Differences in Prevalence of Transfusion Protocols between Critically Ill Neurologic and Non-Neurologic Patient Populations
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- SCCM|Critical Care Statistics. Available online: https://sccm.org/Communications/Critical-Care-Statistics (accessed on 22 April 2019).
- Halpern, N.A.; Pastores, S.M. Critical Care Medicine in the United States 2000-2005: An Analysis of Bed Numbers, Occupancy Rates, Payer Mix, and Costs. Crit. Care Med. 2010, 38, 65–71. [Google Scholar] [CrossRef]
- New York State Dept of Health Sepsis. Available online: https://www.health.ny.gov/diseases/conditions/sepsis/ (accessed on 30 April 2019).
- Lott, J.P.; Iwashyna, T.J.; Christie, J.D.; Asch, D.A.; Kramer, A.A.; Kahn, J.M. Critical Illness Outcomes in Specialty versus General Intensive Care Units. Am. J. Respir. Crit. Care Med. 2009, 179, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Hébert, P.C.; Wells, G.; Blajchman, M.A.; Marshall, J.; Martin, C.; Pagliarello, G.; Tweeddale, M.; Schweitzer, I.; Yetisir, E. A Multicenter, Randomized, Controlled Clinical Trial of Transfusion Requirements in Critical Care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N. Engl. J. Med. 1999, 340, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Hogshire, L.; Carson, J.L. Red Blood Cell Transfusion: What Is the Evidence When to Transfuse? Curr. Opin. Hematol. 2013, 20, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A. Red Blood Cell Transfusion in the Neurological ICU. Neurotherapeutics 2012, 9, 56–64. [Google Scholar] [CrossRef]
- Vincent, J.L.; Baron, J.-F.; Reinhart, K.; Gattinoni, L.; Thijs, L.; Webb, A.; Meier-Hellmann, A.; Nollet, G.; Peres-Bota, D.; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and Blood Transfusion in Critically Ill Patients. JAMA 2002, 288, 1499–1507. [Google Scholar] [CrossRef]
- Chang, T.S.; Jensen, M.B. Haemodilution for Acute Ischaemic Stroke. Cochrane Database Syst. Rev. 2014, 8, CD000103. [Google Scholar] [CrossRef]
- Tseng, M.Y.; Hutchinson, P.J.; Kirkpatrick, P.J. Effects of Fluid Therapy Following Aneurysmal Subarachnoid Haemorrhage: A Prospective Clinical Study. Br. J. Neurosurg. 2008, 22, 257–268. [Google Scholar] [CrossRef]
