1. Introduction
Minimizing ischemic injury of vascularized composite allografts (VCAs) after surgical separation from their physiological blood supply is crucial for the reduction of ischemia–reperfusion injury (IRI) after vascularized composite allotransplantation or replantation. Under optimal conditions of conventional preservation, the allowable ischemia time for VCAs is not more than six hours, as skeletal muscle is vulnerable to IRI [
1].
Ex situ machine perfusion (EMP) facilitates the supply of oxygenated perfusates with essential nutrients and electrolytes for VCAs, exceeding the viable preservation time of static cold storage (SCS) as a preservation method by a multitude, which has been the gold standard preservation method for solid organ transplantation and preservation of amputated limbs [
2,
3]. These findings have been confirmed in small and large animal experiments [
3,
4,
5]. Different EMP strategies evolved, including hypo-subnormothermic and normothermic perfusion. While hypothermic and subnormothermic perfusions decrease the tissues’ metabolic demand, normothermic perfusion allows for viability testing of skeletal muscle and nerves during ex situ perfusion prior to transplantation. As a tissue’s metabolic activity is temperature-dependent and decreases by half for every 10 °C reduction, near-normothermic EMP mimics in vivo conditions, which creates the necessity for blood-based perfusates [
6]. However, those are immunogenic, potentially infectious, require specialized storage in a blood bank, and have a short shelf-life [
7,
8]. Acellular perfusates do not offer a sufficient oxygen-carrying capacity for a satisfactory supply of VCAs with oxygen in normothermic settings. One potential approach to resolve the time-sensitive issue of hypoxia in a normothermic amputated limb is the intravascular administration of artificial oxygen carriers, which can deliver a five-fold amount of oxygen compared to a human red blood cell, adjusted by weight [
9].
In this study, we report our strategy to enhance the beneficial effects of normothermic EMP with rapid-release microparticles for VCAs in a 12 h rat hindlimb perfusion model to reduce the devastating effects of deficient oxygen delivery in skeletal muscle.
2. Methods
2.1. Animal Protocol
This experimental study was approved by the Institutional Review Board and the IACUC (Protocol Number: BCH-18-08-3713R). In this non-survival experiment, male Sprague-Dawley rats of at least 6 weeks of age were used. After weighing the experimental animal, they were put in an induction chamber with isoflurane 2% for 4 min, followed by an intraperitoneal injection of ketamine and xylazine. Sedated rodents were placed on a nose cone with isoflurane to maintain the anesthesia. Electric heating pads with a rectal-probe feedback loop were utilized to prevent hypothermia in the experimental animal and maintain body temperature at 37 °C. After preoxygenation, animals were intubated and ventilated on 30% oxygen during baseline measurements for 15 min. Femoral vasculature was exposed through an inguinal incision (
Figure 1). After microsurgical dissection of the femoral artery and vein below the inguinal ligament, vessels were prepared for ligature, and 100 IU of heparin was administered intraperitoneally 10 min before ligation of the vessels. Baseline Raman spectroscopy measurements were performed on the adductor muscle after dissecting the overlaying fascia. Artery and vein were incised and cannulated with #3 French venous catheters. The artery was immediately flushed with heparinized saline to prevent intravascular clotting.
Animals were euthanized by anesthetic overdose (4–5% isoflurane) and cardiac puncture. Once the animal’s death had been confirmed, transfemoral amputation was performed, and the amputated limb was weighed. The time of ischemia and confirmed death until the beginning of the perfusion or cooling, respectively, were recorded.
2.2. Experimental Setup
Through the catheter, the limb was connected to the perfusion setup via luer lock connectors. Perfusion with a continuous flow rate of 1 mL/min, as assessed in previous experiments as the physiological flow of the femoral artery, was started with (I) oxygenated modified, dextrane-enriched Phoxilium, (ii) non-oxygenated dextrane-enriched Phoxilium, and (iii) Phoxilium enriched with oxygen microcarriers. A detailed overview of the experimental groups can be seen in
Table 1.
