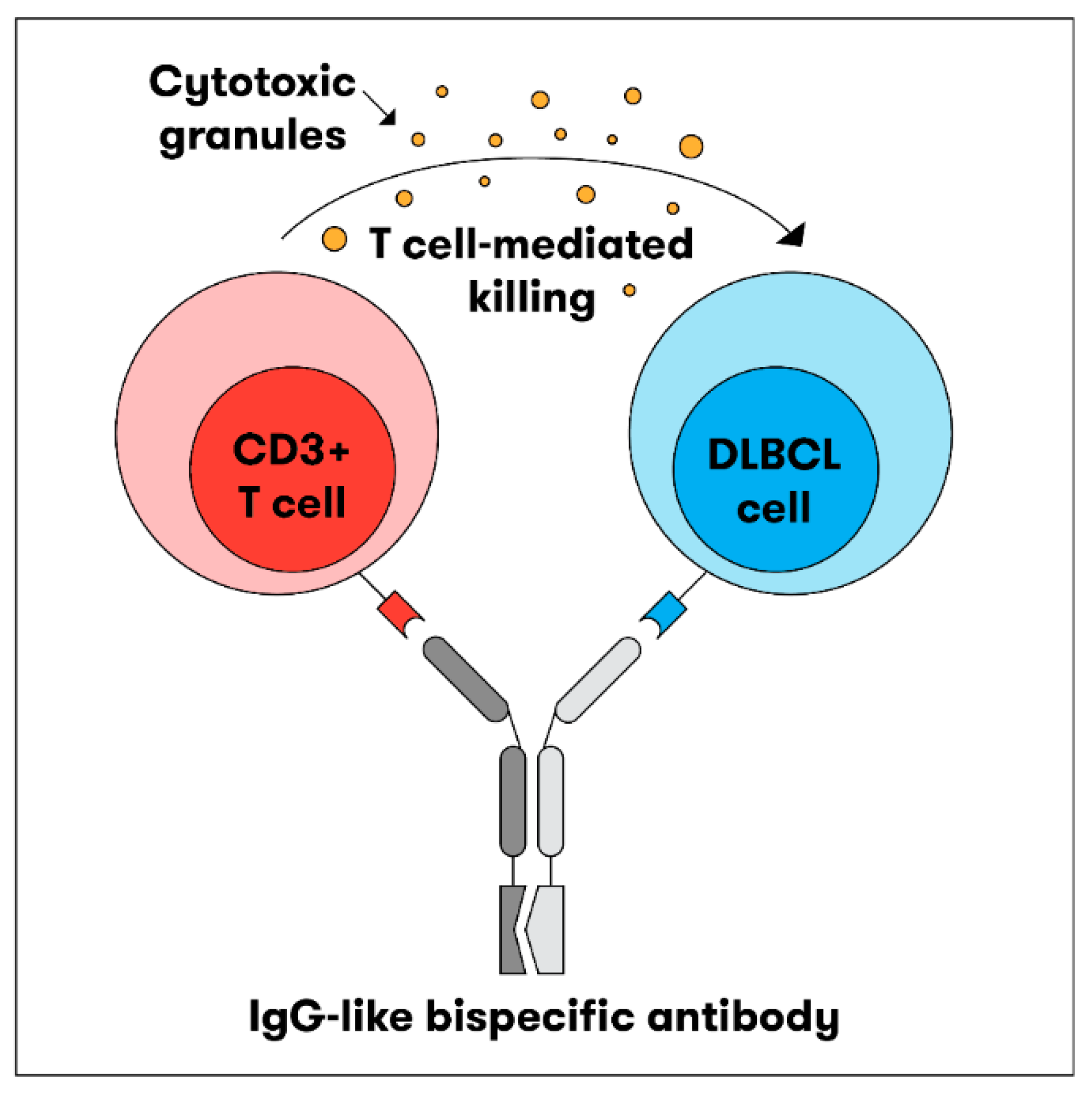
2. Basic Scientific Mechanism
3. Structure: TCEAbs Can Be Divided into Bispecific (BsAbs) and Trispecific Products (TsAbs)
3.1. Bispecific Antibodies
3.1.1. Non-IgG-like BsAbs
3.1.2. IgG-like BsAbs
3.2. Trispecific Antibodies (TsAbs)
4. Clinical Efficacy
4.1. CD19-Directed BsAb
Blinatumomab
4.2. CD20-Directed BsAb
4.2.1. Mosunetuzumab
4.2.2. Epcoritamab
4.2.3. Glofitamab
4.2.4. Odronextamab
4.2.5. Plamotamab
4.3. Trispecific Antibodies
4.3.1. JNJ-80948543
4.3.2. PIT565
5. Role in DLBCL Management
6. Management of TCEAb Mediated Toxicities
7. Mechanisms of Resistance
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morton, L.M.; Wang, S.S.; Devesa, S.S.; Hartge, P.; Weisenburger, D.D.; Linet, M.S. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 2006, 107, 265–276. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Crump, M.; Neelapu, S.S.; Farooq, U.; Van Den Neste, E.; Kuruvilla, J.; Westin, J.; Link, B.K.; Hay, A.; Cerhan, J.R.; Zhu, L.; et al. Outcomes in refractory diffuse large B-cell lymphoma: Results from the international SCHOLAR-1 study. Blood 2017, 130, 1800–1808. [Google Scholar] [CrossRef]
- Salles, G.A.; Pettengell, R.; Cordoba, R.; Dlugosz-Danecka, M.; Jurczak, W.; Tilly, H. Treatment of aggressive B-cell non-Hodgkin lymphoma beyond frontline therapy in patients not eligible for stem cell transplantation: A structured review. Leuk Lymphoma 2019, 60, 1610–1625. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Chalhoub, B.; Suzan, F.; Aloulou, S.; Cainap, C.; Toumi, N.; Ferme, C.; Carde, P.; Ribrag, V. Outcome of elderly patients with aggressive Non-Hodgkin’s lymphoma refractory to or relapsing after first-line CHOP or CHOP-like chemotherapy: A low probability of cure. Leuk Lymphoma 2004, 45, 1391–1394. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, O.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.-A.; Kersten, M.-J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl. J. Med. 2021, 386, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.R.; Dickinson, M.; Purtill, D.; Barba, P.; Santoro, A.; Hamad, N.; Kato, K.; Sureda, A.; Greil, R.; Thieblemont, C.; et al. Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 629–639. [Google Scholar] [CrossRef]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef]
- Kontermann, R.E.; Brinkmann, U. Bispecific antibodies. Drug Discov. Today 2015, 20, 838–847. [Google Scholar] [CrossRef]
- Duell, J.; Lammers, P.E.; Djuretic, I.; Chunyk, A.G.; Alekar, S.; Jacobs, I.; Gill, S. Bispecific Antibodies in the Treatment of Hematologic Malignancies. Clin. Pharmacol. Ther. 2019, 106, 781–791. [Google Scholar] [CrossRef]
- Fangazio, M.; Ladewig, E.; Gomez, K.; Garcia-Ibanez, L.; Kumar, R.; Teruya-Feldstein, J.; Rossi, D.; Filip, I.; Pan-Hammarström, Q.; Inghirami, G.; et al. Genetic mechanisms of HLA-I loss and immune escape in diffuse large B cell lymphoma. Proc. Natl. Acad. Sci. USA 2021, 118, e2104504118. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Dees, S.; Grewal, I.S. Overcoming the challenges associated with CD3+ T-cell redirection in cancer. Br. J. Cancer 2021, 124, 1037–1048. [Google Scholar] [CrossRef]