- Levine, J.; Kofke, A.; Cen, L.; Chen, Z.; Faerber, J.; Elliott, J.P.; Winn, H.R.; Le Roux, P. Red Blood Cell Transfusion Is Associated with Infection and Extracerebral Complications after Subarachnoid Hemorrhage. Neurosurgery 2010, 66, 312–318. [Google Scholar] [CrossRef]
- Festic, E.; Rabinstein, A.A.; Freeman, W.D.; Mauricio, E.A.; Robinson, M.T.; Mandrekar, J.; Zubair, A.C.; Lee, A.S.; Gajic, O. Blood Transfusion Is an Important Predictor of Hospital Mortality among Patients with Aneurysmal Subarachnoid Hemorrhage. Neurocritical Care 2013, 18, 209–215. [Google Scholar] [CrossRef]
- LeRoux, P. Haemoglobin Management in Acute Brain Injury. Curr. Opin. Crit. Care 2013, 19, 83–91. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, L.; Hebert, P.C.; Wells, G.; Fergusson, D.; Marshall, J.; Yetisir, E.; Blajchman, M.J. Is a Restrictive Transfusion Strategy Safe for Resuscitated and Critically Ill Trauma Patients? J. Trauma—Inj. Infect. Crit. Care 2004, 57, 563–568. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, L.A.; Fergusson, D.A.; Hutchison, J.S.; Pagliarello, G.; Marshall, J.C.; Yetisir, E.; Hare, G.M.T.; Hébert, P.C. Effect of a Liberal versus Restrictive Transfusion Strategy on Mortality in Patients with Moderate to Severe Head Injury. Neurocrit. Care 2006, 5, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Lelubre, C.; Bouzat, P.; Crippa, I.A.; Taccone, F.S. Anemia Management after Acute Brain Injury. Crit. Care 2016, 20, 152. [Google Scholar] [CrossRef]
- Kellert, L.; Schrader, F.; Ringleb, P.; Steiner, T.; Bosel, J. The Impact of Low Hemoglobin Levels and Transfusion on Critical Care Patients with Severe Ischemic Stroke: STroke: RelevAnt Impact of HemoGlobin, Hematocrit and Transfusion (STRAIGHT)--an Observational Study. J. Crit. Care 2014, 29, 236–240. [Google Scholar] [CrossRef]
- Sevransky, J.E.; Checkley, W.; Herrera, P.; Pickering, B.W.; Barr, J.; Brown, S.M.; Chang, S.Y.; Chong, D.; Kaufman, D.; Fremont, R.D.; et al. Protocols and Hospital Mortality in Critically Ill Patients: The United States Critical Illness and Injury Trials Group Critical Illness Outcomes Study. Crit. Care Med 2015, 43, 2076–2084. [Google Scholar] [CrossRef]