The detailed composition of the perfusion solution was published in our work in 2020 [
5]. Briefly, the balanced electrolyte solution Phoxilium™ (Baxter, Deerfield, IL, USA) was modified to tailor it to the specific requirements of VCA machine perfusion. Dextran with 40,000 Dalton served as a colloid. Fifty percent Dextrose (Hospira, Inc., Lake Forest, IL, USA) was added to a concentration of 0.1%. Insulin R (Lilly USA, LLC, Indianapolis, IN, USA) was added to a concentration of 0.0075%. Further additives were 125 mg of methylprednisolone and 2500 units/L of heparin (both Fresenius Kabi, Lake Zurich, IL, USA).
Calibrated Baxter i.v. pumps (Baxter, Deerfield, IL, USA) were used to transport the preheated perfusate into the limb. Pressure was assessed through an in-line luer-lock pressure sensor (Pendar, Cambridge, MA, USA).
Limbs of the control group were stored in an ice slurry at 4 °C (static cold storage (SCS)) for the entire duration (12 h) of the experiment.
Temperature, pressure, and tissue pO2 were continuously recorded using LabChart Pro-8 software (ADInstruments) software. Perfusate samples were drawn from the cannulated femoral vein.
Osmotic pressure of the perfusate was measured with a vapor pressure osmometer (Wescor Vapro, Logan Utah, UT, USA) and averaged around 305 mOsmol/kg.
2.3. Oxygen Microcarriers
The fabrication of polymeric microbubbles (mean size = 5 μm) was thoroughly reported in our previous work [
10].
Briefly, nanoprecipitation of biocompatible dextran, as the base of the polymer, was utilized to produce stable microbubbles. The 100% oxygen loading of oxygen microcarriers was achieved by passively purging the headspace of microbubble suspension in 10% dextrose solution, as measured with a dissolved pO2 > 750 mmHg. The oxygenated microbubbles can be stored in a capped syringe to maintain oxygen content and are stable for months at room temperature.
2.4. Raman Spectroscopy
A Resonance Raman Spectrometer was used to quantify the mitochondrial redox state in the skeletal muscle of the limb in real time, as described previously for myocardial muscle [
11].
