The Expression of Activation Markers CD25 and CD69 Increases during Biologic Treatment of Psoriasis
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Group
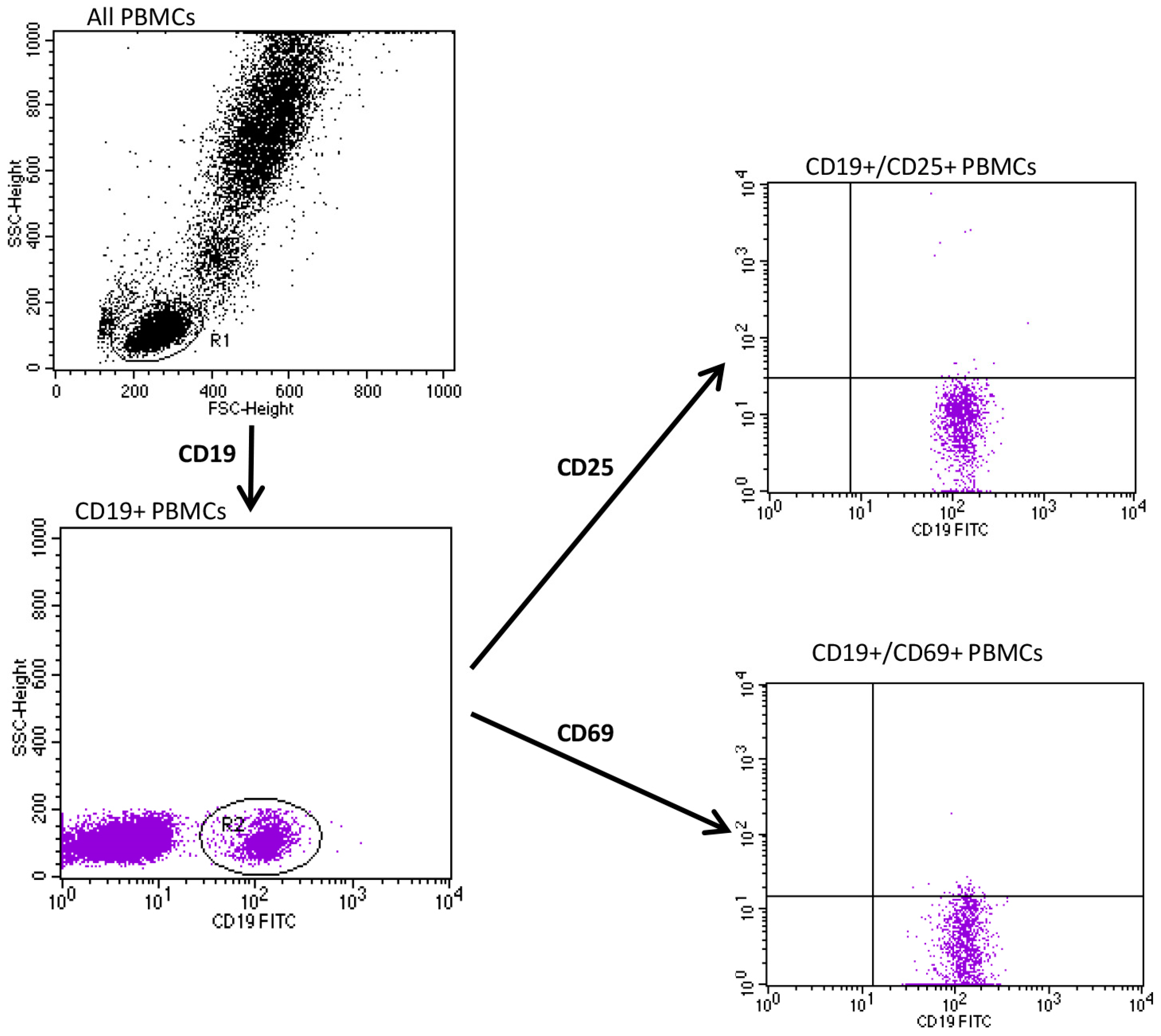
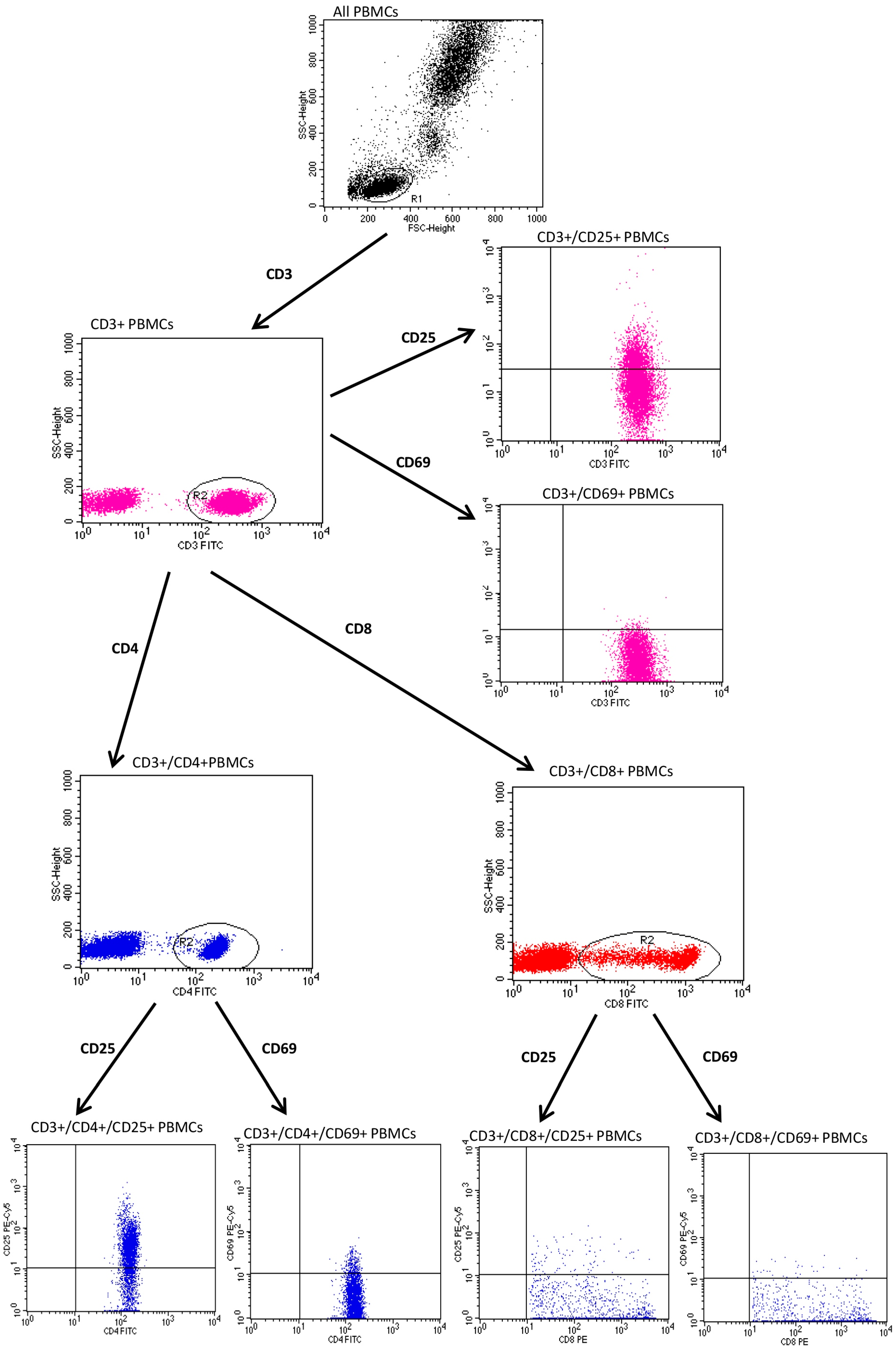
2.2. Evaluation of CD25 and CD69
2.3. Statistical Methods
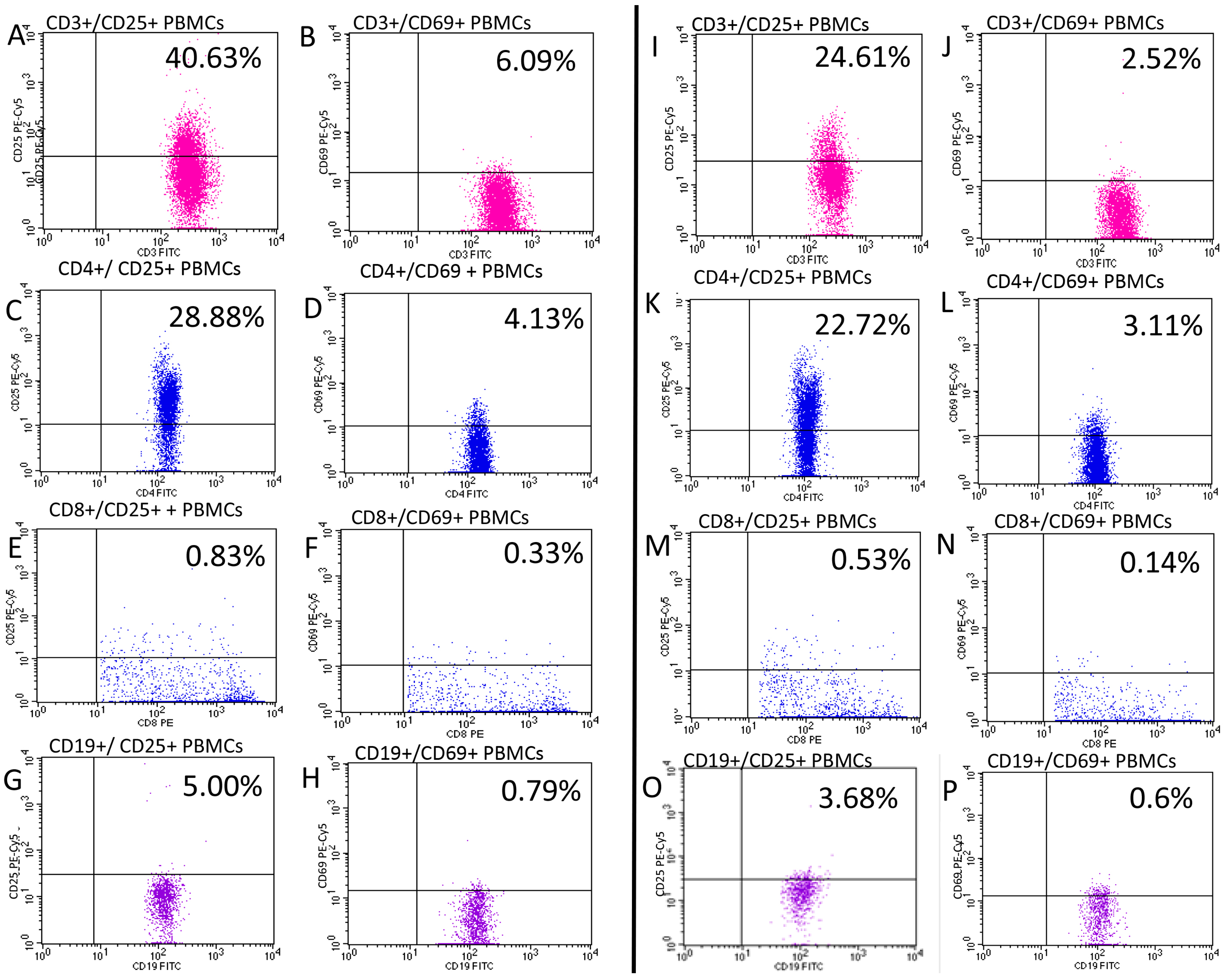
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armstrong, A.W.; Read, C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Samotij, D.; Nedoszytko, B.; Bartosińska, J.; Batycka-Baran, A.; Czajkowski, R.; Dobrucki, I.T.; Dobrucki, L.W.; Górecka-Sokołowska, M.; Janaszak-Jasienicka, A.; Krasowska, D.; et al. Pathogenesis of psoriasis in the “omic” era. Part I. Epidemiology, clinical manifestation, immunological and neuroendocrine disturbances. Postep. Dermatol. Alergol. 2020, 37, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, Y.; Yu, Q.; Shi, Y. Biologic and Small-Molecule Therapies for Moderate-to-Severe Psoriasis: Focus on Psoriasis Comorbidities. BioDrugs 2023, 37, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Tao, Y.; Zhang, Y.; Wang, J.; Yang, J.; Wang, Y. CD25: A potential tumor therapeutic target. Int. J. Cancer 2023, 152, 1290–1303. [Google Scholar] [CrossRef] [PubMed]
- Panackel, C.; Mathew, J.F.; Fawas, N.M.; Jacob, M. Immunosuppressive Drugs in Liver Transplant: An Insight. J. Clin. Exp. Hepatol. 2022, 12, 1557–1571. [Google Scholar] [CrossRef]
- Kanda, N.; Hoashi, T.; Saeki, H. The Defect in Regulatory T Cells in Psoriasis and Therapeutic Approaches. J. Clin. Med. 2021, 10, 3880. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Hajighasemi, S.; Kiaie, N.; Gheibi Hayat, S.M.; Jamialahmadi, T.; Johnston, T.P.; Sahebkar, A. The pivotal role of CD69 in autoimmunity. J. Autoimmun. 2020, 111, 102453. [Google Scholar] [CrossRef]