- Falchi, L.; Vardhana, S.A.; Salles, G.A. Bispecific antibodies for the treatment of B-cell lymphoma: Promises, unknowns, and opportunities. Blood 2023, 141, 467–480. [Google Scholar] [CrossRef]
- Tavarozzi, R.; Manzato, E. The Role of Bispecific Antibodies in Non-Hodgkin’s Lymphoma: From Structure to Prospective Clinical Use. Antibodies 2022, 11, 16. [Google Scholar] [CrossRef]
- Bates, A.; Power, C.A. David vs. Goliath: The Structure, Function, and Clinical Prospects of Antibody Fragments. Antibodies 2019, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.G.; Thomopoulos, T.P.; Liaskas, A.; Vassilakopoulos, T.P. Monoclonal Antibodies in the Treatment of Diffuse Large B-Cell Lymphoma: Moving beyond Rituximab. Cancers 2022, 14, 1917. [Google Scholar] [CrossRef]
- Li, H.; Er Saw, P.; Song, E. Challenges and strategies for next-generation bispecific antibody-based antitumor therapeutics. Cell Mol. Immunol. 2020, 17, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Salvaris, R.; Ong, J.; Gregory, G.P. Bispecific Antibodies: A Review of Development, Clinical Efficacy and Toxicity in B-Cell Lymphomas. J. Pers. Med. 2021, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Kuchnio, A.; Yang, D.; Vloemans, N.; Lowenstein, C.; Cornelissen, I.; Amorim, R.; Han, C.; Sukumaran, S.; Janssen, L.; Suls, T.; et al. Characterization of JNJ-80948543, a Novel CD79bxCD20xCD3 Trispecific T-Cell Redirecting Antibody for the Treatment of B-Cell Non-Hodgkin Lymphoma. Blood 2022, 140, 3105–3106. [Google Scholar] [CrossRef]
- Lu, H.; Oka, A.; Coulson, M.; Polli, J.R.; Aardalen, K.; Ramones, M.; Walker, D.B.; Carrion, A.; Alexander, D.; Klopfenstein, M.; et al. PIT565, a First-in-Class Anti-CD19, Anti-CD3, Anti-CD2 Trispecific Antibody for the Treatment of B Cell Malignancies. Blood 2022, 140, 3148. [Google Scholar] [CrossRef]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef]
- Gokbuget, N.; Dombret, H.; Bonifacio, M.; Reichle, A.; Graux, C.; Faul, C.; Diedrich, H.; Topp, M.S.; Bruggemann, M.; Horst, H.A.; et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 2018, 131, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Dufner, V.; Sayehli, C.M.; Chatterjee, M.; Hummel, H.D.; Gelbrich, G.; Bargou, R.C.; Goebeler, M.E. Long-term outcome of patients with relapsed/refractory B-cell non-Hodgkin lymphoma treated with blinatumomab. Blood Adv. 2019, 3, 2491–2498. [Google Scholar] [CrossRef]
- Coyle, L.; Morley, N.J.; Rambaldi, A.; Mason, K.D.; Verhoef, G.; Furness, C.L.; Zhang, A.; Jung, A.S.; Cohan, D.; Franklin, J.L. Open-Label, phase 2 study of blinatumomab as second salvage therapy in adults with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Leuk Lymphoma 2020, 61, 2103–2112. [Google Scholar] [CrossRef]
