Nightmares and Sleep Disturbances in Children with PTSD: A Polysomnographic and Actigraphy Approach Evaluation
Abstract
1. Introduction
1.1. Post-Traumatic Stress Disorder in Children and Adolescents
1.2. Sleep Disturbances in Children and Adolescents with PTSD
2. Materials and Methods
2.1. Participants and Study Setting
2.2. Variables and Assessment Measures
- Assessment of PTSD and its comorbidities
- Subjective assessment of sleep and circadian rhythms
- Objective assessment of sleep and circadian rhythms
- Actigraphy
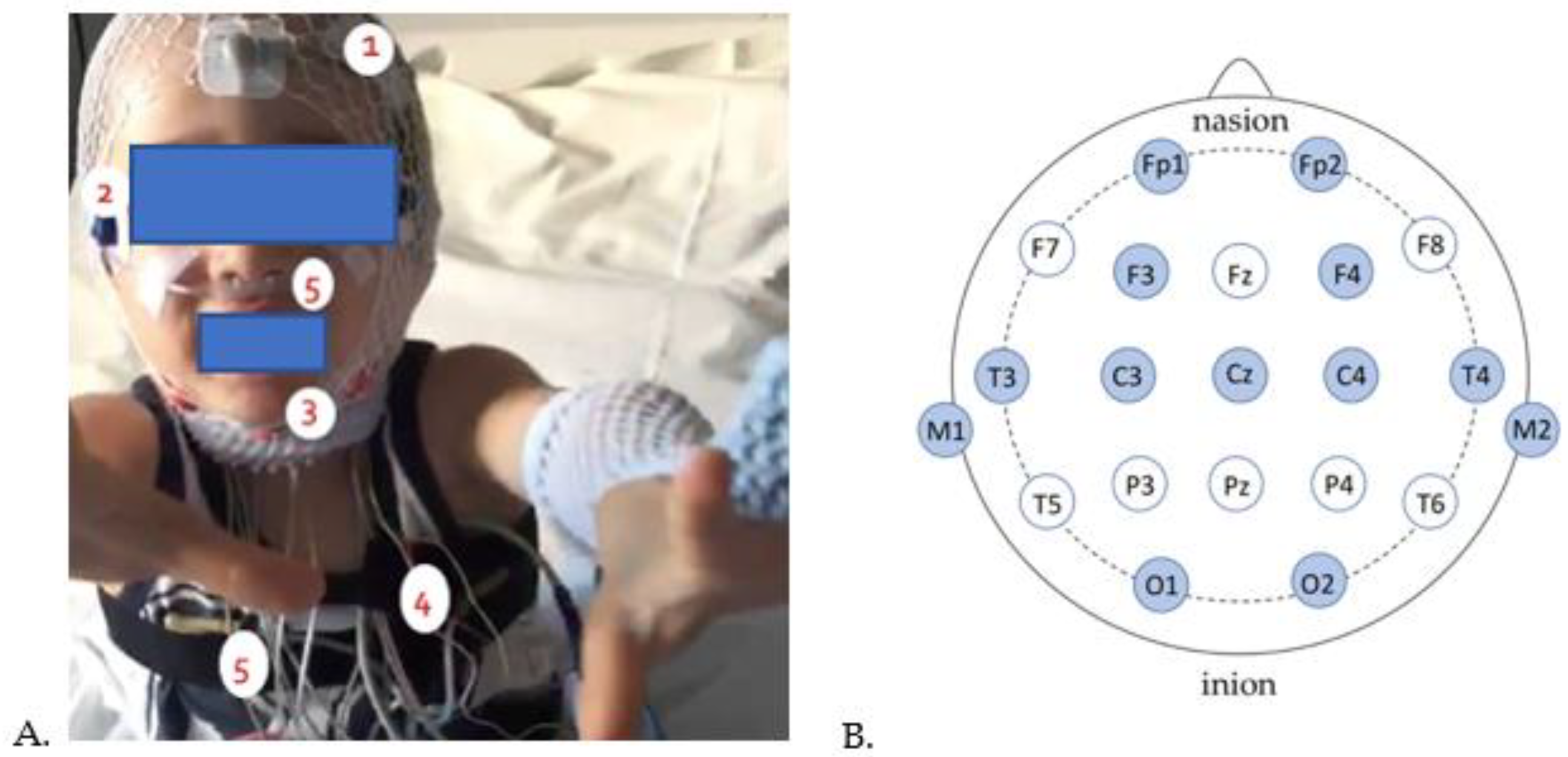
- Polysomnography
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Subjective Parameters of PTSD, Sleep, and Circadian Rhythms in Children with PTSD
3.2. Characteristics of Objective PSG and Actigraphy Derived Sleep and Circadian Rhythm Parameters for Children with PTSD and Controls
3.3. Correlation Analyses
3.4. Sleep Quality under Controlled Compared to Ecological Conditions in Children with PTSD
3.5. Characteristics of Circadian Parameters in Children with PTSD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shalev, A.; Liberzon, I.; Marmar, C. Post-Traumatic Stress Disorder. N. Engl. J. Med. 2017, 376, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Arseneault, L.; Caspi, A.; Fisher, H.L.; Matthews, T.; Moffitt, T.E.; Odgers, C.L.; Stahl, D.; Teng, J.Y.; Danese, A. The Epidemiology of Trauma and Post-Traumatic Stress Disorder in a Representative Cohort of Young People in England and Wales. Lancet Psychiatry 2019, 6, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Alisic, E.; Zalta, A.K.; van Wesel, F.; Larsen, S.E.; Hafstad, G.S.; Hassanpour, K.; Smid, G.E. Rates of Post-Traumatic Stress Disorder in Trauma-Exposed Children and Adolescents: Meta-Analysis. Br. J. Psychiatry 2014, 204, 335–340. [Google Scholar] [CrossRef]
- Truskauskaite, I.; Dumarkaite, A.; Petrauskaite, G.; Andersson, G.; Brailovskaia, J.; Karatzias, T.; Margraf, J.; Kazlauskas, E. ICD-11 PTSD and Complex PTSD in Lithuanian University Students: Prevalence and Associations with Trauma Exposure. Psychol. Trauma Theory Res. Pract. Policy 2023, 15, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Gindt, M.; Fernandez, A.; Zeghari, R.; Ménard, M.-L.; Nachon, O.; Richez, A.; Auby, P.; Battista, M.; Askenazy, F. A 3-Year Retrospective Study of 866 Children and Adolescent Outpatients Followed in the Nice Pediatric Psychotrauma Center Created after the 2016 Mass Terror Attack. Front. Psychiatry 2022, 13, 1010957. [Google Scholar] [CrossRef] [PubMed]
- Kovachy, B.; O’Hara, R.; Hawkins, N.; Gershon, A.; Primeau, M.M.; Madej, J.; Carrion, V. Sleep Disturbance in Pediatric PTSD: Current Findings and Future Directions. J. Clin. Sleep Med. 2013, 9, 501–510. [Google Scholar] [CrossRef]