Briefly, a single-mode laser light source with a specific wavelength of 441 nm excites specific molecular bonds to obtain a spectroscopical read-out. In this study, we used resonance Raman reduced mitochondrial ratio (3RMR) to quantify the mitochondrial redox state of the skeletal muscle within the limb during the ex situ perfusion.
Before amputation, baseline measurements of the physiologically perfused skeletal muscle were performed to generate a spectral library of the different components.
2.5. Ultrastructural Analysis
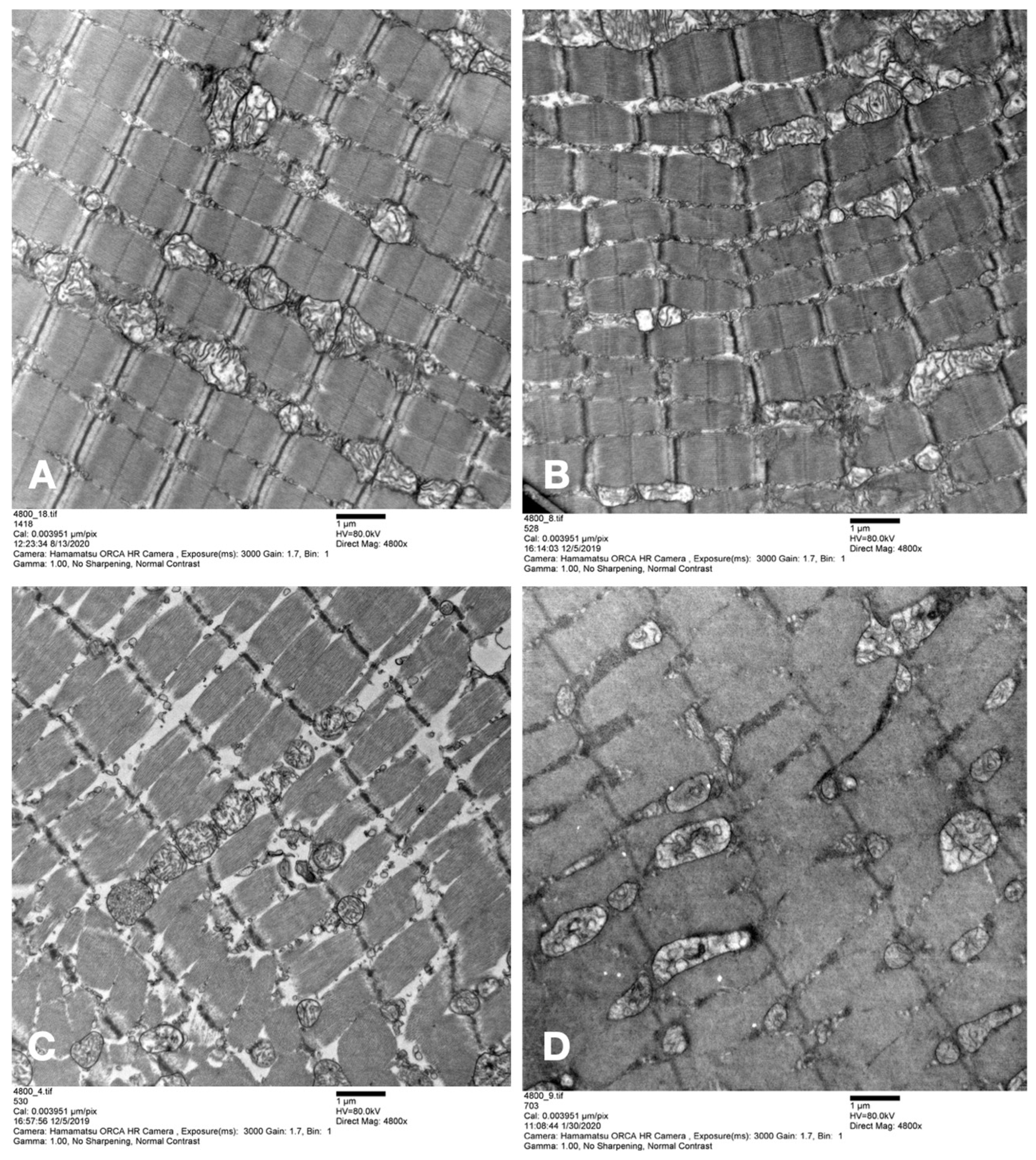
Skeletal muscle samples were harvested before amputation of the hindlimb directly after amputation, as well as at 6 and 12 h post-amputation. Samples were fixed in 2.5% glutaraldehyde, 1.25% PFA, and 0.03% picric acid in 0.1 M sodium cacodylate buffer (pH 7.4) overnight at 4 °C. Samples were then washed with 0.1 M phosphate buffer, post-fixed with 1% OsO4 dissolved in 0.1 M phosphate-buffered saline (PBS) for 2 h, dehydrated in ascending gradual series (50–100%) of ethanol, and infiltrated with propylene oxide. Samples were embedded using the Poly/Bed 812 kit (Polysciences) according to the manufacturer’s instructions. After pure fresh resin embedding and polymerization in a 65 °C oven (TD-700, DOSAKA, Kyoto, Japan) for 24 h, sections of approximately 200–250 nm thickness were cut and stained with toluidine blue for light microscopy. Sections of 70 nm thickness were double-stained with 6% uranyl acetate (EMS, 22400; Hatfield, PA, USA) for 20 min and lead citrate (Fisher) for 10 min for contrast staining. The sections were cut using Reichert Ultracut-S/LEICA EM UC-7 (Leica, Deer Park, IL, USA) with a diamond knife (Diatome Ltd., Nidau, Switzerland) and transferred onto copper and nickel grids. All the sections were observed by transmission electron microscopy (JEOL 1200EX, Bronx, NY, USA) at an acceleration voltage of 80 kV. All steps, including image acquisition, were performed in a blinded manner by independent persons (F.B., A.W., B.T.). Image analysis was performed using the open-source application Image J 1.52 k (Wayne Rasband, NIH, USA).