- Viardot, A.; Goebeler, M.E.; Hess, G.; Neumann, S.; Pfreundschuh, M.; Adrian, N.; Zettl, F.; Libicher, M.; Sayehli, C.; Stieglmaier, J.; et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood 2016, 127, 1410–1416. [Google Scholar] [CrossRef]
- Poh, C.; Frankel, P.; Ruel, C.; Abedi, M.; Schwab, E.; Costello, C.L.; Zain, J.; Budde, L.E.; William, B.M.; Foss, F.M.; et al. Blinatumomab/Lenalidomide in Relapsed/Refractory Non-Hodgkin’s Lymphoma: A Phase I California Cancer Consortium Study of Safety, Efficacy and Immune Correlative Analysis. Blood 2019, 134, 760. [Google Scholar] [CrossRef]
- Ghobadi, A.; Rettig, M.; Cashen, A.; Gehrs, L.; Christ, S.; Mehta-Shah, N.; Westervelt, P.; Kahl, B.; Bartlett, N.; Dipersio, J. Blinatumomab Consolidation Post Autologous Hematopoietic Stem Cell Transplantation in Patients with Diffuse Large B Cell Lymphoma. Blood 2020, 136, 3–4. [Google Scholar] [CrossRef]
- Budde, L.E.; Assouline, S.; Sehn, L.H.; Schuster, S.J.; Yoon, S.S.; Yoon, D.H.; Matasar, M.J.; Bosch, F.; Kim, W.S.; Nastoupil, L.J.; et al. Single-Agent Mosunetuzumab Shows Durable Complete Responses in Patients with Relapsed or Refractory B-Cell Lymphomas: Phase I Dose-Escalation Study. J. Clin. Oncol. 2022, 40, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.; Mous, R.; Clausen, M.R.; Johnson, P.; Linton, K.M.; Chamuleau, M.E.D.; Lewis, D.J.; Sureda Balari, A.; Cunningham, D.; Oliveri, R.S.; et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: An open-label, phase 1/2 study. Lancet 2021, 398, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Thieblemont, C.; Phillips, T.; Ghesquieres, H.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Do, Y.R.; Feldman, T.; Gasiorowski, R.; Jurczak, W.; et al. Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J. Clin. Oncol. 2023, 41, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Thieblemont, C.; Karimi, Y.; Jurczak, W.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Do, Y.R.; Lewis, D.J.; Gasiorowski, R.; Kim, T.M.; et al. Subcutaneous epcoritamab induces deep, durable complete remissions in relapsed/refractory large B-cell lymphoma: Longer follow-up from the pivotal epcore NHL-1 trial. Hematol. Oncol. 2023, 41, 142–144. [Google Scholar] [CrossRef]
- Bacac, M.; Colombetti, S.; Herter, S.; Sam, J.; Perro, M.; Chen, S.; Bianchi, R.; Richard, M.; Schoenle, A.; Nicolini, V.; et al. CD20-TCB with Obinutuzumab Pretreatment as Next-Generation Treatment of Hematologic Malignancies. Clin. Cancer Res. 2018, 24, 4785–4797. [Google Scholar] [CrossRef]
- Dickinson, M.J.; Carlo-Stella, C.; Morschhauser, F.; Bachy, E.; Corradini, P.; Iacoboni, G.; Khan, C.; Wrobel, T.; Offner, F.; Trneny, M.; et al. Glofitamab for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 387, 2220–2231. [Google Scholar] [CrossRef]