- American Psychiatric Association. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; (DSM-5); American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Zhou, X.; Wu, X.; Chen, Q.; Zhen, R. Why Did Adolescents Have Sleep Problems after Earthquakes? Understanding the Role of Traumatic Exposure, Fear, and PTSD. Scand. J. Psychol. 2017, 58, 221–227. [Google Scholar] [CrossRef]
- Sateia, M.J. International Classification of Sleep Disorders-Third Edition. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- Phelps, A.J.; Kanaan, R.A.A.; Worsnop, C.; Redston, S.; Ralph, N.; Forbes, D. An Ambulatory Polysomnography Study of the Post-Traumatic Nightmares of Post-Traumatic Stress Disorder. Sleep 2018, 41, zsx188. [Google Scholar] [CrossRef]
- Pynoos, R.; Nader, K.; Fairbanks, L.; Frederick, C. Children’s PTSD Reactions One Year after a Sniper Attack at Their School. Am. J. Psychiatry 1990, 147, 1526–1530. [Google Scholar] [CrossRef]
- Thabet, A.A.; Vostanis, P. Post Traumatic Stress Disorder Reactions in Children of War: A Longitudinal Study. Child Abus. Negl. 2000, 24, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Mghaieth, S.; Othman, S.; Bouden, A.; Halayem, M.B. État de stress post-traumatique chez l’enfant: Sémiologie et comorbidité. L’Encéphale 2007, 33, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.H.; Mellman, T.A.; Alfano, C.A.; Weems, C.F. Sleep Fears, Sleep Disturbance, and PTSD Symptoms in Minority Youth Exposed to Hurricane Katrina. J. Trauma. Stress 2011, 24, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Noll, J.G.; Trickett, P.K.; Susman, E.J.; Putnam, F.W. Sleep Disturbances and Childhood Sexual Abuse. J. Pediatr. Psychol. 2006, 31, 469–480. [Google Scholar] [CrossRef]
- Thordardottir, E.B.; Hansdottir, I.; Valdimarsdottir, U.A.; Shipherd, J.C.; Resnick, H.; Gudmundsdottir, B. The Manifestations of Sleep Disturbances 16 Years Post-Trauma. Sleep 2016, 39, 1551–1554. [Google Scholar] [CrossRef][Green Version]
- Brownlow, J.A.; McLean, C.P.; Gehrman, P.R.; Harb, G.C.; Ross, R.J.; Foa, E.B. Influence of Sleep Disturbance on Global Functioning After Posttraumatic Stress Disorder Treatment: PTSD Treatment, Sleep, Global Functioning. J. Trauma. Stress 2016, 29, 515–521. [Google Scholar] [CrossRef]
- Kobayashi, I.; Boarts, J.M.; Delahanty, D.L. Polysomnographically Measured Sleep Abnormalities in PTSD: A Meta-Analytic Review. Psychophysiology 2007, 44, 660–669. [Google Scholar] [CrossRef]
- Wallace, D.M.; Shafazand, S.; Ramos, A.R.; Carvalho, D.Z.; Gardener, H.; Lorenzo, D.; Wohlgemuth, W.K. Insomnia Characteristics and Clinical Correlates in Operation Enduring Freedom/Operation Iraqi Freedom Veterans with Post-Traumatic Stress Disorder and Mild Traumatic Brain Injury: An Exploratory Study. Sleep Med. 2011, 12, 850–859. [Google Scholar] [CrossRef]
