Gender Difference in REM Sleep Behavior Disorder in Japanese Population: Polysomnography and Sleep Questionnaire Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
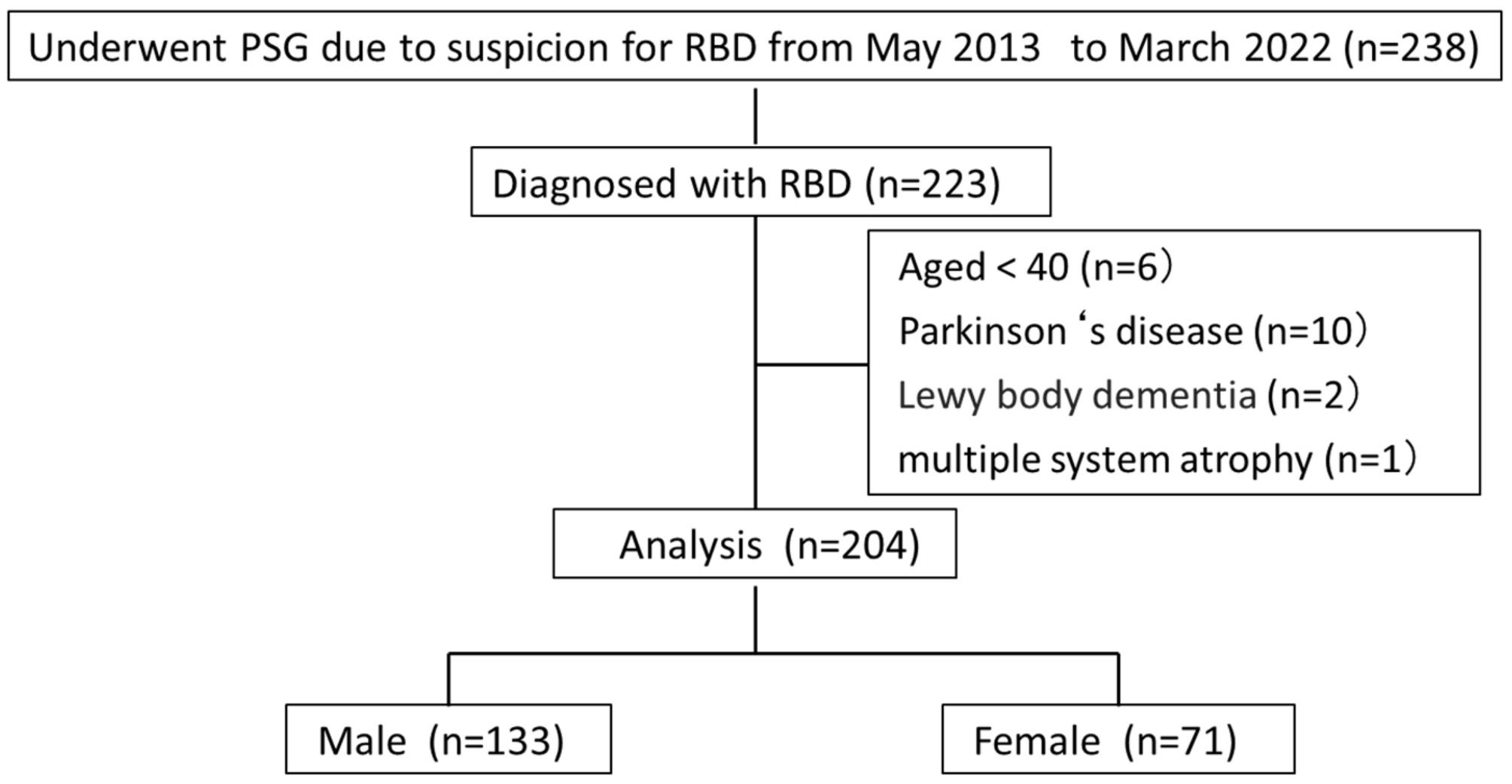
2.2. Patients
2.3. Sleep Questionnaires
2.4. Polysomnography
2.5. Diagnosis of RBD
- Repeated episodes of sleep-related vocalizations and/or complex motor behaviors.
- These behaviors are documented by polysomnography to occur during REM sleep or, based on the clinical history of dream enactment, are presumed to occur during REM sleep.
- Polysomnographic recording demonstrates REM sleep without atonia (RWA).
- The sleep disturbance is not better explained by another sleep disorder, mental disorder, medication, or substance use.