- Hutchings, M.; Avigdor, A.; Sureda, A.; Terol, M.J.; Bosch, F.; Corradini, P.; Larsen, T.S.; Domínguez, A.R.; Skarbnik, A.; Jørgensen, J.; et al. Glofitamab plus polatuzumab vedotin demonstrates durable responses and a manageable safety profile in patients with relapsed/refractory diffuse large B-cell lymphoma. Hematol. Oncol. 2023, 41, 138–140. [Google Scholar] [CrossRef]
- Smith, E.J.; Olson, K.; Haber, L.J.; Varghese, B.; Duramad, P.; Tustian, A.D.; Oyejide, A.; Kirshner, J.R.; Canova, L.; Menon, J.; et al. A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys. Sci. Rep. 2015, 5, 17943. [Google Scholar] [CrossRef]
- Bannerji, R.; Arnason, J.E.; Advani, R.H.; Brown, J.R.; Allan, J.N.; Ansell, S.M.; Barnes, J.A.; O’Brien, S.M.; Chavez, J.C.; Duell, J.; et al. Odronextamab, a human CD20xCD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): Results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022, 9, e327–e339. [Google Scholar] [CrossRef]
- Poon, M.; Walewski, J.; Kim, T.M.; Cho, S.; Jarque, I.; Iskierka-Jażdżewska, E.; Prince, H.M.; Oh, S.Y.; Lim, F.; Carpio, C.; et al. Odronextamab in patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL): Results from a prespecified analysis of the pivotal phase II study ELM-2. Hematol. Oncol. 2023, 41, 141–142. [Google Scholar] [CrossRef]
- Patel, K.; Riedell, P.A.; Tilly, H.; Ahmed, S.; Michot, J.-M.; Ghesquieres, H.; Schiano de Collela, J.M.; Chanan-Khan, A.; Bouabdallah, K.; Tessoulin, B.; et al. A Phase 1 Study of Plamotamab, an Anti-CD20 x Anti-CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Non-Hodgkin’s Lymphoma: Recommended Dose Safety/Efficacy Update and Escalation Exposure-Response Analysis. Blood 2022, 140, 9470–9472. [Google Scholar] [CrossRef]
- Patel, K.; Koh, Y.; Ayyappan, S.; Karimi, Y.; Lossos, I.S.; Merchant, A.; Lee, P.; Jin, J.; Clynes, R.; Kanodia, J.; et al. Phase 2 Randomized, Open-Label, Multicenter Study to Evaluate the Efficacy and Safety of Plamotamab Combined with Tafasitamab (Tafa) + Lenalidomide (Len) Vs Tafa+Len in Relapsed or Refractory DLBCL. Blood 2022, 140, 12066–12067. [Google Scholar] [CrossRef]
- Tilly, H.; Morschhauser, F.; Sehn, L.H.; Friedberg, J.W.; Trneny, M.; Sharman, J.P.; Herbaux, C.; Burke, J.M.; Matasar, M.; Rai, S.; et al. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 351–363. [Google Scholar] [CrossRef]
- Kamdar, M.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F.; et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): Results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 2022, 399, 2294–2308. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transpl. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- Shimabukuro-Vornhagen, A.; Godel, P.; Subklewe, M.; Stemmler, H.J.; Schlosser, H.A.; Schlaak, M.; Kochanek, M.; Boll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef]
- Winkler, U.; Jensen, M.; Manzke, O.; Schulz, H.; Diehl, V.; Engert, A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood 1999, 94, 2217–2224. [Google Scholar] [CrossRef]
