Mental Health for All: The Case for Investing in Digital Mental Health to Improve Global Outcomes, Access, and Innovation in Low-Resource Settings
Abstract
:1. Introduction
2. Methodology
3. Evidence, Efficacy, and Limitations
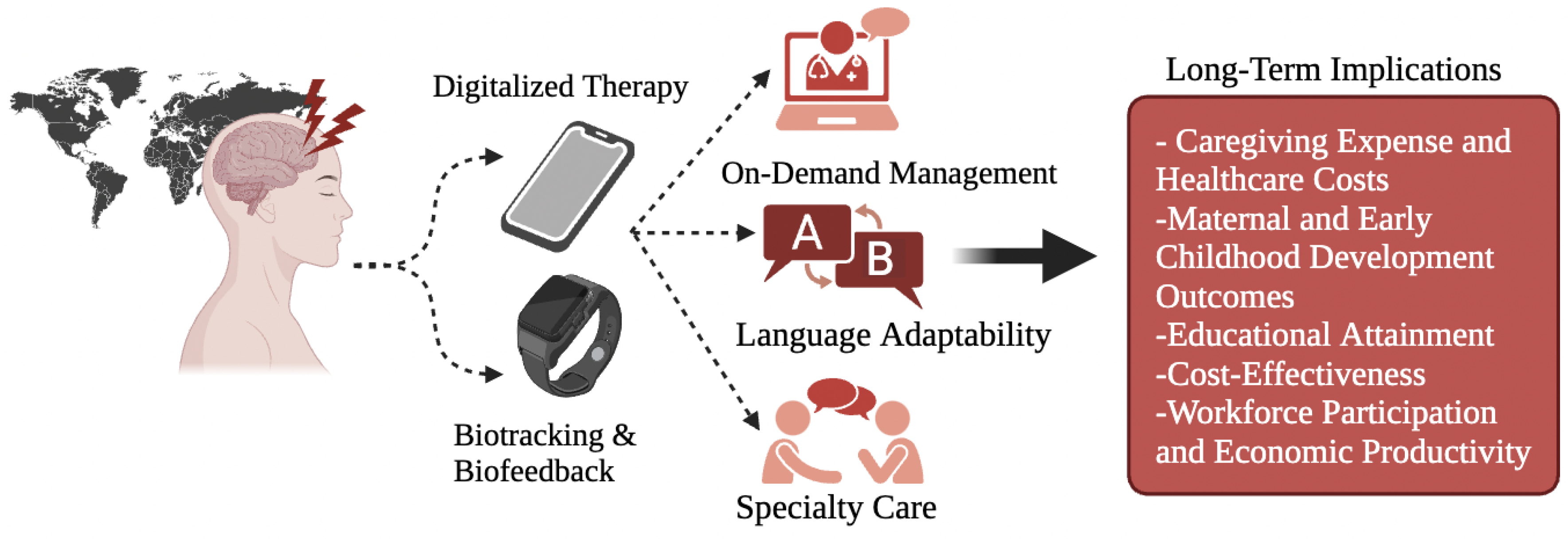
4. Innovation and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). The WHO Special Initiative for Mental Health (2019–2023): Universal Health Coverage for Mental Health; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimate; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Dattani, S.; Ritchie, H.; Roser, M. Mental Health—Our World in Data. 2021. Available online: https://ourworldindata.org/mental-health (accessed on 1 August 2022).
- Mental Health and COVID-19: Early Evidence of the Pandemic’s Impact: Scientific Brief, 2 March 2022. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Mental_health-2022.1 (accessed on 2 August 2023).
- Fahmy, H.; Tarun, D. (Eds.) Mental Health Atlas 2020; World Health Organization: Geneva, Switzerland, 2021; ISBN 9789240036703. [Google Scholar]
- COVID-19 Pandemic Triggers 25% Increase in Prevalence of Anxiety and Depression Worldwide. Available online: https://www.who.int/news/item/02-03-2022-covid-19-pandemic-triggers-25-increase-in-prevalence-of-anxiety-and-depression-worldwide (accessed on 2 August 2023).