- Germain, A.; Nielsen, T.A. Sleep Pathophysiology in Posttraumatic Stress Disorder and Idiopathic Nightmare Sufferers. Biol. Psychiatry 2003, 54, 1092–1098. [Google Scholar] [CrossRef]
- Mellman, T.A.; Bustamante, V.; Fins, A.I.; Pigeon, W.R.; Nolan, B. REM Sleep and the Early Development of Posttraumatic Stress Disorder. Am. J. Psychiatry 2002, 159, 1696–1701. [Google Scholar] [CrossRef]
- Ross, R.J.; Ball, W.A.; Dinges, D.F.; Kribbs, N.B.; Morrison, A.R.; Silver, S.M.; Mulvaney, F.D. Rapid Eye Movement Sleep Disturbance in Posttraumatic Stress Disorder. Biol. Psychiatry 1994, 35, 195–202. [Google Scholar] [CrossRef]
- Sadeh, A.; McGuire, J.P.D.; Sachs, H.; Seifer, R.; Tremblay, A.; Civita, R.; Hayden, R.M. Sleep and Psychological Characteristics of Children on a Psychiatric Inpatient Unit. J. Am. Acad. Child Adolesc. Psychiatry 1995, 34, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Glod, C.A.; Teicher, M.H.; Hartman, C.R.; Harakal, T. Increased Nocturnal Activity and Impaired Sleep Maintenance in Abused Children. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Sanabria, F.; Bruno, S.; Bazzani, A.; Bonelli, C.; Violi, M.; Frumento, P.; Faraguna, U. Associations between Post-Traumatic Stress Symptoms and Sleep/Circadian Parameters: Exploring the Effect of Chronotype as a Moderator Variable. Chronobiol. Int. 2023, 40, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Geracioti, T.D.; Baker, D.G.; Ekhator, N.N.; West, S.A.; Hill, K.K.; Bruce, A.B.; Schmidt, D.; Rounds-Kugler, B.; Yehuda, R.; Keck, P.E.; et al. CSF Norepinephrine Concentrations in Posttraumatic Stress Disorder. Am. J. Psychiatry 2001, 158, 1227–1230. [Google Scholar] [CrossRef]
- Corsi-Cabrera, M.; Velasco, F.; del Río-Portilla, Y.; Armony, J.L.; Trejo-Martínez, D.; Guevara, M.A.; Velasco, A.L. Human Amygdala Activation during Rapid Eye Movements of Rapid Eye Movement Sleep: An Intracranial Study. J. Sleep Res. 2016, 25, 576–582. [Google Scholar] [CrossRef]
- Ferrafiat, V.; Soleimani, M.; Chaumette, B.; Martinez, A.; Guilé, J.-M.; Keeshin, B.; Gerardin, P. Use of Prazosin for Pediatric Post-Traumatic Stress Disorder With Nightmares and/or Sleep Disorder: Case Series of 18 Patients Prospectively Assessed. Front. Psychiatry 2020, 11, 724. [Google Scholar] [CrossRef]
- Akinsanya, A.; Marwaha, R.; Tampi, R.R. Prazosin in Children and Adolescents with Posttraumatic Stress Disorder Who Have Nightmares: A Systematic Review. J. Clin. Psychopharmacol. 2017, 37, 84–88. [Google Scholar] [CrossRef]
- Casement, M.D.; Swanson, L.M. A Meta-Analysis of Imagery Rehearsal for Post-Trauma Nightmares: Effects on Nightmare Frequency, Sleep Quality, and Posttraumatic Stress. Clin. Psychol. Rev. 2012, 32, 566–574. [Google Scholar] [CrossRef]
- Terr, L.C. Childhood Traumas: An Outline and Overview. Am. J. Psychiatry 1991, 148, 10–20. [Google Scholar] [CrossRef]