- Polito, C.C.; Cribbs, S.K.; Martin, G.S.; O’Keeffe, T.; Herr, D.; Rice, T.W.; Sevransky, J.E. Navigating the Institutional Review Board Approval Process in a Multicenter Observational Critical Care Study. Crit. Care Med. 2014, 42, 1105–1109. [Google Scholar] [CrossRef]
- Seitz, K.P.; Sevransky, J.E.; Martin, G.S.; Roback, J.D.; Murphy, D.J. Evaluation of RBC Transfusion Practice in Adult ICUs and the Effect of Restrictive Transfusion Protocols on Routine Care. Crit. Care Med. 2017, 45, 271–281. [Google Scholar] [CrossRef]
- Corwin, H.L.; Gettinger, A.; Pearl, R.G.; Fink, M.P.; Levy, M.M.; Abraham, E.; MacIntyre, N.R.; Shabot, M.M.; Duh, M.-S.; Shapiro, M.J. The CRIT Study: Anemia and Blood Transfusion in the Critically Ill--Current Clinical Practice in the United States. Crit. Care Med. 2004, 32, 39–52. [Google Scholar] [CrossRef]
- Prabhakaran, S.; Ruff, I.; Bernstein, R.A. Acute Stroke Intervention: A Systematic Review. JAMA 2015, 313, 1451–1462. [Google Scholar] [CrossRef]
- Khandelwal, P.; Yavagal, D.R.; Sacco, R.L. Acute Ischemic Stroke Intervention. J. Am. Coll. Cardiol. 2016, 67, 2631–2644. [Google Scholar] [CrossRef] [PubMed]
- Pierot, L.; Jayaraman, M.V.; Szikora, I.; Hirsch, J.A.; Baxter, B.; Miyachi, S.; Mahadevan, J.; Chong, W.; Mitchell, P.J.; Coulthard, A.; et al. Standards of Practice in Acute Ischemic Stroke Intervention: International Recommendations. J. Neurointerv. Surg. 2018, 10, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.S.; Hannay, H.J.; Yamal, J.M.; Gopinath, S.; Goodman, J.C.; Tilley, B.C.; Epo Severe TBI Trial Investigators; Baldwin, A.; Rivera Lara, L.; Saucedo-Crespo, H.; et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: A randomized clinical trial. JAMA 2014, 312, 36–47. [Google Scholar] [CrossRef] [PubMed]
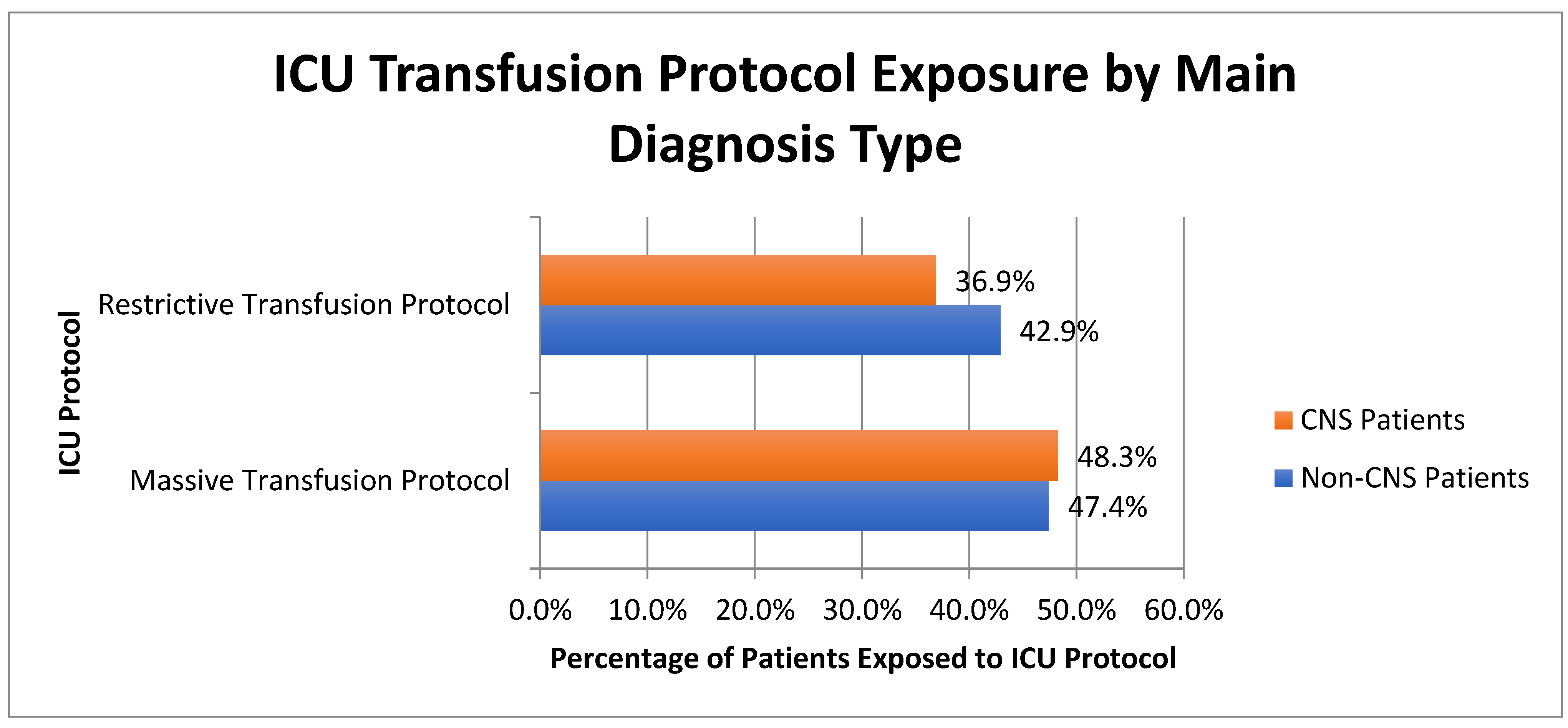
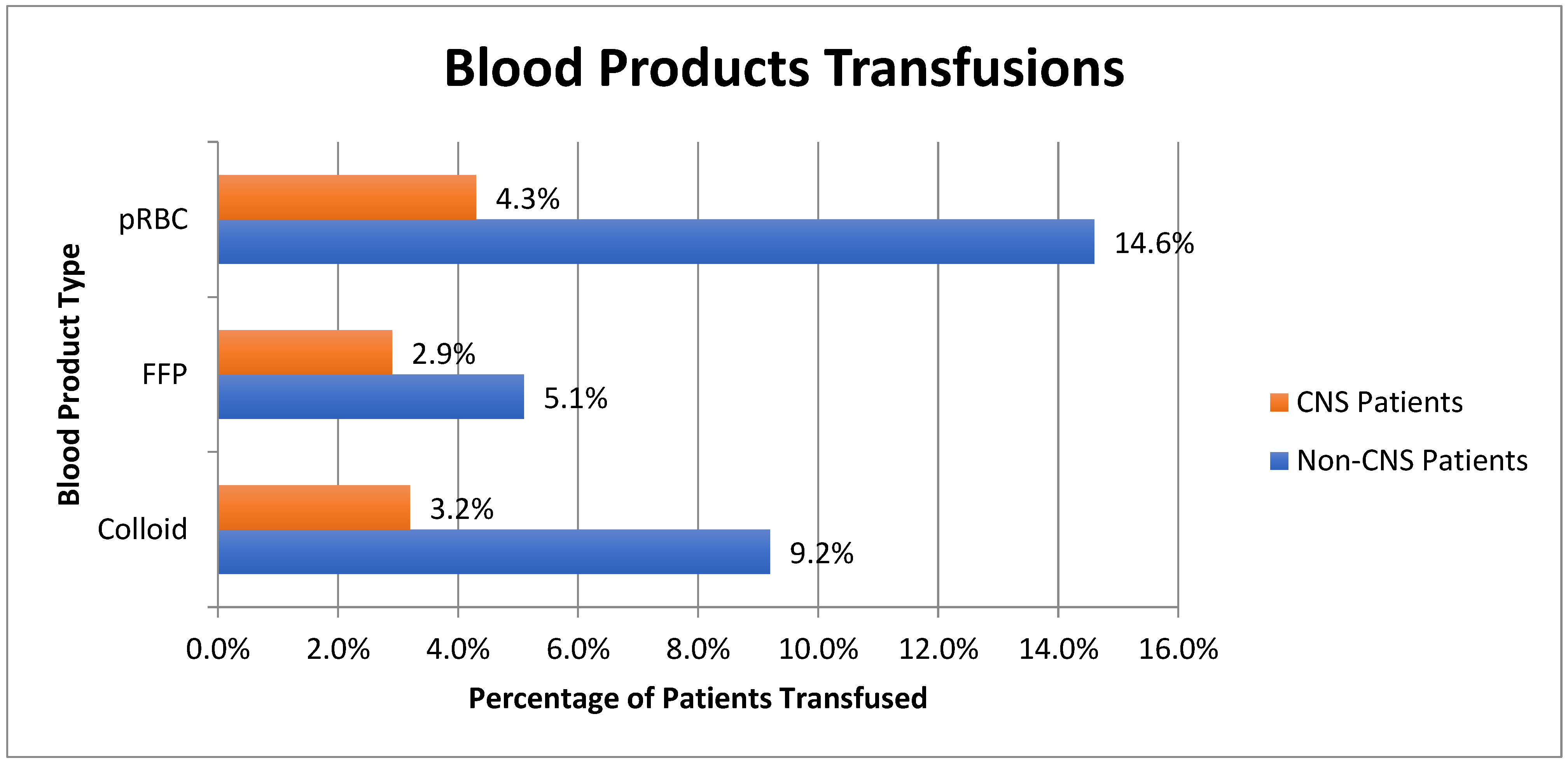
| Demographics | CNS Diagnosis | Non-CNS Diagnosis | p-Value |
|---|---|---|---|
| 1266 (20.4%) | 4913 (79.6%) | ||
| Age (mean) | 58 (SD = 18) | 60 (SD = 17) | p < 0.001 |