- Wainberg, M.L.; Scorza, P.; Shultz, J.M.; Helpman, L.; Mootz, J.J.; Johnson, K.A.; Neria, Y.; Bradford, J.-M.E.; Oquendo, M.A.; Arbuckle, M.R. Challenges and Opportunities in Global Mental Health: A Research-to-Practice Perspective. Curr. Psychiatry Rep. 2017, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, T.A.; Scheffler, R.M.; Shen, G.; Yoon, J.; Chisholm, D.; Morris, J.; Fulton, B.D.; Dal Poz, M.R.; Saxena, S. The mental health workforce gap in low- and middle-income countries: A needs-based approach. Bull. World Health Organ. 2011, 89, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Lattie, E.G.; Stiles-Shields, C.; Graham, A.K. An overview of and recommendations for more accessible digital mental health services. Nat. Rev. Psychol. 2022, 1, 87–100. [Google Scholar] [CrossRef]
- Ghebreyesus, T.A. Use of Appropriate Digital Technologies for Public Health: Report by the Director-General; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Goldman, M.L.; Druss, B.G.; Horvitz-Lennon, M.; Norquist, G.S.; Kroeger Ptakowski, K.; Brinkley, A.; Greiner, M.; Hayes, H.; Hepburn, B.; Jorgensen, S.; et al. Mental Health Policy in the Era of COVID-19. Psychiatr. Serv. 2020, 71, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Global Internet Penetration Rate by Region 2023|Statista. Available online: https://www.statista.com/statistics/269329/penetration-rate-of-the-internet-by-region/ (accessed on 2 August 2023).
- World Health Organization Providing Guidance and Support for National mHealth Programming Since 2012. Available online: https://www.who.int/initiatives/behealthy/digital-health (accessed on 2 August 2023).
- Freeman, M. The World Mental Health Report: Transforming mental health for all. World Psychiatry 2022, 21, 391–392. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Lam, S.U.; Simonsson, O.; Torous, J.; Sun, S. Mobile phone-based interventions for mental health: A systematic meta-review of 14 meta-analyses of randomized controlled trials. PLOS Digit Health 2022, 1, e0000002. [Google Scholar] [CrossRef]
- Lehtimaki, S.; Martic, J.; Wahl, B.; Foster, K.T.; Schwalbe, N. Evidence on digital mental health interventions for adolescents and young people: Systematic overview. JMIR Ment Health 2021, 8, e25847. [Google Scholar] [CrossRef]
- Philippe, T.J.; Sikder, N.; Jackson, A.; Koblanski, M.E.; Liow, E.; Pilarinos, A.; Vasarhelyi, K. Digital Health Interventions for Delivery of Mental Health Care: Systematic and Comprehensive Meta-Review. JMIR Ment. Health 2022, 9, e35159. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, S.; Schofield, P.; Fisher, A.; Firth, J.; Jackson, S.E.; Stubbs, B.; Smith, L. Digital behavior change interventions to promote physical activity and/or reduce sedentary behavior in older adults: A systematic review and meta-analysis. Exp. Gerontol. 2019, 120, 68–87. [Google Scholar] [CrossRef]
- Brigden, A.; Anderson, E.; Linney, C.; Morris, R.; Parslow, R.; Serafimova, T.; Smith, L.; Briggs, E.; Loades, M.; Crawley, E. Digital behavior change interventions for younger children with chronic health conditions: Systematic review. J. Med. Internet Res. 2020, 22, e16924. [Google Scholar] [CrossRef]
- Olthuis, J.V.; Watt, M.C.; Bailey, K.; Hayden, J.A.; Stewart, S.H. Therapist-supported Internet cognitive behavioural therapy for anxiety disorders in adults. Cochrane Database Syst. Rev. 2015, CD011565. [Google Scholar] [CrossRef]