- Olliac, B.; Birmes, P.; Bui, E.; Allenou, C.; Brunet, A.; Claudet, I.; Sales de Gauzy, J.; Grandjean, H.; Raynaud, J.-P. Validation of the French Version of the Child Post-Traumatic Stress Reaction Index: Psychometric Properties in French Speaking School-Aged Children. PLoS ONE 2014, 9, e112603. [Google Scholar] [CrossRef] [PubMed]
- Boyes, M.E.; Cluver, L.D.; Gardner, F. Psychometric Properties of the Child PTSD Checklist in a Community Sample of South African Children and Adolescents. PLoS ONE 2012, 7, e46905. [Google Scholar] [CrossRef] [PubMed]
- Brewin, C.R.; Rose, S.; Andrews, B.; Green, J.; Tata, P.; McEvedy, C.; Turner, S.; Foa, E.B. Brief Screening Instrument for Post-Traumatic Stress Disorder. Br. J. Psychiatry 2002, 181, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, M. The Children’s Depression, Inventory (CDI). Psychopharmacol. Bull. 1985, 21, 995–998. [Google Scholar]
- Spielberg, C.D.; Gorsuch, R.L.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manualfor the State-Trait Anxiety Inventory (STAI); Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef]
- Lecuelle, F.; Gustin, M.-P.; Leslie, W.; Mindell, J.A.; Franco, P.; Putois, B. French Validation of the Sleep Disturbance Scale for Children (SDSC) in Young Children (Aged 6 Months to 4 Years). Sleep Med. 2020, 67, 56–65. [Google Scholar] [CrossRef]
- Belicki, K. The Relationship of Nightmare Frequency to Nightmare Suffering with Implications for Treatment and Research. Dreaming 1992, 2, 143–148. [Google Scholar] [CrossRef]
- Werner, H.; LeBourgeois, M.K.; Geiger, A.; Jenni, O.G. Assessment of Chronotype in Four- to Eleven-Year-Old Children: Reliability and Validity of the Children’s ChronoType Questionnaire (CCTQ). Chronobiol. Int. 2009, 26, 992–1014. [Google Scholar] [CrossRef]
- Roenneberg, T.; Kuehnle, T.; Juda, M.; Kantermann, T.; Allebrandt, K.; Gordijn, M.; Merrow, M. Epidemiology of the Human Circadian Clock. Sleep Med. Rev. 2007, 11, 429–438. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Smith, M.T.; McCrae, C.S.; Cheung, J.; Martin, J.L.; Harrod, C.G.; Heald, J.L.; Carden, K.A. Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J. Clin. Sleep Med. 2018, 14, 1209–1230. [Google Scholar] [CrossRef] [PubMed]
- Auger, R.R.; Burgess, H.J.; Emens, J.S.; Deriy, L.V.; Thomas, S.M.; Sharkey, K.M. Clinical Practice Guideline for the Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2015, 11, 1199–1236. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Lloyd, R.M.; Marcus, C.L.; Vaughn, B.V. The American Association of Sleep Medicine Manual for the Scoring of Sleep and Associated Events: Scoring Manual; Version 2.2; 2015. Available online: https://aasm.org/resources/pdf/scoring-manual-preface.pdf (accessed on 20 July 2023).