- Adamczyk, M.; Bartosińska, J.; Raczkiewicz, D.; Michalak-Stoma, A.; Krasowska, D. The Impact of Biologic Treatment on PD-1/PD-L1 Pathway Disturbances in Psoriasis. J. Clin. Med. 2023, 12, 4179. [Google Scholar] [CrossRef]
- Mrowietz, U.; Zhu, K.; Christophers, E. Treatment of severe psoriasis with anti-CD25 monoclonal antibodies. Arch. Dermatol. 2000, 136, 675–676. [Google Scholar] [CrossRef]
- Fan, Z.; Li, L.; Wang, X.; Miao, G. Dysfunction of regulatory T cells mediated by AKT-FOXO1 signaling pathway occurs during the development of psoriasis. Int. J. Clin. Exp. Pathol. 2020, 13, 799–809. [Google Scholar]
- Li, B.; Lei, J.; Yang, L.; Gao, C.; Dang, E.; Cao, T.; Xue, K.; Zhuang, Y.; Shao, S.; Zhi, D.; et al. Dysregulation of Akt-FOXO1 Pathway Leads to Dysfunction of Regulatory T Cells in Patients with Psoriasis. J. Investig. Dermatol. 2019, 139, 2098–2107. [Google Scholar] [CrossRef]
- Su, Q.Y.; Zhang, S.X.; Yang, L.; Luo, J.; Li, X.F.; Zhang, J.Q.; Zhang, Y.; Liu, J.Q.; Shi, L. Peripheral Treg Levels and Transforming Growth Factor-β (TGFβ) in Patients with Psoriatic Arthritis: A Systematic Review Meta-Analysis. Adv. Ther. 2023, 40, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H.; Mashiko, S.; Angsana, J.; Rubio, M.; Hsieh, Y.M.; Maari, C.; Reich, K.; Blauvelt, A.; Bissonnette, R.; Muñoz-Elías, E.J.; et al. Differential Changes in Inflammatory Mononuclear Phagocyte and T-Cell Profiles within Psoriatic Skin during Treatment with Guselkumab vs. Secukinumab. J. Investig. Dermatol. 2021, 141, 1707–1718.e9. [Google Scholar] [CrossRef]
- Quaglino, P.; Ortoncelli, M.; Comessatti, A.; Ponti, R.; Novelli, M.; Bergallo, M.; Costa, C.; Cicchelli, S.; Savoia, P.; Bernengo, M.G. Circulating CD4+CD25 bright FOXP3+ T cells are up-regulated by biological therapies and correlate with the clinical response in psoriasis patients. Dermatology 2009, 219, 250–258. [Google Scholar] [CrossRef] [PubMed]