- Karyotaki, E.; Efthimiou, O.; Miguel, C.; Bermpohl, F.M.G.; Furukawa, T.A.; Cuijpers, P.; Individual Patient Data Meta-Analyses for Depression (IPDMA-DE) Collaboration; Riper, H.; Patel, V.; Mira, A.; et al. Internet-Based Cognitive Behavioral Therapy for Depression: A Systematic Review and Individual Patient Data Network Meta-analysis. JAMA Psychiatry 2021, 78, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Olthuis, J.V.; Wozney, L.; Asmundson, G.J.G.; Cramm, H.; Lingley-Pottie, P.; McGrath, P.J. Distance-delivered interventions for PTSD: A systematic review and meta-analysis. J. Anxiety Disord. 2016, 44, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, N.S.; Mascia, M.L.; Penna, M.P. Digital treatment paths for substance use disorders (suds). Int. J. Environ. Res. Public Health 2022, 19, 7322. [Google Scholar] [CrossRef] [PubMed]
- Linardon, J.; Shatte, A.; Messer, M.; Firth, J.; Fuller-Tyszkiewicz, M. E-mental health interventions for the treatment and prevention of eating disorders: An updated systematic review and meta-analysis. J. Consult. Clin. Psychol. 2020, 88, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Zachariae, R.; Lyby, M.S.; Ritterband, L.M.; O’Toole, M.S. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia—A systematic review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 2016, 30, 1–10. [Google Scholar] [CrossRef]
- Wootton, B.M. Remote cognitive-behavior therapy for obsessive-compulsive symptoms: A meta-analysis. Clin. Psychol. Rev. 2016, 43, 103–113. [Google Scholar] [CrossRef]
- Kennard, B.D.; Biernesser, C.; Wolfe, K.L.; Foxwell, A.A.; Craddock Lee, S.J.; Rial, K.V.; Patel, S.; Cheng, C.; Goldstein, T.; McMakin, D.; et al. Developing a brief suicide prevention intervention and mobile phone application: A qualitative report. J. Technol. Hum. Serv. 2015, 33, 345–357. [Google Scholar] [CrossRef]
- Carolan, S.; Harris, P.R.; Cavanagh, K. Improving Employee Well-Being and Effectiveness: Systematic Review and Meta-Analysis of Web-Based Psychological Interventions Delivered in the Workplace. J. Med. Internet Res. 2017, 19, e271. [Google Scholar] [CrossRef]
- Taylor, H.; Strauss, C.; Cavanagh, K. Can a little bit of mindfulness do you good? A systematic review and meta-analyses of unguided mindfulness-based self-help interventions. Clin. Psychol. Rev. 2021, 89, 102078. [Google Scholar] [CrossRef]
- Smith, E.N.; Santoro, E.; Moraveji, N.; Susi, M.; Crum, A.J. Integrating wearables in stress management interventions: Promising evidence from a randomized trial. Int. J. Stress Manag. 2020, 27, 172–182. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Park, M.J.; Jeon, H.J. Current advances in wearable devices and their sensors in patients with depression. Front. Psychiatry 2021, 12, 672347. [Google Scholar] [CrossRef]
- Lautman, Z.; Lev-Ari, S. The use of smart devices for mental health diagnosis and care. J. Clin. Med. 2022, 11, 5359. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Pei, J.; Hudec, K.; Shams, F.; Munthali, R.; Vigo, D. The Effects of Nonclinician Guidance on Effectiveness and Process Outcomes in Digital Mental Health Interventions: Systematic Review and Meta-analysis. J. Med. Internet Res. 2022, 24, e36004. [Google Scholar] [CrossRef]
- World Health Organization. mhGAP Humanitarian Intervention Guide (mhGAP-HIG): Clinical Management of Mental, Neurological and Substance Use Conditions in Humanitarian Emergencies; World Health Organization: Geneva, Switzerland, 2015; p. 60. ISBN 9789241548922. [Google Scholar]
- Mental Health and Substance Use. Available online: https://www.who.int/teams/mental-health-and-substance-use/policy-law-rights/qr-e-training (accessed on 2 August 2023).