- Sack, W.H.; Him, C.; Dickason, D. Twelve-Year Follow-up Study of Khmer Youths Who Suffered Massive War Trauma as Children. J. Am. Acad. Child Adolesc. Psychiatry 1999, 38, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Zhou, Y.; Liu, X. Sleep Disturbance Predicts Posttraumatic Stress Disorder and Depressive Symptoms: A Cohort Study of Chinese Adolescents. J. Clin. Psychiatry 2017, 78, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, I.; Lavela, J.; Mellman, T.A. Nocturnal Autonomic Balance and Sleep in PTSD and Resilience: Nocturnal Autonomic Balance and Sleep in PTSD. J. Trauma. Stress 2014, 27, 712–716. [Google Scholar] [CrossRef]
- Cohen, H.; Benjamin, J.; Geva, A.B.; Matar, M.A.; Kaplan, Z.; Kotler, M. Autonomic Dysregulation in Panic Disorder and in Post-Traumatic Stress Disorder: Application of Power Spectrum Analysis of Heart Rate Variability at Rest and in Response to Recollection of Trauma or Panic Attacks. Psychiatry Res. 2000, 96, 1–13. [Google Scholar] [CrossRef]
- Ahmed, S.; Gorman, G.H.; Susi, A.; Robertson, B.D.; Collen, J.F.; Hisle-Gorman, E.J. Impact of Parental Injury on Adolescent Sleep. J. Clin. Sleep Med. 2020, 16, 1437–1444. [Google Scholar] [CrossRef]
- Mellman, T.A.; Nolan, B.; Hebding, J.; Kulick-Bell, R.; Dominguez, R. A Polysomnographic Comparison of Veterans with Combat-Related PTSD, Depressed Men, and Non-Ill Controls. Sleep 1997, 20, 46–51. [Google Scholar] [CrossRef][Green Version]
- Ulmer, C.S.; Hall, M.H.; Dennis, P.A.; Beckham, J.C.; Germain, A. Posttraumatic Stress Disorder Diagnosis Is Associated with Reduced Parasympathetic Activity during Sleep in US Veterans and Military Service Members of the Iraq and Afghanistan Wars. Sleep 2018, 41, zsy174. [Google Scholar] [CrossRef]
- Gieselmann, A.; Ait Aoudia, M.; Carr, M.; Germain, A.; Gorzka, R.; Holzinger, B.; Kleim, B.; Krakow, B.; Kunze, A.E.; Lancee, J.; et al. Aetiology and Treatment of Nightmare Disorder: State of the Art and Future Perspectives. J. Sleep Res. 2019, 28, e12820. [Google Scholar] [CrossRef]