2.6. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schenck, C.H.; Hurwitz, T.D.; Mahowald, M.W. Symposium: Normal and abnormal REM sleep regulation: REM sleep behaviour disorder: An update on a series of 96 patients and a review of the world literature. J. Sleep Res. 1993, 2, 224–231. [Google Scholar] [CrossRef]
- Schenck, C.H.; Mahowald, M.W. REM sleep behavior disorder: Clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep 2002, 25, 120–138. [Google Scholar] [CrossRef]
- Claassen, D.; Josephs, K.; Ahlskog, J.; Silber, M.H.; Tippmann-Peikert, M.; Boeve, B.F. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010, 75, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, J.F.; Postuma, R.B.; Mazza, S.; Doyon, J.; Montplaisir, J. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006, 5, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Boeve, B.F.; Silber, M.H.; Saper, C.B.; Ferman, T.J.; Dickson, D.W.; Parisi, J.E.; Benarroch, E.E.; Ahlskog, J.E.; Smith, G.E.; Caselli, R.C.; et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 2007, 130, 2770–2788. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Iranzo, A.; Hu, M.; Högl, B.; Boeve, B.F.; Manni, R.; Oertel, W.H.; Arnulf, I.; Ferini-Strambi, L.; Puligheddu, M.; et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain 2019, 142, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Schenck, C.; Bundlie, S.; Mahowald, M. REM sleep behaviour disorder (RBD) delayed emergence of parkinsonism and/or dementia in 65% of older men initially diagnosed with idiopathic RBD, and an analysis of the maximum and minimum tonic and/or phasic electromyographic abnormalities found during REM sleep. Sleep 2003, 26, A316. [Google Scholar]
- Tippman-Peikert, M.; Olson, E.J.; Boeve, B.; Silber, M.H. Idiopathic REM sleep behavior disorder: A follow-up of 39 patients. Sleep 2006, 29, A272. [Google Scholar]
- Iranzo, A.; Molinuevo, J.; Santamaria, J.; Serradell, M.; Marti, M.; Valledeoriola, F. Sixty-four percent of patients with idiopathic REM sleep behavior disorder developed a neurological disorder after a mean clinical follow-up of seven years. Sleep 2008, 31, A280. [Google Scholar]
- Postuma, R.B.; Gagnon, J.F.; Vendette, M.; Fantini, M.L.; Massicotte-Marquez, J.; Montplaisir, J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 2009, 72, 1296–1300. [Google Scholar] [CrossRef]
- Nishikawa, N.; Murata, M.; Hatano, T.; Mukai, Y.; Saitoh, Y.; Sakamoto, T.; Hanakawa, T.; Kamei, Y.; Tachimori, H.; Hatano, K.; et al. Idiopathic rapid eye movement sleep behavior disorder in Japan: An observational study. Park. Relat. Disord. 2022, 103, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lawton, M.A.; Lo, C.; Bowring, F.; Klein, J.C.; Querejeta-Coma, A.; Scotton, S.; Welch, J.; Rassaque, J.; Barber, T.; et al. Longitudinal changes in Parkinson’s disease symptoms with and without rapid eye movement sleep behavior disorder: The oxford discovery cohort study. Mov. Disord. 2021, 36, 2821–2832. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Yoon, I.Y.; Lee, S.D.; Han, J.W.; Kim, T.H.; Kim, K.W. REM sleep behavior disorder in the Korean elderly population: Prevalence and clinical characteristics. Sleep 2013, 36, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Haba-Rubio, J.; Frauscher, B.; Marques-Vidal, P.; Toriel, J.; Tobback, N.; Andries, D.; Preisig, M.; Vollenweider, P.; Postuma, R.; Heinzer, R. Prevalence and determinants of REM sleep behavior disorder in the general population. Sleep 2017, 41, zsx197. [Google Scholar] [CrossRef]