- Cuijpers, P.; Heim, E.; Abi Ramia, J.; Burchert, S.; Carswell, K.; Cornelisz, I.; Knaevelsrud, C.; Noun, P.; van Klaveren, C.; Van’t Hof, E.; et al. Effects of a WHO-guided digital health intervention for depression in Syrian refugees in Lebanon: A randomized controlled trial. PLoS Med. 2023, 19, e1004025, Erratum in PLoS Med. 2023, 20, e1004231. [Google Scholar] [CrossRef]
- Salisbury, C.; O’Cathain, A.; Edwards, L.; Thomas, C.; Gaunt, D.; Hollinghurst, S.; Nicholl, J.; Large, S.; Yardley, L.; Lewis, G.; et al. Effectiveness of an integrated telehealth service for patients with depression: A pragmatic randomised controlled trial of a complex intervention. Lancet Psychiatry 2016, 3, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Burger, H.; Arjadi, R.; Bockting, C.L.H. Effectiveness of digital psychological interventions for mental health problems in low-income and middle-income countries: A systematic review and meta-analysis. Lancet Psychiatry 2020, 7, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, D.; Sweeny, K.; Sheehan, P.; Rasmussen, B.; Smit, F.; Cuijpers, P.; Saxena, S. Scaling-up treatment of depression and anxiety: A global return on investment analysis. Lancet Psychiatry 2016, 3, 415–424. [Google Scholar] [CrossRef]
- Gentili, A.; Failla, G.; Melnyk, A.; Puleo, V.; Tanna GL, D.; Ricciardi, W.; Cascini, F. The cost-effectiveness of digital health interventions: A systematic review of the literature. Front. Public Health 2022, 10, 787135. [Google Scholar] [CrossRef]
- Linardon, J.; Cuijpers, P.; Carlbring, P.; Messer, M.; Fuller-Tyszkiewicz, M. The efficacy of app-supported smartphone interventions for mental health problems: A meta-analysis of randomized controlled trials. World Psychiatry 2019, 18, 325–336. [Google Scholar] [CrossRef]
- Cuijpers, P.; Noma, H.; Karyotaki, E.; Cipriani, A.; Furukawa, T.A. Effectiveness and Acceptability of Cognitive Behavior Therapy Delivery Formats in Adults with Depression: A Network Meta-analysis. JAMA Psychiatry 2019, 76, 700–707. [Google Scholar] [CrossRef]
- Gan, D.Z.Q.; McGillivray, L.; Han, J.; Christensen, H.; Torok, M. Effect of Engagement with Digital Interventions on Mental Health Outcomes: A Systematic Review and Meta-Analysis. Front. Digit. Health 2021, 3, 764079. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guideline Recommendations on Digital Interventions for Health System Strengthening; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2019; ISBN 9789241550505. [Google Scholar]
- Dunn, J.; Kidzinski, L.; Runge, R.; Witt, D.; Hicks, J.L.; Schüssler-Fiorenza Rose, S.M.; Li, X.; Bahmani, A.; Delp, S.L.; Hastie, T.; et al. Wearable sensors enable personalized predictions of clinical laboratory measurements. Nat. Med. 2021, 27, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Smets, E.; Rios Velazquez, E.; Schiavone, G.; Chakroun, I.; D’Hondt, E.; De Raedt, W.; Cornelis, J.; Janssens, O.; Van Hoecke, S.; Claes, S.; et al. Large-scale wearable data reveal digital phenotypes for daily-life stress detection. NPJ Digit. Med. 2018, 1, 67. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; Simpkin, A.J.; Roshan, D.; Glynn, N.; Killilea, J.; Walsh, J.; Molloy, G.; Ganly, S.; Ryman, H.; Coen, E.; et al. Stress Monitoring Using Wearable Sensors: A Pilot Study and Stress-Predict Dataset. Sensors 2022, 22, 8135. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; El Atrache, R.; Yu, S.; Asif, U.; Jackson, M.; Roy, S.; Mirmomeni, M.; Cantley, S.; Sheehan, T.; Schubach, S.; et al. Seizure detection using wearable sensors and machine learning: Setting a benchmark. Epilepsia 2021, 62, 1807–1819. [Google Scholar] [CrossRef]
- Warrington, D.J.; Shortis, E.J.; Whittaker, P.J. Are wearable devices effective for preventing and detecting falls: An umbrella review (a review of systematic reviews). BMC Public Health 2021, 21, 2091. [Google Scholar] [CrossRef]
- Bayoumy, K.; Gaber, M.; Elshafeey, A.; Mhaimeed, O.; Dineen, E.H.; Marvel, F.A.; Martin, S.S.; Muse, E.D.; Turakhia, M.P.; Tarakji, K.G.; et al. Smart wearable devices in cardiovascular care: Where we are and how to move forward. Nat. Rev. Cardiol. 2021, 18, 581–599. [Google Scholar] [CrossRef]
- Sara, J.D.S.; Orbelo, D.; Maor, E.; Lerman, L.O.; Lerman, A. Guess What We Can Hear-Novel Voice Biomarkers for the Remote Detection of Disease. Mayo Clin. Proc. 2023, 98, 1353–1375. [Google Scholar] [CrossRef]
| Study | WHO Step-by-Step Intervention |
|---|---|