2.6. Perfusion Solution Sample Analysis
Collection from the venous cannulation was performed at timepoint 0, directly after starting the perfusion, and then every two hours until timepoint 12 h. Perfusion solution samples were analyzed for markers of muscle injury, such as CK, LDH, and myoglobin. Additionally, blood gas analysis was performed for pH, pO
2 and pCO
2, potassium (K+), glucose, and lactate no later than 1 h after collection. Blood gas analysis was performed with an 837 Flex Radiometer (Radiometer Inc., Brea, CA, USA). Analysis of myoglobin was carried out with Cobas e602, lactate analysis with Cobas c702, and LDH and CK with Cobas c502 (Roche Diagnostics, IL, USA), as previously published [
2].
2.7. Statistical Analysis
For the comparison between multiple groups, a single ANOVA test was performed.
Differences amongst multiple groups and different timepoints were calculated in a mixed effect model or a two-way ANOVA. To account for multiple comparisons, p-values were adjusted via Tukey post hoc correction. A p-value of <0.05 was considered statistically significant. Results are shown as the mean with standard deviation. All statistical analyses and visualizations were carried out using GraphPad Prism version 9.4.1 for MacOS (GraphPad Software, La Jolla, CA, USA).
4. Tissue Oxygenation
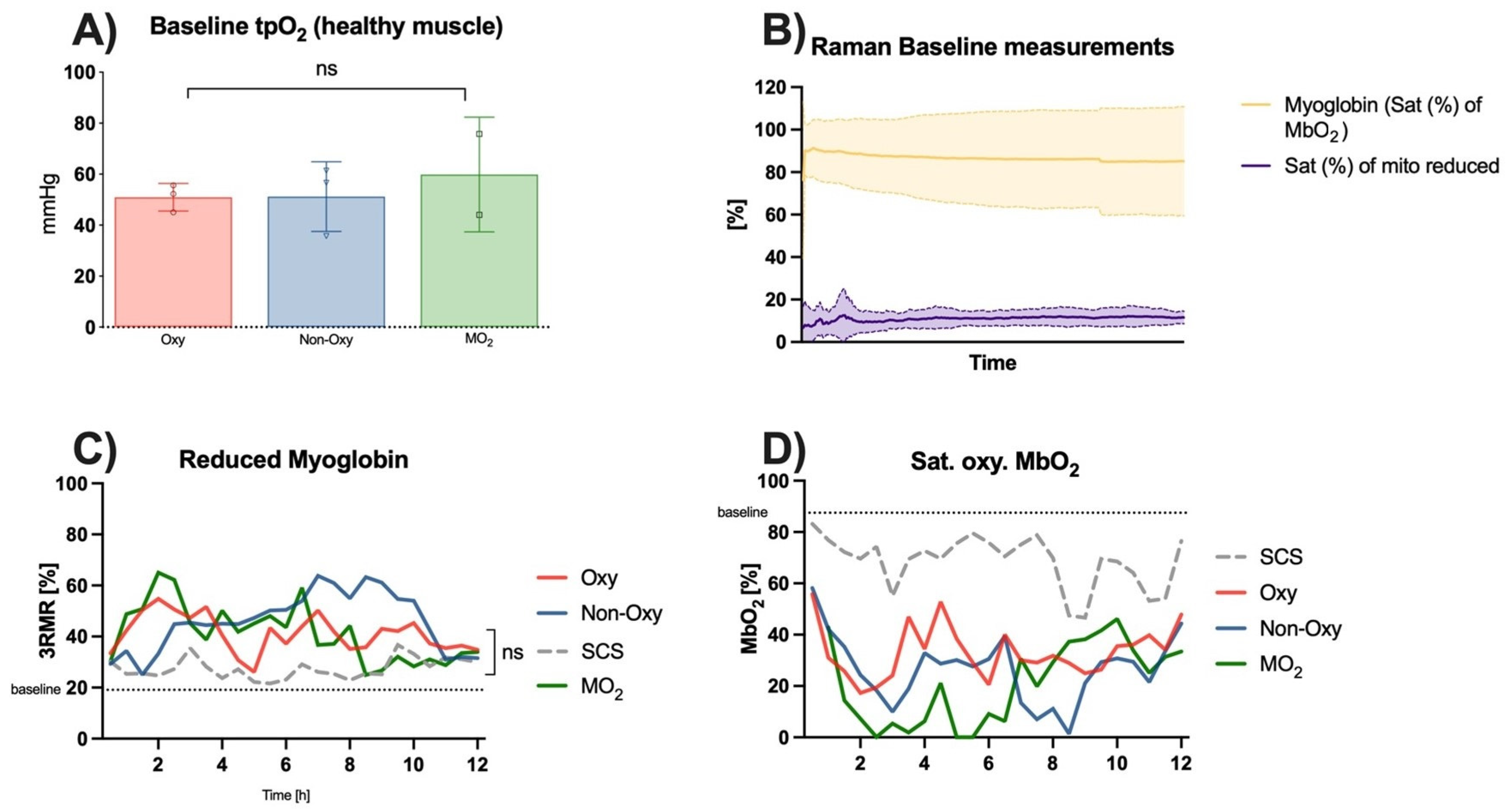
Raman Spectroscopy and Tissue pO2
Measurements of healthy skeletal muscle perfused in the anesthetized rat showed a mean baseline tissue pO
2 value of 53.3 ± 12.3 mmHg prior to cannulation and perfusion (
Figure 7A). Raman spectroscopy showed a saturation of the oxygenated myoglobin of 87.47 ± 11.6% and a saturation of the reduced myoglobin of 19.12 ± 5.51% (
Figure 7B).
3RMR signals of skeletal muscle during either perfusion or static cold storage over 12 h did not show significant differences within the groups. Values in the SCS group were the closest to the aforementioned baseline values. Values of reduced myoglobin in the perfusion groups peaked after 2 h of the experiment in the MO
2 group and the Oxy group and then gradually decreased to twofold baseline values after 12 h. Values in the Non-Oxy group peaked after 7–9 h of perfusion and then decreased as well towards the end of the 12 h (
Figure 7C). Statistical analysis did not show substantial differences among the different groups.