- Pujol, M.; Pujol, J.; Alonso, T.; Fuentes, A.; Pallerola, M.; Freixenet, J.; Barbé, F.; Salamero, M.; Santamaría, J.; Iranzo, A. Idiopathic REM sleep behavior disorder in the elderly Spanish community: A primary care center study with a two-stage design using video-polysomnography. Sleep Med. 2017, 40, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Sasai-Sakuma, T.; Takeuchi, N.; Asai, Y.; Inoue, Y.; Inoue, Y. Prevalence and clinical characteristics of REM sleep behavior disorder in Japanese elderly people. Sleep 2020, 43, zsaa024. [Google Scholar] [CrossRef]
- Lee, W.-J.; Baek, S.-H.; Im, H.-J.; Lee, S.-K.; Yoon, J.-E.; Thomas, R.J.; Wing, Y.-K.; Shin, C.; Yun, C.-H. REM sleep behavior disorder and its possible prodromes in general population: Prevalence, polysomnography findings, and associated factors. Neurology 2023, 101, e2364–e2375. [Google Scholar] [CrossRef]
- Olson, E.J.; Boeve, B.F.; Silber, M.H. Rapid eye movement sleep behaviour disorder: Demographic, clinical and laboratory findings in 93 cases. Brain 2000, 123, 331–339. [Google Scholar] [CrossRef]
- Wing, Y.K.; Lam, S.P.; Li, S.X.; Yu, M.W.; Fong, S.Y.; Tsoh, J.M.; Ho, C.K.; Lam, V.K. REM sleep behavior disorder in Hong Kong Chinese: Clinical outcome and gender comparison. J. Neurol. Neurosurg. Psychiatry 2008, 79, 1415–1416. [Google Scholar] [CrossRef]
- Fernández-Arcos, A.; Iranzo, A.; Serradell, M.; Gaig, C.; Santamaria, I. The Clinical Phenotype of Idiopathic Rapid Eye Movement Sleep Behavior Disorder at Presentation: A Study in 203 Consecutive Patients. Sleep 2016, 39, 121–132. [Google Scholar] [CrossRef]
- Wong, J.C.; Li, J.; Pavlova, M.; Chen, S.; Wu, A.; Wu, S.; Gao, X. Risk factors for probable REM sleep behavior disorder: A community-based study. Neurology 2016, 86, 1306–1312. [Google Scholar] [CrossRef]
- Bjørnara, K.A.; Dietrichs, E.; Toft, M. REM sleep behavior disorder in Parkinson’s disease—Is there a gender difference? Park. Relat. Disord. 2013, 19, 120–122. [Google Scholar] [CrossRef]
- Takeuchi, N.; Sasai-Sakuma, T.; Inoue, Y. Gender differences in clinical findings and a-synucleiopathy-related markers in patients with idiopathic REM sleep behavior disorder. Sleep Med. 2020, 66, 216–219. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Zung, W.W.K. A self-rating depression scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Stiasny-Kolster, K.; Mayer, G.; Schäfer, S.; Möller, J.C.; Heinzel-Gutenbrunner, M.; Oertel, W.H. The REM sleep behavior disorder screening questionnaire—A new diagnostic instrument. Mov. Disord. 2007, 22, 2386–2393, (RBDSQ). [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Tangredi, M.M. Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Sateia, M.J. International Classification of Sleep Disorders, 3rd ed.; ICSD-3; American Academy of Sleep Medicine: Darien, CT, USA, 2014. [Google Scholar]
- Stanton, B.; Baldwin, R.M.; Rachuba, L. A quarter century of violence in the United States. An epidemiologic assessment. Psychiatr. Clin. N. Am. 1997, 20, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Sumi, Y.; Masuda, F.; Kadotani, H.; Ozeki, Y. The prevalence of depression in isolated/idiopathic rapid eye movement sleep behavior disorder: A systematic review and meta-analysis. Sleep Med. Rev. 2022, 65, 101684. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mao, S.; Xiang, D.; Fang, C. Association between depression and the subsequent risk of Parkinson’s disease: A meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 86, 186–192. [Google Scholar] [CrossRef]