- De Pità, O.; Ruffelli, M.; Cadoni, S.; Frezzolini, A.; Biava, G.F.; Simom, R.; Bottari, V.; De Sanctis, G. Psoriasis: Comparison of immunological markers in patients with acute and remission phase. J. Dermatol. Sci. 1996, 13, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Porto Ferreira, C.; Gomes da Silva, A.; Martins, C.J.; Da-Cruz, A.M. CD25+CD8+, CLA+CD4+, CD11a+ CD4+, and CD11a+ CD8+ T cell counts are elevated in the blood of Brazilian patients with chronic plaque psoriasis. Actas Dermosifiliogr. 2011, 102, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Economidou, J.; Barkis, J.; Demetriou, Z.; Avgerinou, G.; Psarra, K.; Degiannis, D.; Vareltzidis, A.; Katsambas, A. Effects of cyclosporin A on immune activation markers in patients with active psoriasis. Dermatology 1999, 199, 144–148. [Google Scholar] [CrossRef]
- Langewouters, A.M.; Bovenschen, H.J.; De jong, E.M.; Van Erp, P.E.; Van De Kerkhof, P.C. The effect of topical corticosteroids in combination with alefacept on circulating T-cell subsets in psoriasis. J. Dermatol. Treat. 2007, 18, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.; Torres, G.; McCormick, T.; Marano, C.; Cooper, K.; Yeilding, N.; Wang, Y.; Pendley, C.; Prabhakar, U.; Wong, J.; et al. Positive treatment effects of ustekinumab in psoriasis: Analysis of lesional and systemic parameters. J. Dermatol. 2010, 37, 413–425. [Google Scholar] [CrossRef]
- Gertel, S.; Polachek, A.; Furer, V.; Levartovsky, D.; Sidis, H.; Pel, S.; Paran, D.; Elkayam, O. T cell functions of psoriatic arthritis patients are regulated differently by TNF, IL-17A and IL-6 receptor blockades in vitro. Clin. Exp. Rheumatol. 2022, 40, 120–128. [Google Scholar] [CrossRef]