- Mellman, T.A.; David, D.; Bustamante, V.; Torres, J.; Fins, A. Dreams in the Acute Aftermath of Trauma and Their Relationship to PTSD. J. Trauma. Stress 2001, 14, 241–247. [Google Scholar] [CrossRef]
- Iglowstein, I.; Jenni, O.G.; Molinari, L.; Largo, R.H. Sleep Duration from Infancy to Adolescence: Reference Values and Generational Trends. Pediatrics 2003, 111, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.C.; Herringa, R.J. Prefrontal-Amygdala Dysregulation to Threat in Pediatric Posttraumatic Stress Disorder. Neuropsychopharmacol. Publ. Am. Coll. Neuropsychopharmacol. 2016, 41, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.N.; Walker, M.P. The Role of Sleep in Emotional Brain Function. Annu. Rev. Clin. Psychol. 2014, 10, 679–708. [Google Scholar] [CrossRef]
- Sivertsen, B.; Harvey, A.G.; Pallesen, S.; Hysing, M. Mental Health Problems in Adolescents with Delayed Sleep Phase: Results from a Large Population-Based Study in Norway. J. Sleep Res. 2015, 24, 11–18. [Google Scholar] [CrossRef]
| Description of PTSD Population (n = 11) | |||||||
|---|---|---|---|---|---|---|---|
| Variables | Mean | Median | Standard Deviation | Minimum | Maximum | Scale Cut-Off | % Population with Score+ |
| Child trauma assessment | |||||||
| CPTS-RI total | 58.9 | 58 | 5.9 | 51 | 67 | _ | 100 |
| Mild PTSD | _ | _ | _ | _ | _ | 12–24 | 0 |
| Moderate PTSD | _ | _ | _ | _ | _ | 25–39 | 0 |
| Severe PTSD | 58.9 | 58 | 5 | 43 | 58 | 40–59 | 60 |
| Very severe PTSD | 62 | 63 | 2.5 | 61 | 67 | >60 | 40 |
| CPC total | 62.9 | 64 | 18.1 | 32 | 93 | ||
| CPC PTSD diagnosis | 52 | 48.5 | 18.2 | 22 | 83 | >20 | 100 |
| CPC functional repercussions | 12.8 | 13 | 5.3 | 4 | 20 | >4 | 100 |
| Child sleep assessment | |||||||
| ISI total | 18.3 | 18 | 4 | 12 | 25 | Moderate insomnia > 15 | 90 |
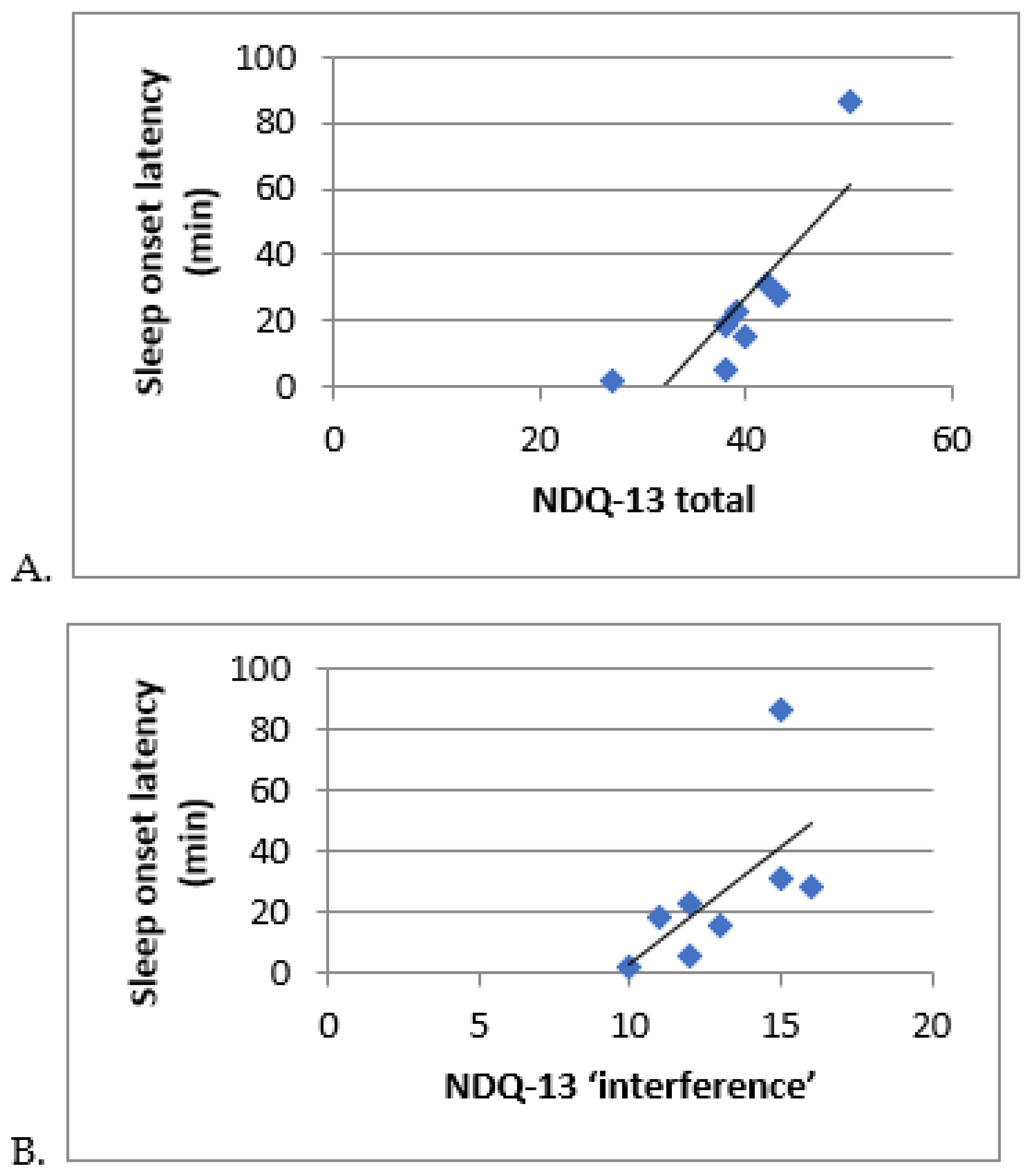
| NDQ-13 total | 37.9 | 39 | 7.9 | 24 | 50 | >20 | 100 |
| NDQ-13 fear items | 17.3 | 19 | 4.8 | 8 | 24 | >10 | 90 |
| NDQ-13 interference items | 12.6 | 12 | 2.4 | 9 | 16 | >7 | 100 |
| NDQ-13 premonition items | 4.8 | 4 | 1.6 | 3 | 7 | >4 | 80 |
| SDSC total | 61.1 | 62 | 7.2 | 47 | 68 | >45 | 100 |
| SDSC insomnia | 23.2 | 24.5 | 4 | 15 | 27 | >21 | 85.7 |