| Country, region | Lebanon, WHO Eastern Mediterranean region |
| Summary | A single-blind, 2-arm pragmatic randomized clinical trial, comparing guided Step-by-Step with enhanced care as usual (ECAU) among displaced Syrians suffering from depression and impaired functioning in Lebanon |
| Conditions | Depression, impaired functioning |
| Intervention | Step-by-Step is a 5-session intervention, designed to treat depression through an internet-connected device (hybrid app for iOS, Android, and web browsers). It includes 5 story sessions (divided into 3 smaller parts; 20 min taken to read altogether) which are illustrated, and audio recorded. Users are recommended to complete 1 session per week, amounting to a total period of 5 to 8 weeks. It is psychoeducation and training in behavioral activation through an illustrated narrative, with additional therapeutic techniques such as stress management, gratitude exercise, positive self-talk, strengthening social support, and relapse prevention. Users were supported by trained non-specialists (“e-helpers”) who offered weekly phone or message-based contact with users to provide support (up to 15 min per week). Users who accessed the intervention received email or phone notifications. |
| Outcome measure | Primary outcomes: depression (Patient Health Questionnaire, PHQ-9) and impaired functioning (WHO Disability Assessment Schedule-12, WHODAS) at post-treatment. Secondary outcomes: subjective well-being, anxiety, post-traumatic stress, and self-described problems. |
| Study size | A total of 569 participants were included in the study, with 283 randomized to the intervention and 286 to ECAU (enhanced care as usual). |
| Impact | Significant treatment effects were seen for both primary outcomes, depression, and functional impairment. Effect sizes were moderate for both primary outcomes. At 3 months follow-up, the intervention effect was maintained for depression (moderate to large effect sizes) and functional impairment (moderate effect size). For secondary outcomes, significant treatment effects were seen on all outcomes with moderate effect sizes. At 3 months follow-up, the intervention continues to be more significantly effective than ECAU. |
| Data collected | Primary Outcomes: baseline, post-test, and follow-up mean and standard deviation of scores for PHQ-9 and WHODAS. Secondary Outcomes: baseline, post-test, and follow-up mean and standard deviation of scores for WHO-5, GAD-7, PCL-5, and PSYCLOPS. |
| Fit-for-purpose | Relevance: the study addresses the pressing need for accessible mental health care for displaced populations, particularly in low- and middle-income countries where such services are scarce. Completeness: the study design is comprehensive, employing a single-blind, 2-arm pragmatic randomized clinical trial and a good sample size (n = 569). The tools used are realistic and forward-thinking. Accuracy: the study followed a well-defined protocol, and the intervention was delivered consistently across participants. Use of validated instruments, intention-to-treat analyses, multiple imputation methods to address missing data, and sensitivity analyses to evaluate bias. Timeliness: the study was conducted during the COVID-19 pandemic and addressed the urgent need for scalable mental health care in crisis-affected regions like Lebanon. Interpretability: clear and comprehensive results, with effects sized for primary and secondary outcomes. The intervention is shown to be effective in reducing mental health problems among displaced Syrians in Lebanon. The study emphasized need for further research. |
| Study | The Healthline Services: Integrated Telehealth Service for Patients with Depression |
|---|---|
| Country, region | England, WHO/Europe |
| Summary | A pragmatic, multicenter, randomized controlled trial comparing integrated Healthline Services with usual care among participants recruited from 43 general practices in 3 areas of England. |
| Conditions | Major Depression |
| Intervention | The Healthline Service consisted of regular telephone calls from non-clinical, trained health advisers who followed standardized scripts generated by interactive software. After an initial assessment and goal-setting telephone call, the advisers called each participant on six occasions over 4 months, and then made up to three more calls at intervals of roughly 2 months to provide reinforcement and to detect relapse. Advisers supported participants in the use of online resources (including computerized cognitive behavioral therapy) and sought to encourage healthier lifestyles, optimize medication, and improve treatment adherence. To be eligible, participants needed to have access to the internet and email. |