- Khalil, S.; Bardawil, T.; Kurban, M.; Abbas, O. Tissue-resident memory T cells in the skin. Inflamm. Res. 2020, 69, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Cibrian, D.; Saiz, M.L.; de la Fuente, H.; Sánchez-Díaz, R.; Moreno-Gonzalo, O.; Jorge, I.; Ferrarini, A.; Vázquez, J.; Punzón, C.; Fresno, M.; et al. CD69 controls the uptake of L-tryptophan through LAT1-CD98 and AhR-dependent secretion of IL-22 in psoriasis. Nat. Immunol. 2016, 17, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bissonnette, R.; Lee, J.; Correa da Rosa, J.; Suárez-Fariñas, M.; Lowes, M.A.; Krueger, J.G. The Spectrum of Mild to Severe Psoriasis Vulgaris Is Defined by a Common Activation of IL-17 Pathway Genes, but with Key Differences in Immune Regulatory Genes. J. Investig. Dermatol. 2016, 136, 2173–2182. [Google Scholar] [CrossRef]
- Diani, M.; Casciano, F.; Marongiu, L.; Longhi, M.; Altomare, A.; Pigatto, P.D.; Secchiero, P.; Gambari, R.; Banfi, G.; Manfredi, A.A.; et al. Increased frequency of activated CD8+ T cell effectors in patients with psoriatic arthritis. Sci. Rep. 2019, 9, 10870. [Google Scholar] [CrossRef]
- Cameron, A.L.; Kirby, B.; Griffiths, C.E. Circulating natural killer cells in psoriasis. Br. J. Dermatol. 2003, 149, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Lankford, K.V.; Mosunjacm, M.; Hillyer, C.D. Effects of UVB radiation on cytokine generation, cell adhesion molecules, and cell activation markers in T-lymphocytes and peripheral blood HPCs. Transfusion 2000, 40, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Gaál, J.; Lakos, G.; Szodoray, P.; Kiss, J.; Horváth, I.; Horkay, E.; Nagy, G.; Szegedi, A. Immunological and clinical effects of alphacalcidol in patients with psoriatic arthropathy: Results of an open, follow-up pilot study. Acta Derm. Venereol. 2009, 89, 140–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haider, A.S.; Lowes, M.A.; Gardner, H.; Bandaru, R.; Darabi, K.; Chamian, F.; Kikuchi, T.; Gilleaudeau, P.; Whalen, M.S.; Cardinale, I.; et al. Novel insight into the agonistic mechanism of alefacept in vivo: Differentially expressed genes may serve as biomarkers of response in psoriasis patients. J. Immunol. 2007, 178, 7442–7449. [Google Scholar] [CrossRef] [PubMed]