| SDSC parasomnia | 17.3 | 17 | 3.8 | 13 | 23 | >17 | 57.1 |
| SDSC respiratory disturbances | 8.8 | 8.5 | 1.7 | 7 | 11 | >12 | 0 |
| SDSC non-restorative sleep | 6.9 | 7 | 2.1 | 3 | 10 | >11 | 0 |
| SDSC daytime sleepiness | 5 | 5 | 0.8 | 4 | 6 | >5 | 71.5 |
| Children’s comorbidities | |||||||
| Children’s CDI | 28.1 | 27.5 | 4.8 | 23 | 35 | _ | 100 |
| Description of parent population (n = 5) | |||||||
| Evaluation trauma parents | |||||||
| TSQ total | 7.2 | 8 | 2.1 | 5 | 10 | >5 | 100 |
| Parents sleep assessment | |||||||
| ISI | 14.7 | 13.5 | 5.12 | 10 | 22 | Moderate insomia > 15 | 25 |
| PSQI | 12.7 | 13 | 0.6 | 12 | 13 | >5 | 100 |
| STAI parents | 53.2 | 59 | 11.7 | 33 | 66 | Moderate anxiety > 46 | 87.5 |
| Description of PSG Characteristics of PTSD Population and Controls | |||
|---|---|---|---|
| Variables | PTSD (n = 11) | Controls (n = 11) | p |
| Total Sleep Time, TST (min) | 565.6 (±117.7) | 557.3 (±64.5) | 0.82 |
| Sleep Efficiency (TTS/PTS × 100) (%) | 90.1 (±6.4) | 93.6 (±4.3) | 0.30 |
| Sleep efficiency (%) | 86.6 (±7.8) | 88.0 (±5.8) | 0.82 |
| Sleep latency, LE (min) | 21.7 (±19.3) | 22.1 (±23.6) | 0.91 |
| Time awake after sleep, WASO (min) | 62.2 (±35.6) | 52.3 (±29.6) | 0.65 |
| Total sleep time, DTV (LE + WASO) (min) | 59.2 (±35.6) | 38.2 (±27.9) | 0.30 |
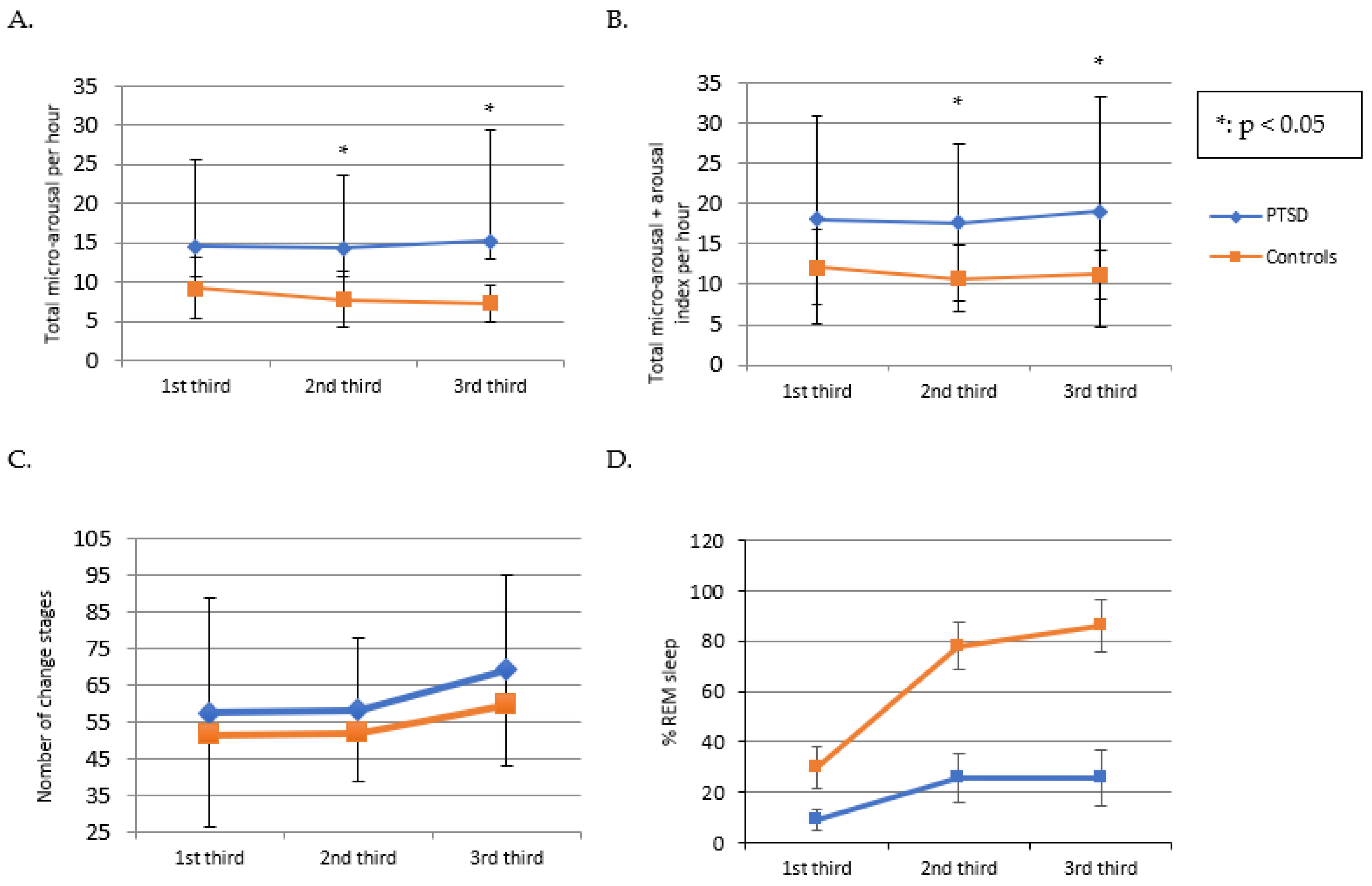
| Number Changes Stages | 184.1 (±70.7) | 158.5 (±87.4) | 0.11 |
| % N1 | 8.6 (±6.7) | 3.9 (±2.4) | 0.08 |
| % N2 | 43.6 (±10.6) | 43.5 (±13.0) | 0.73 |
| % N3 | 25.0 (±11.1) | 26.4 (±10.5) | 0.86 |
| REM | 20.9 (±5.0) | 24.3 (±6.6) | 0.77 |
| % Total NREM | 78.1 (±5.0) | 74.7 (±6.6) | 0.77 |
| Total micro-arousals index (index/h) | 14.8 (±10.8) | 8.2 (±2.5) | 0.039 * |
| Total arousals index (index/h) | 13.3 (±1.5) | 12.6 (±13.9) | 0.06 |
| Index micro-arousals + arousals total (index/h) | 18.1 (±11.5) | 11.5 (±3.2) | 0.042 * |
| Average HR TTS (bpm) | 71.6 (±10.6) | 76.1 (±11.9) | 0.21 |
| Comparison of Sleep Parameters from PSG and Actimetry in Laboratory and Home Conditions for Children with PTSD | |||||
|---|---|---|---|---|---|