- Brudno, J.N.; Kochenderfer, J.N. Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood 2016, 127, 3321–3330. [Google Scholar] [CrossRef]
- Maude, S.L.; Teachey, D.T.; Porter, D.L.; Grupp, S.A. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 2015, 125, 4017–4023. [Google Scholar] [CrossRef]
- Hutchings, M.; Morschhauser, F.; Iacoboni, G.; Carlo-Stella, C.; Offner, F.C.; Sureda, A.; Salles, G.; Martinez-Lopez, J.; Crump, M.; Thomas, D.N.; et al. Glofitamab, a Novel, Bivalent CD20-Targeting T-Cell-Engaging Bispecific Antibody, Induces Durable Complete Remissions in Relapsed or Refractory B-Cell Lymphoma: A Phase I Trial. J. Clin. Oncol. 2021, 39, 1959–1970. [Google Scholar] [CrossRef]
- Gust, J.; Hay, K.A.; Hanafi, L.A.; Li, D.; Myerson, D.; Gonzalez-Cuyar, L.F.; Yeung, C.; Liles, W.C.; Wurfel, M.; Lopez, J.A.; et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017, 7, 1404–1419. [Google Scholar] [CrossRef]
- Santomasso, B.D.; Park, J.H.; Salloum, D.; Riviere, I.; Flynn, J.; Mead, E.; Halton, E.; Wang, X.; Senechal, B.; Purdon, T.; et al. Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia. Cancer Discov. 2018, 8, 958–971. [Google Scholar] [CrossRef]
- Kennedy, A.D.; Beum, P.V.; Solga, M.D.; DiLillo, D.J.; Lindorfer, M.A.; Hess, C.E.; Densmore, J.J.; Williams, M.E.; Taylor, R.P. Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J. Immunol. 2004, 172, 3280–3288. [Google Scholar] [CrossRef]
- Foran, J.M.; Norton, A.J.; Micallef, I.N.; Taussig, D.C.; Amess, J.A.; Rohatiner, A.Z.; Lister, T.A. Loss of CD20 expression following treatment with rituximab (chimaeric monoclonal anti-CD20): A retrospective cohort analysis. Br. J. Haematol. 2001, 114, 881–883. [Google Scholar] [CrossRef] [PubMed]
- Brouwer-Visser, J.; Fiaschi, N.; Deering, R.; Dhanik, A.; Cygan, K.; Zhang, W.; Jeong, S.; Pourpe, S.; Boucher, L.; Hamon, S.; et al. Baseline Biomarkers of T-Cell Function Correlate with Clinical Responses to Odronextamab (REGN1979), and Loss of CD20 Target Antigen Expression Identified As a Mechanism of Treatment Resistance. Blood 2020, 136, 10–11. [Google Scholar] [CrossRef]
- Broske, A.E.; Korfi, K.; Belousov, A.; Wilson, S.; Ooi, C.H.; Bolen, C.R.; Canamero, M.; Alcaide, E.G.; James, I.; Piccione, E.C.; et al. Pharmacodynamics and molecular correlates of response to glofitamab in relapsed/refractory non-Hodgkin lymphoma. Blood Adv. 2022, 6, 1025–1037. [Google Scholar] [CrossRef]
- Pascual, M.; Mena-Varas, M.; Robles, E.F.; Garcia-Barchino, M.J.; Panizo, C.; Hervas-Stubbs, S.; Alignani, D.; Sagardoy, A.; Martinez-Ferrandis, J.I.; Bunting, K.L.; et al. PD-1/PD-L1 immune checkpoint and p53 loss facilitate tumor progression in activated B-cell diffuse large B-cell lymphomas. Blood 2019, 133, 2401–2412. [Google Scholar] [CrossRef]
- Shouval, R.; Alarcon Tomas, A.; Fein, J.A.; Flynn, J.R.; Markovits, E.; Mayer, S.; Olaide Afuye, A.; Alperovich, A.; Anagnostou, T.; Besser, M.J.; et al. Impact of TP53 Genomic Alterations in Large B-Cell Lymphoma Treated with CD19-Chimeric Antigen Receptor T-Cell Therapy. J. Clin. Oncol. 2022, 40, 369–381. [Google Scholar] [CrossRef] [PubMed]