- Boot, B.P.; Boeve, B.F.; Roberts, R.O.; Ferman, T.J.; Geda, Y.E.; Pankratz, V.S.; Ivnik, R.J.; Smith, G.E.; McDade, E.; Christianson, T.J.; et al. Probable rapid eye movement sleep behavior disorder increases risk for mild cognitive impairment and Parkinson disease: A population-based study. Ann. Neurol. 2012, 71, 49–56. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.X.; Lam, S.P.; Wing, Y.K. REM sleep behavior disorder and obstructive sleep apnea: Does one “evil” make the other less or more “evil”? Sleep Med. 2017, 37, 216–217. [Google Scholar] [CrossRef]
- Lee, S.A.; Paek, J.H.; Han, S.H.; Ryu, H.U. The utility of a Korean version of the REM sleep behavior disorder screening questionnaire in patients with obstructive sleep apnea. J. Neurol. Sci. 2015, 358, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Sasai-Sakuma, T.; Kayaba, M.; Inoue, Y.; Nakayama, H. Prevalence, clinical symptoms and polysomnographic findings of REM-related sleep disordered breathing in Japanese population. Sleep Med. 2021, 80, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Bugalho, P.; Salavisa, M. Factors influencing the presentation of REM sleep behavior disorder: The relative importance of sex, associated neurological disorder, and context of referral to polysomnography. J. Clin. Sleep Med. 2019, 15, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, J.; Li, Y.; Du, L.; Li, Z.; Lei, F.; Wing, Y.; Kushida, C.; Zhou, D.; Tang, X. Gender differences in REM sleep behavior disorder: A clinical and polysomnographic study in China. Sleep Med. 2015, 16, 414–418. [Google Scholar] [CrossRef]
- Peppard, P.; Szklo-Coxe, M.; Mae Hla, K.; Young, T. Longitudinal association of sleep-related breathing disorder and depression. Arch. Intern. Med. 2006, 166, 1709–17915. [Google Scholar] [CrossRef]
- Zarranz, J.; Tijero, B.; Velasco, F.; Barcena, J.; Rouco, I.; Lezcano, E.; Lachen, M.; Jauregui, A.; Ugarte, A. Restless legs syndrome in Parkinson’s disease. Mov. Disord. 2007, 22, 1912–1916. [Google Scholar] [CrossRef]
- McCarter, S.J.; St Louis, E.K.; Boswell, C.L.; Dueffert, L.G.; Slocumb, N.; Boeve, B.F.; Silber, M.H.; Olson, E.J.; Morgenthaler, T.I.; Tippmann-Peikert, M. Factors associated with injury in REM sleep behavior disorder. Sleep Med. 2014, 15, 1332–1338. [Google Scholar] [CrossRef]
| Characteristic | Total (n = 204) | Male (n = 133) | Female (n = 71) | p Value |
|---|---|---|---|---|
| Age (years) | 68.8 ± 8.1 | 67.9 ± 8.0 | 70.5 ± 8.2 | <0.05 |
| BMI (kg/mm2) | 23.1 ± 2.9 | 23.5 ± 2.7 | 22.5 ± 3.3 | <0.05 |
| Waist circumference (cm) | 87.4 ± 9.6 | 88.7 ± 9.4 | 84.9 ± 3.3 | <0.05 |
| Neck circumference (cm) | 36.2 ± 4.9 | 38.0 ± 4.8 | 32.7 ± 2.4 | <0.001 |
| Current Smoking (%) | 17.2 | 21.2 | 9.9 | <0.05 |
| Alcohol intake (%) | 32.8 | 39.2 | 21.2 | <0.05 |
| Questionnaire | Total (n = 204) | Male (n = 133) | Female (n = 71) | p Value |
|---|---|---|---|---|
| ESS | 6.6 ± 4.2 | 6.7 ± 4.1 | 6.5 ± 4.4 | 0.63 |
| PSQI | 6.3 ± 3.8 | 5.9 ± 3.8 | 7.2 ± 3.6 | <0.001 |
| PSQI ≥ 5.5 | 52.0 (%) | 45.1 (%) | 64.8 (%) | <0.05 |
| RBDSQ | 8.3 ± 3.0 | 8.6 ± 2.9 | 7.7 ± 3.1 | <0.05 |
| SDS | 39.2 ± 8.8 | 38.0 ± 8.7 | 41.7 ± 8.5 | <0.001 |