- Rubant, S.A.; Ludwig, R.J.; Diehl, S.; Hardt, K.; Kaufmann, R.; Pfeilschifter, J.M.; Boehncke, W.H. Dimethylfumarate reduces leukocyte rolling in vivo through modulation of adhesion molecule expression. J. Investig. Dermatol. 2008, 128, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Torres-Alvarez, B.; Castanedo-Cazares, J.P.; Fuentes-Ahumada, C.; Moncada, B. The effect of methotrexate on the expression of cell adhesion molecules and activation molecule CD69 in psoriasis. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 334–339. [Google Scholar] [CrossRef]
- Sigmundsdottir, H.; Gudjonsson, J.E.; Valdimarsson, H. The effects of ultraviolet B treatment on the expression of adhesion molecules by circulating T lymphocytes in psoriasis. Br. J. Dermatol. 2003, 148, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Owczarczyk-Saczonek, A.; Kasprowicz-Furmańczyk, M.; Czerwińska, J.; Krajewska-Włodarczyk, M.; Placek, W. The effect of therapy on TRM in psoriatic lesions. Adv. Dermatol. Allergol./Postępy Dermatol. Alergologii 2022, 39, 209–220. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Unit or Category | Results |
|---|---|---|
| Patients with psoriasis (N = 84): | ||
| Age, min–max, Median (IQR) | Years | 18–68, 43 (34–52) |
| Gender, n (%) | Male | 60 (71.4) |
| Female | 24 (28.6) | |
| Weight, min–max, Median (IQR) | kg | 47–125, 85 (74–95) |
| BMI, min–max, Median (IQR) | kg/m2 | 17.72–46.88, 27.51 (24.44–31.25) |
| Smoking status, n (%) | Non-smokers | 30 (38.0) |
| Smokers | 54 (62.0) | |
| Psoriasis type, n (%) * | I | 72 (88.1) |
| II | 12 (11.9) | |
| Duration of psoriasis, min–max, Median (IQR) | Years | 1–50, 20 (14–26) |
| PASI, min–max, Median (IQR) | 5.5–47.0, 18.0 (13.1–21.3) | |