| PSG in the Laboratory | Actimetry in Laboratory | Home Actimetry | Home/Laboratory Actimetry | PSG/Home Actimetry | |
| Variables | Average | Average | Average | p | p |
| TST (min) | 585.6 | 586.6 | 464.3 | 0.018 * | 0.19 |
| Efficacity (%) | 90.8 | 86.6 | 79.2 | 0.17 | 0.014 * |
| SOL (min) | 18.9 | 25.6 | 79.2 | 0.55 | 0.43 |
| Circadian Data for PTSD | |||
|---|---|---|---|
| Variables | Mean | Standard Deviation | Median |
| Objective circadian data (NPCRA) | |||
| L5 onset (h) | 03:12:00 | 06:18:00 | 01:30:00 |
| M10 onset (h) | 09:30:00 | 01:18:00 | 09:30:00 |
| RA (Relative Amplitude) | 0.91 | 0.05 | 0.91 |
| IS (Inter-daily stability) | 0.48 | 0.13 | 0.45 |
| IV (Intraday variability) | 0.73 | 0.15 | 0.67 |
| Subjective circadian data CCTQ) | |||
| MSFsc | 3.10 | 1.39 | 2.82 |
| Total MSFsc (h) | 03:05:49 | 01:23:17 | 02:35:54 |
| Sleep debt (h) | 01:38:00 | 00:47:00 | 02:16:00 |
| Sleep debt (% of total) | 50 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolling, J.; Rabot, J.; Reynaud, E.; Kolb, O.; Bourgin, P.; Schroder, C.M. Nightmares and Sleep Disturbances in Children with PTSD: A Polysomnographic and Actigraphy Approach Evaluation. J. Clin. Med. 2023, 12, 6570. https://doi.org/10.3390/jcm12206570
Rolling J, Rabot J, Reynaud E, Kolb O, Bourgin P, Schroder CM. Nightmares and Sleep Disturbances in Children with PTSD: A Polysomnographic and Actigraphy Approach Evaluation. Journal of Clinical Medicine. 2023; 12(20):6570. https://doi.org/10.3390/jcm12206570
Chicago/Turabian StyleRolling, Julie, Juliette Rabot, Eve Reynaud, Oriane Kolb, Patrice Bourgin, and Carmen M. Schroder. 2023. "Nightmares and Sleep Disturbances in Children with PTSD: A Polysomnographic and Actigraphy Approach Evaluation" Journal of Clinical Medicine 12, no. 20: 6570. https://doi.org/10.3390/jcm12206570
APA StyleRolling, J., Rabot, J., Reynaud, E., Kolb, O., Bourgin, P., & Schroder, C. M. (2023). Nightmares and Sleep Disturbances in Children with PTSD: A Polysomnographic and Actigraphy Approach Evaluation. Journal of Clinical Medicine, 12(20), 6570. https://doi.org/10.3390/jcm12206570