- God, J.M.; Cameron, C.; Figueroa, J.; Amria, S.; Hossain, A.; Kempkes, B.; Bornkamm, G.W.; Stuart, R.K.; Blum, J.S.; Haque, A. Elevation of c-MYC disrupts HLA class II-mediated immune recognition of human B cell tumors. J. Immunol. 2015, 194, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Budde, E.; Gopal, A.K.; Kim, W.S.; Flinn, I.W.; Cheah, C.Y.Y.; Nastoupil, L.; Matasar, M.J.; Diefenbach, C.S.; Gregory, G.P.; Qazi, I.; et al. A Phase 1 Dose Escalation Study of Igm-2323, a Novel Anti-CD20 x Anti-CD3 IgM T Cell Engager (TCE) in Patients with Advanced B-Cell Malignancies. Blood 2021, 138, 132. [Google Scholar] [CrossRef]
| Drug Name | Structure | Epitopes (Ratio) | Format | ½ Life | Technology | Administration/Schedule |
|---|---|---|---|---|---|---|
| Blinatumomab [27] | 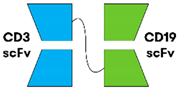 | CD19 × CD3 | IgG1 | 2.1 h | BiTE® (Amgen, Southend Oaks, CA, USA) | IV 4W continuous infusion (2W treatment-free interval) |
| Mosunetuzumab [16,31] | 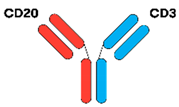 | CD20 × CD3 | IgG1 | 6–11 days | Knobs-into-holes | IV or SC 8–17 cycles SUD C1–C2 3W C3 onwards |
| Epcoritamab [16,33] | 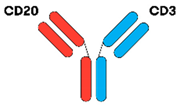 | CD20 × CD3 | IgG1 | 8.7 days | Controlled Fab-arm exchange | SC SUD C1 1W C1–C3. 2W C4–9. 4W C10 onwards |
| Glofitamab [16,36] | 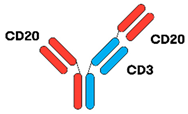 | (CD20)2 × CD3 | IgG1 | 10 days | Head-to-tail fusion | IV 12 cycles 1 × obinutuzumab (1000 mg) 7 days prior to first dose of glofitamab |
| Odronextamab [16,39] | 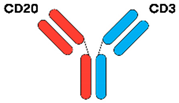 | CD20 × CD3 | IgG4 | 14 days | VelociSuite® (Regeneron, Tarrytown, NY, USA) | IV or SC SUD C1 W1 C2–C4 W2 C5 onwards |
| Plamotamab [16,41] |  | CD20 × CD3 | IgG1 | NR | XmAb® (Amgen, Southend Oaks, CA, USA) | IV or SC Weekly dosing |
| Imvotamab [16,61] | 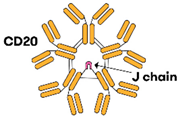 | (CD20)10 × CD3 | IgM | NR | IGM biosciences® (IGM biosciences, Mountain View, CA, USA) | IV Weekly or 3W dosing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balendran, S.; Tam, C.; Ku, M. T-Cell Engaging Antibodies in Diffuse Large B Cell Lymphoma—An Update. J. Clin. Med. 2023, 12, 6737. https://doi.org/10.3390/jcm12216737
Balendran S, Tam C, Ku M. T-Cell Engaging Antibodies in Diffuse Large B Cell Lymphoma—An Update. Journal of Clinical Medicine. 2023; 12(21):6737. https://doi.org/10.3390/jcm12216737
Chicago/Turabian StyleBalendran, Shalini, Constantine Tam, and Matthew Ku. 2023. "T-Cell Engaging Antibodies in Diffuse Large B Cell Lymphoma—An Update" Journal of Clinical Medicine 12, no. 21: 6737. https://doi.org/10.3390/jcm12216737
APA StyleBalendran, S., Tam, C., & Ku, M. (2023). T-Cell Engaging Antibodies in Diffuse Large B Cell Lymphoma—An Update. Journal of Clinical Medicine, 12(21), 6737. https://doi.org/10.3390/jcm12216737