| BSA, min–max, Median (IQR) | 6–80.0, 23.3 (15.0–39.8) | |
| PsA, n (%) | Yes | 27 (30.9) |
| Control group (N = 29): | ||
| Age, min-max, Median (IQR) | Years | 24–65, 40 (33–52) |
| Gender, n (%) | Male | 20 (69.0) |
| Female | 9 (31.0) | |
| Specificity | Fluorochrome | Producer | Clone | Isotype |
|---|---|---|---|---|
| Mouse anti human-CD3 | FITC | BD Biosciences, USA | MEM-57 | Mouse IgG2a, κ |
| Mouse anti human-CD4 | FITC | BD Biosciences, USA | RPA-T4 | Mouse IgG1, κ |
| Mouse anti human-CD8 | FITC | BD Biosciences, USA | SK1 | Mouse BALB/c IgG1, κ |
| Mouse anti human-CD19 | FITC | BD Biosciences, USA | HIB19 | Mouse IgG1, κ |
| Mouse anti human-CD25 | PE-CyTM5 | BD Biosciences, USA | M-A251 | Mouse BALB/c IgG1, κ |
| Mouse anti human-CD69 | PE-CyTM5 | BD Biosciences, USA | FN50 | Mouse IgG1, κ |
| PBMC Subtype | Psoriasis | Control | p |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| CD3/CD69 | 3.22 (2.18–5.81) | 2.07 (1.68–3.00) | 0.006 |
| CD3/CD69 within CD3 | 4.19 (2.87–7.75) | 3.17 (2.33–4.40) | 0.018 |
| CD3/CD25 | 27.97 (21.87–33.59) | 24.94 (21.41–29.00) | 0.107 |
| CD3/CD25 within CD3 | 37.74 (30.43–44.68) | 35.95 (31.00–41.88) | 0.349 |
| CD4/CD69 | 2.13 (1.27–3.59) | 1.80 (1.36–2.13) | 0.071 |
| CD4/CD69 within CD4 | 4.49 (2.85–7.84) | 3.61 (2.92–5.25) | 0.090 |
| CD4/CD25 | 23.74 (18.83–28.79) | 22.88 (18.36–27.27) | 0.740 |
| CD4/CD25 within CD4 | 56.53 (47.77–64.50) | 54.20 (50.50–57.08) | 0.363 |
| CD8/CD69 | 0.27 (0.16–0.55) | 0.22 (0.14–0.26) | 0.017 |
| CD8/CD69 within CD8 | 1.09 (0.55–1.78) | 0.53 (0.32–0.73) | <0.001 |
| CD8/CD25 | 0.57 (0.37–0.97) | 0.54 (0.43–0.82) | 0.723 |
| CD8/CD25 within CD8 | 1.86 (1.27–3.34) | 1.56 (1.12–2.20) | 0.210 |
| CD19/CD69 | 0.66 (0.32–1.13) | 0.42 (0.28–0.60) | 0.035 |
| CD19/CD69 within CD19 | 8.08 (3.99–12.54) | 6.50 (3.14–9.90) | 0.068 |
| CD19/CD25 | 1.88 (1.27–2.67) | 1.98 (1.28–2.43) | 0.989 |