Consistent with these results, saturation of oxygenated myoglobin showed a sudden decrease in the values in all groups after the first 2–3 h of the experiment. Apart from peaks around hour 4 of perfusion, the saturation of oxygenated myoglobin remained low, particularly in the Oxy group and the MO
2 group. These groups showed a recovery from hour 6 until the end of the experiment. Values in the Non-Oxy group only decreased between hours 8–10. The trend in the values in the static cold storage group was similar to that in the Non-Oxy group; however, they were closer to baseline values (
Figure 7D).
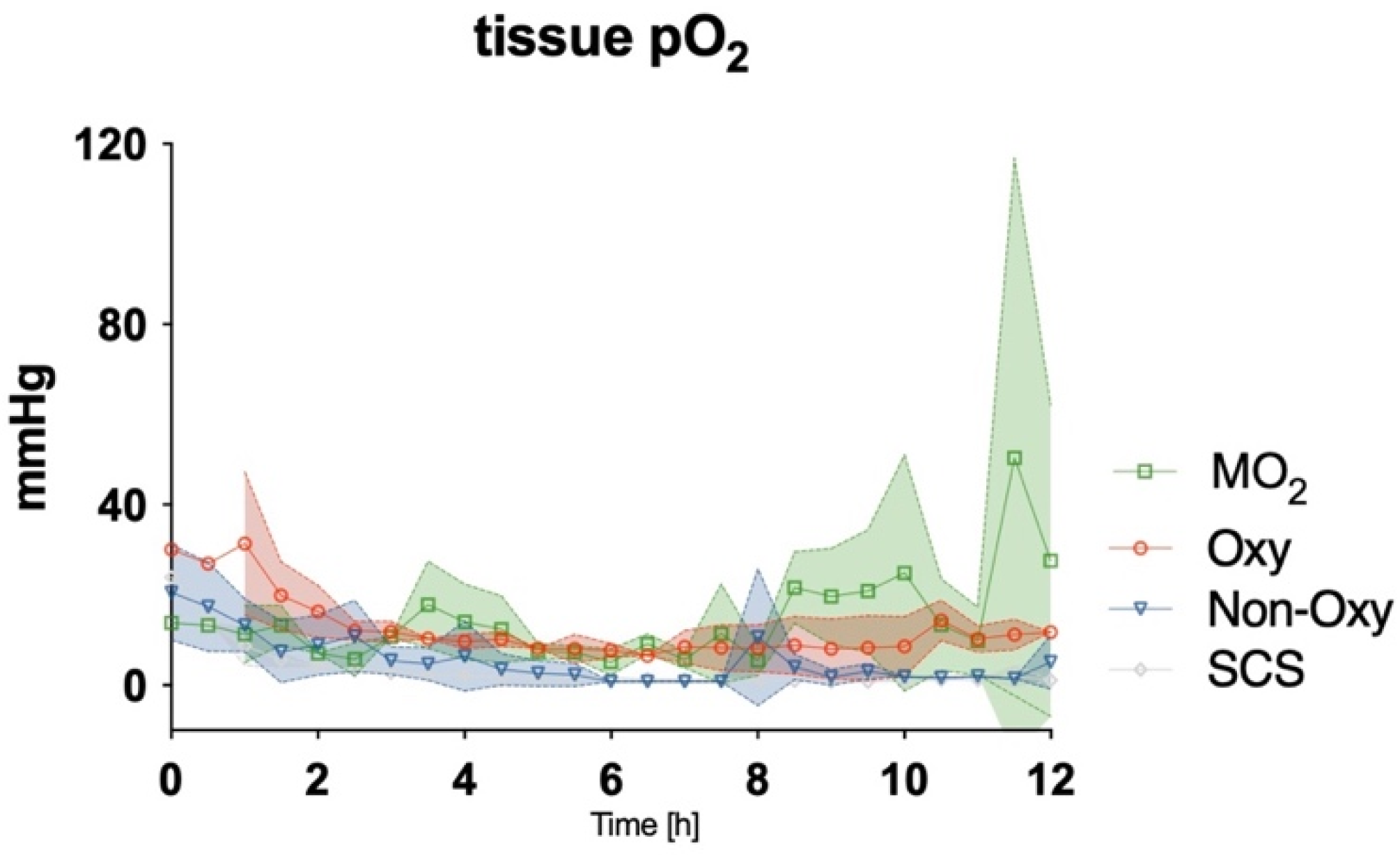
After detaching the cannulated limb and either storing it on ice or attaching it to the perfusion devices, levels of tissue pO
2 were assessed with an oxygen probe. A gradual decrease was observed in all groups during the first 2–3 h of the experiment. While values for tissue pO
2 remained low in the SCS group and the Non-Oxy group for the entire duration of the procedure, tissue pO
2 in the Oxy group and the MO
2 group remained at circa 20% of the baseline values from hour 3 to 8. After hour 8, a gradual increase in tissue pO
2 could be observed in the MO
2 group, up to 50% of baseline values (
Figure 8). Statistical differences were not observed among the groups.