| Gender (M) | 693 (54.7%) | 2760 (52.2%) | p = 0.036 |
| Race | p < 0.001 | ||
| Black | 323 (25.5%) | 1029 (20.9%) | |
| White | 722 (57%) | 3458 (70.4%) | |
| Other | 157 (12.4%) | 288 (5.9%) | |
| Not reported | 64 (5.1%) | 138 (2.8%) | |
| ICU Classification | p < 0.001 | ||
| Medical | 422 (33.3%) | 2237 (45.5%) | |
| Surgical | 555 (43.8%) | 1624 (33.1%) | |
| Other | 289 (22.8%) | 1052 (21.4%) | |
| APACHE II (mean) | 16.2 (SD = 7.3) | 16.8 (SD = 7.4) | p = 0.047 |
| Admission Source | p < 0.01 | ||
| Emergency Department | 724 (57.2%) | 2006 (40.8%) | |
| Hospital | 162 (12.8%) | 990 (20.2%) | |
| Operating Room | 139 (11.0%) | 1092 (22.2%) | |
| Outside Hospital | 210 (16.6%) | 607 (12.4%) | |
| Other | 31 (2.4%) | 218 (4.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, T.M.; Billington, M.E.; Seethala, R.R.; Hou, P.C.; Askari, R.; Aisiku, I.P., on behalf of USIITG-CIOS Investigators. Differences in Prevalence of Transfusion Protocols between Critically Ill Neurologic and Non-Neurologic Patient Populations. J. Clin. Med. 2023, 12, 6633. https://doi.org/10.3390/jcm12206633
Oliveira TM, Billington ME, Seethala RR, Hou PC, Askari R, Aisiku IP on behalf of USIITG-CIOS Investigators. Differences in Prevalence of Transfusion Protocols between Critically Ill Neurologic and Non-Neurologic Patient Populations. Journal of Clinical Medicine. 2023; 12(20):6633. https://doi.org/10.3390/jcm12206633
Chicago/Turabian StyleOliveira, Thiago M., Michael E. Billington, Raghu R. Seethala, Peter C. Hou, Reza Askari, and Imoigele P. Aisiku on behalf of USIITG-CIOS Investigators. 2023. "Differences in Prevalence of Transfusion Protocols between Critically Ill Neurologic and Non-Neurologic Patient Populations" Journal of Clinical Medicine 12, no. 20: 6633. https://doi.org/10.3390/jcm12206633
APA StyleOliveira, T. M., Billington, M. E., Seethala, R. R., Hou, P. C., Askari, R., & Aisiku, I. P., on behalf of USIITG-CIOS Investigators. (2023). Differences in Prevalence of Transfusion Protocols between Critically Ill Neurologic and Non-Neurologic Patient Populations. Journal of Clinical Medicine, 12(20), 6633. https://doi.org/10.3390/jcm12206633