| CD19/CD25 within CD19 | 23.49 (16.26–31.38) | 24.91 (19.41–29.30) | 0.404 |
| PBMC Subtype | Before Treatment | During Treatment | p |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| CD3/CD69 | 2.86 (1.69–3.68) | 3.73 (1.61–18.83) | 0.052 |
| CD3/CD69 within CD3 | 3.74 (2.36–4.79) | 5.01 (2.16–37.84) | 0.044 |
| CD3/CD25 | 25.15 (22.99–34.79) | 29.26 (22.27–33.98) | 0.755 |
| CD3/CD25 within CD3 | 37.09 (33.28–44.26) | 41.75 (31.87–45.95) | 0.280 |
| CD4/CD69 | 1.69 (0.90–2.43) | 2.87 (1.02–11.70) | 0.019 |
| CD4/CD69 within CD4 | 3.80 (2.25–5.68) | 7.16 (2.60–42.45) | 0.005 |
| CD4/CD25 | 23.53 (18.05–32.33) | 25.11 (21.41–29.50) | 0.471 |
| CD4/CD25 within CD4 | 55.33 (49.03–66.15) | 59.65 (51.83–66.83) | 0.041 |
| CD8/CD69 | 0.21 (0.15–0.36) | 0.27 (0.11–5.72) | 0.094 |
| CD8/CD69 z CD8 | 0.94 (0.43–1.28) | 1.06 (0.34–18.06) | 0.073 |
| CD8/CD25 | 0.57 (0.42–0.97) | 0.73 (0.43–0.98) | 0.638 |
| CD8/CD25 z CD8 | 2.15 (1.35–2.76) | 2.40 (1.23–3.10) | 0.981 |
| CD19/CD69 | 0.39 (0.28–0.77) | 0.99 (0.19–4.80) | 0.004 |
| CD19/CD69 within CD19 | 6.16 (2.78–9.82) | 9.40 (2.58–45.89) | 0.006 |
| CD19/CD25 | 1.94 (1.20–2.83) | 2.34 (1.33–3.66) | 0.088 |
| CD19/CD25 within CD19 | 24.42 (16.10–30.66) | 24.46 (15.53–33.06) | 0.220 |
| PBMC Subtype | PASI | BSA | Duration of Psoriasis | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| CD3/CD69 | 0.178 | 0.105 | −0.031 | 0.776 | −0.046 | 0.682 |
| CD3/CD69 within CD3 | 0.174 | 0.113 | −0.051 | 0.643 | −0.030 | 0.788 |
| CD3/CD25 | −0.063 | 0.576 | 0.143 | 0.200 | 0.122 | 0.280 |
| CD3/CD25 within CD3 | −0.138 | 0.209 | 0.051 | 0.648 | 0.224 | 0.043 |
| CD4/CD69 | 0.188 | 0.087 | −0.038 | 0.732 | −0.059 | 0.599 |
| CD4/CD69 within CD4 | 0.221 | 0.043 | −0.013 | 0.909 | −0.083 | 0.457 |
| CD4/CD25 | −0.094 | 0.397 | 0.063 | 0.572 | 0.043 | 0.702 |
| CD4/CD25 within CD4 | −0.157 | 0.154 | −0.019 | 0.867 | 0.144 | 0.197 |