| Outcome measure | Primary outcome: proportion of participants responding to the intervention at 4 months after start of intervention (achieved PHQ-9 < 10 and reduction in PHQ-9 of ≥5 points). Baseline score of participants was PHQ-9 of at least 10. |
| Study size | A total of 609 participants were recruited, with 307 randomly assigned to Healthline Services plus usual care and 302 to usual care. Final sample size, n = 525 |
| Impact | The primary outcome showed that 27% of participants in the intervention group responded to treatment at 4 months, compared with 19% of 270 participants in the control group (adjusted odds ratio 1.7, 95% CI 1.1–2.5, p = 0.019). There was an overall treatment effect from all follow-up data at 4, 8, and 12 months in two repeated measures analyses, binary and continuous, suggesting a positive average effect of the intervention over the 12-month follow-up period. Mean PHQ-9 scores also decreased over time for both groups. Participants in the intervention group expressed greater satisfaction with access to health care, treatment, and amount of support they received. There was no evidence of improved adherence to antidepressant medication or improved care coordination or that participants made more use of other health-related technologies. |
| Data Collected | Primary outcome: baseline, post-test, and follow-up at 4, 8, and 12 months mean and standard deviation of scores for PHQ-9. Secondary outcome: baseline, post-test and follow-up at 4, 8, and 12 months mean and standard deviation of scores for GAD-7, EQ-5D-5L, heiQ, Morisky, eHEALS, and Haggerty. |
| Fit-for-Purpose | Relevance: the study design and intervention were relevant to address the need for expanding mental health care and improving outcomes for patients with depression. Completeness: the intervention was comprehensive and studied, addressing various components of the TECH model, which included realistic telehealth tools with evidence of effectiveness for depression. It included a good sample size of n = 525. Accuracy: the study followed a well-defined protocol, and the intervention was delivered consistently across participants. It used validated instruments, multiple imputation methods to address missing data, repeated measures analyses, mixes-effects logistic regressions, and sensitivity analyses. The study reported adjusted odds rations with confidence intervals as well as effect sizes for the secondary outcomes. Timeliness: the study was published recently and addressed ongoing challenges in mental health service delivery, at a time when mental health issues continue to be significant public health concerns worldwide. Interpretability: clear and comprehensive results, showing the intervention to be effective as a mental health care tool for patients with depression. The study highlighted the challenges in achieving sustained engagement with the intervention. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, M.; Zin, S.T.P.; Chestnov, R.; Novak, A.M.; Lev-Ari, S.; Snyder, M. Mental Health for All: The Case for Investing in Digital Mental Health to Improve Global Outcomes, Access, and Innovation in Low-Resource Settings. J. Clin. Med. 2023, 12, 6735. https://doi.org/10.3390/jcm12216735
Faria M, Zin STP, Chestnov R, Novak AM, Lev-Ari S, Snyder M. Mental Health for All: The Case for Investing in Digital Mental Health to Improve Global Outcomes, Access, and Innovation in Low-Resource Settings. Journal of Clinical Medicine. 2023; 12(21):6735. https://doi.org/10.3390/jcm12216735
Chicago/Turabian StyleFaria, Manuel, Stella Tan Pei Zin, Roman Chestnov, Anne Marie Novak, Shahar Lev-Ari, and Michael Snyder. 2023. "Mental Health for All: The Case for Investing in Digital Mental Health to Improve Global Outcomes, Access, and Innovation in Low-Resource Settings" Journal of Clinical Medicine 12, no. 21: 6735. https://doi.org/10.3390/jcm12216735
APA StyleFaria, M., Zin, S. T. P., Chestnov, R., Novak, A. M., Lev-Ari, S., & Snyder, M. (2023). Mental Health for All: The Case for Investing in Digital Mental Health to Improve Global Outcomes, Access, and Innovation in Low-Resource Settings. Journal of Clinical Medicine, 12(21), 6735. https://doi.org/10.3390/jcm12216735