| SDS ≥ 40 | 48.0 (%) | 41.4 (%) | 59.2 (%) | <0.05 |
| PSG Parameter | Total (n = 204) | Male (n = 133) | Female (n = 71) | p Value |
|---|---|---|---|---|
| Time In Bed (min) | 485.4 ± 32.6 | 485.4 ± 34.0 | 485.2 ± 29.9 | 0.79 |
| Total Sleep Time (min) | 365.5 ± 64.9 | 363.9 ± 66.5 | 368.4 ± 62.1 | 0.79 |
| WASO (min) | 94.5 ± 57.2 | 97.3 ± 57.3 | 89.0 ± 57.0 | 0.28 |
| Sleep Efficiency (%) | 75.4 ± 13.2 | 75.1 ± 13.3 | 76.2 ± 13.1 | 0.58 |
| Sleep Latency (min) | 17.2 ± 19.2 | 16.7 ± 20.8 | 18.27 ± 16.2 | 0.71 |
| REM Sleep Latency (min) | 145.4 ± 88.7 | 133.5 ± 87.7 | 167.6 ± 86.9 | 0.55 |
| REM sleep time/TST (%) | 18.1 ± 7.3 | 18.7 ± 7.7 | 17.1 ± 6.6 | 0.17 |
| Stage N1 sleep time/TST (%) | 44.6 ± 18.8 | 48.8 ± 17.7 | 36.5 ± 18.2 | <0.001 |
| Stage N2 sleep time/TST (%) | 36.7 ± 17.9 | 32.1 ± 16.4 | 45.4 ± 17.4 | <0.001 |
| Stage N3 sleep time/TST (%) | 0.6 ± 2.1 | 0.4 ± 1.4 | 1.0 ± 2.9 | 0.07 |
| AHI (events/h) | 12.4 ± 15.4 | 15.1 ± 17.6 | 7.2 ± 7.9 | <0.001 |
| AI (events/h) | 3.8 ± 9.2 | 5.4 ± 11.0 | 0.7 ± 1.8 | <0.001 |
| HI (events/h) | 8.6 ± 9.2 | 9.7 ± 10.0 | 6.5 ± 7.0 | <0.05 |
| 3%ODI (events/h) | 9.6 ± 13.9 | 11.7 ± 15.8 | 5.8 ± 7.9 | <0.05 |
| Arousal index (events/h) | 27.0 ± 15.3 | 29.5 ± 16.3 | 22.3 ± 11.8 | <0.001 |
| Lowest SpO2 (%) | 89.9 ± 5.2 | 89.6 ± 5.4 | 90.5 ± 4.8 | 0.23 |
| CT90 (%) | 0.76 ± 3.3 | 0.98 ± 3.9 | 0.36 ± 1.5 | 0.19 |
| NREM-AHI (events/h) | 12.6 ± 15.9 | 15.5 ± 18.1 | 7.1 ± 7.7 | <0.001 |
| REM-AHI (events/h) | 11.1 ± 16.3 | 13.0 ± 17.4 | 7.8 ± 13.7 | <0.05 |
| SUP-AHI (events/h) | 16.7 ± 19.5 | 20.8 ± 21.9 | 9.0 ± 10.1 | <0.001 |
| NSUP-AHI (events/h) | 4.3 ± 10.0 | 5.4 ± 11.9 | 2.1 ± 3.5 | 0.25 |
| Proportion of AHI ≥ 5 (events/h) (%) | 60.3 | 68.4 | 44.4 | <0.05 |
| Periodic limb movement index (events/h) | 20.2 ± 28.0 | 17.5 ± 26.3 | 25.4 ± 30.5 | <0.05 |
| Model | OR | 95% CI | p Value | |
|---|---|---|---|---|
| sleep quality | unadjusted | 2.24 | 1.235–4.058 | <0.05 |
| Model 1 | 2.19 | 1.230–4.001 | <0.05 | |
| Model 2 | 2.10 | 1.145–3.855 | <0.05 | |
| Model 3 | 2.12 | 1.137–3.939 | <0.05 | |
| Model 4 | 2.03 | 1.082–3.796 | <0.001 | |
| depression | unadjusted | 1.99 | 1.109–3.575 | <0.05 |
| Model 1 | 2.06 | 1.135–3.724 | <0.05 | |
| Model 2 | 2.16 | 1.178–3.954 | <0.05 | |
| Model 3 | 2.38 | 1.276–4.437 | <0.001 | |
| Model 4 | 2.34 | 1.251–4.371 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mano, M.; Nomura, A.; Sasanabe, R. Gender Difference in REM Sleep Behavior Disorder in Japanese Population: Polysomnography and Sleep Questionnaire Study. J. Clin. Med. 2024, 13, 914. https://doi.org/10.3390/jcm13030914
Mano M, Nomura A, Sasanabe R. Gender Difference in REM Sleep Behavior Disorder in Japanese Population: Polysomnography and Sleep Questionnaire Study. Journal of Clinical Medicine. 2024; 13(3):914. https://doi.org/10.3390/jcm13030914
Chicago/Turabian StyleMano, Mamiko, Atsuhiko Nomura, and Ryujiro Sasanabe. 2024. "Gender Difference in REM Sleep Behavior Disorder in Japanese Population: Polysomnography and Sleep Questionnaire Study" Journal of Clinical Medicine 13, no. 3: 914. https://doi.org/10.3390/jcm13030914
APA StyleMano, M., Nomura, A., & Sasanabe, R. (2024). Gender Difference in REM Sleep Behavior Disorder in Japanese Population: Polysomnography and Sleep Questionnaire Study. Journal of Clinical Medicine, 13(3), 914. https://doi.org/10.3390/jcm13030914