| CD8/CD69 | 0.133 | 0.228 | −0.172 | 0.118 | −0.047 | 0.674 |
| CD8/CD69 within CD8 | 0.101 | 0.363 | −0.147 | 0.183 | 0.001 | 0.990 |
| CD8/CD25 | −0.070 | 0.530 | −0.032 | 0.771 | 0.039 | 0.725 |
| CD8/CD25 within CD8 | −0.145 | 0.189 | −0.068 | 0.540 | 0.055 | 0.625 |
| CD19/CD69 | 0.213 | 0.052 | 0.015 | 0.891 | −0.144 | 0.310 |
| CD19/CD69 within CD19 | 0.125 | 0.259 | −0.033 | 0.766 | −0.147 | 0.188 |
| CD19/CD25 | 0.069 | 0.533 | 0.043 | 0.702 | 0.033 | 0.771 |
| CD19/CD25 within CD19 | −0.010 | 0.927 | −0.003 | 0.979 | 0.013 | 0.907 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk, M.; Bartosińska, J.; Raczkiewicz, D.; Kowal, M.; Surdacka, A.; Krasowska, D.; Michalak-Stoma, A.; Krasowska, D. The Expression of Activation Markers CD25 and CD69 Increases during Biologic Treatment of Psoriasis. J. Clin. Med. 2023, 12, 6573. https://doi.org/10.3390/jcm12206573
Adamczyk M, Bartosińska J, Raczkiewicz D, Kowal M, Surdacka A, Krasowska D, Michalak-Stoma A, Krasowska D. The Expression of Activation Markers CD25 and CD69 Increases during Biologic Treatment of Psoriasis. Journal of Clinical Medicine. 2023; 12(20):6573. https://doi.org/10.3390/jcm12206573
Chicago/Turabian StyleAdamczyk, Michał, Joanna Bartosińska, Dorota Raczkiewicz, Małgorzata Kowal, Agata Surdacka, Danuta Krasowska, Anna Michalak-Stoma, and Dorota Krasowska. 2023. "The Expression of Activation Markers CD25 and CD69 Increases during Biologic Treatment of Psoriasis" Journal of Clinical Medicine 12, no. 20: 6573. https://doi.org/10.3390/jcm12206573
APA StyleAdamczyk, M., Bartosińska, J., Raczkiewicz, D., Kowal, M., Surdacka, A., Krasowska, D., Michalak-Stoma, A., & Krasowska, D. (2023). The Expression of Activation Markers CD25 and CD69 Increases during Biologic Treatment of Psoriasis. Journal of Clinical Medicine, 12(20), 6573. https://doi.org/10.3390/jcm12206573