5. Discussion
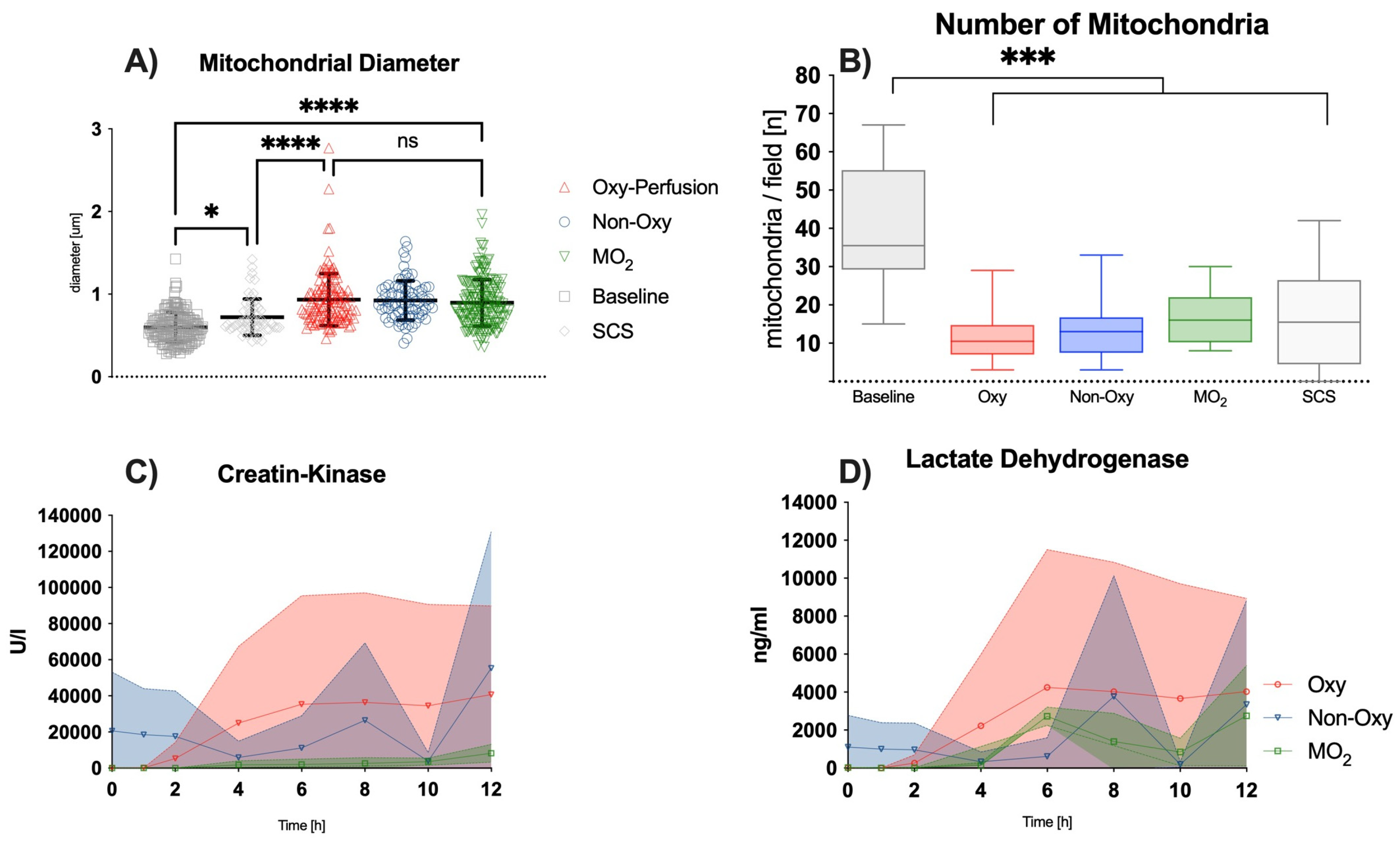
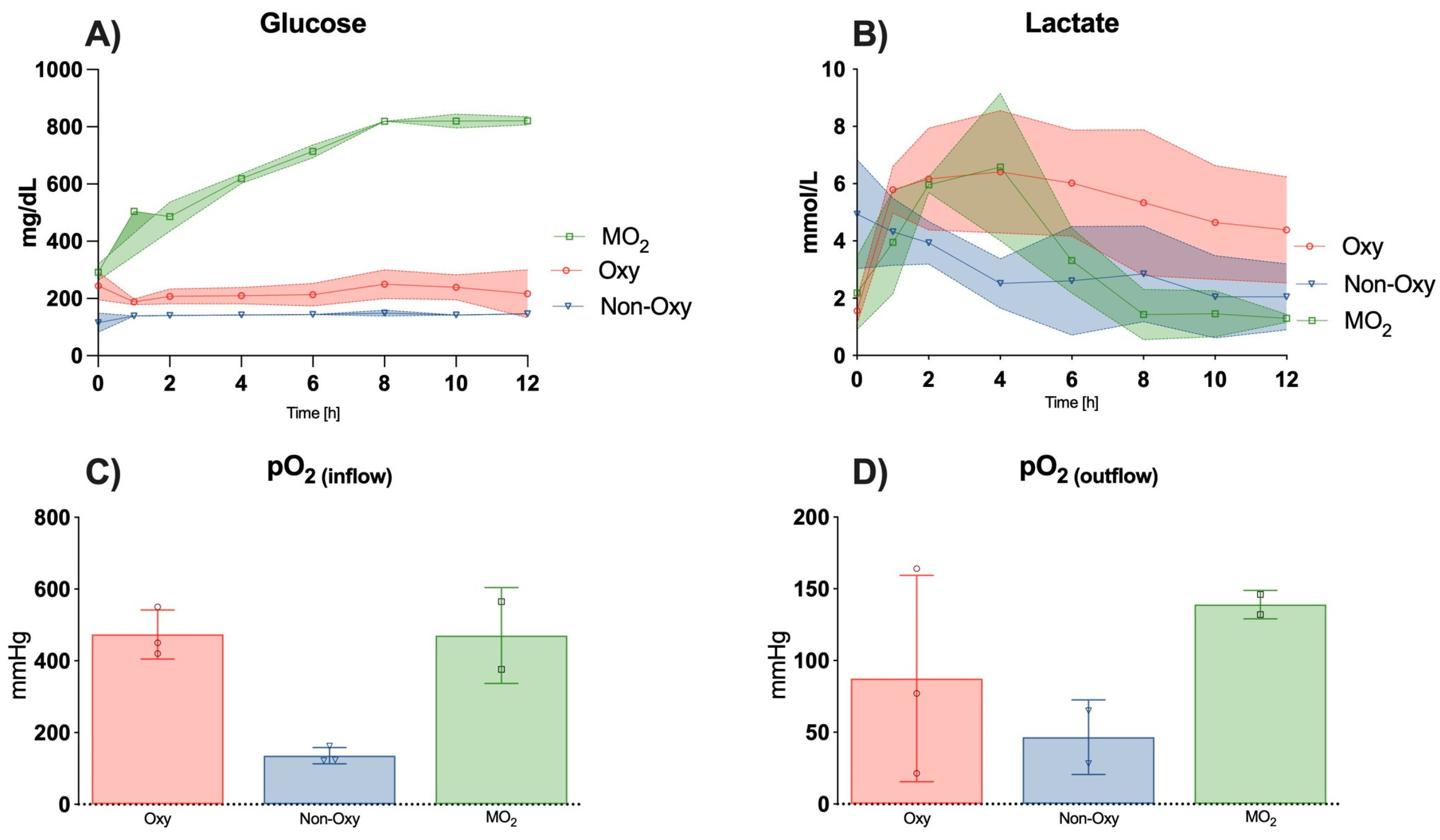
The use of dextrane polymer oxygen microcarriers for the normothermic ex situ perfusion of rat hindlimbs proved to be beneficial, with (i) higher levels of tissue pO2 and (ii) lower levels of lactate after 8–12 h as compared to groups in which acellular perfusion solutions were used as perfusate, while ischemia times and temperatures were comparable. Mitochondrial diameter remained comparable to the values in the other perfusion groups after 12 h of perfusion and was significantly higher as compared to the static cold storage group, while the total number of mitochondria decreased significantly in all ex situ perfusion groups.
Raman spectroscopy of the skeletal muscle mitochondria redox state during the ex situ perfusion or static cold storage did not show significant differences between the different preservation techniques. After 8 h of perfusion, however, a trend towards values closer to baseline could be observed in the MO2 group, both for reduced and oxygenated mitochondria. Given the amount of scattered data and the multitude of measurements, statistical differences between the perfusion groups were not found.
In previous studies, we have demonstrated the feasibility of ex situ perfusion of VCAs with acellular solutions under hypothermic conditions [
2,
5]. Given the tissue’s increased metabolic demand under normothermic conditions, we are now able to demonstrate non-inferiority towards conventional tissue conservation methods, such as static cold storage and hypothermic ex situ perfusion. Normothermic perfusion enables the testing of skeletal muscle during the experiment and directly prior to transplantation.
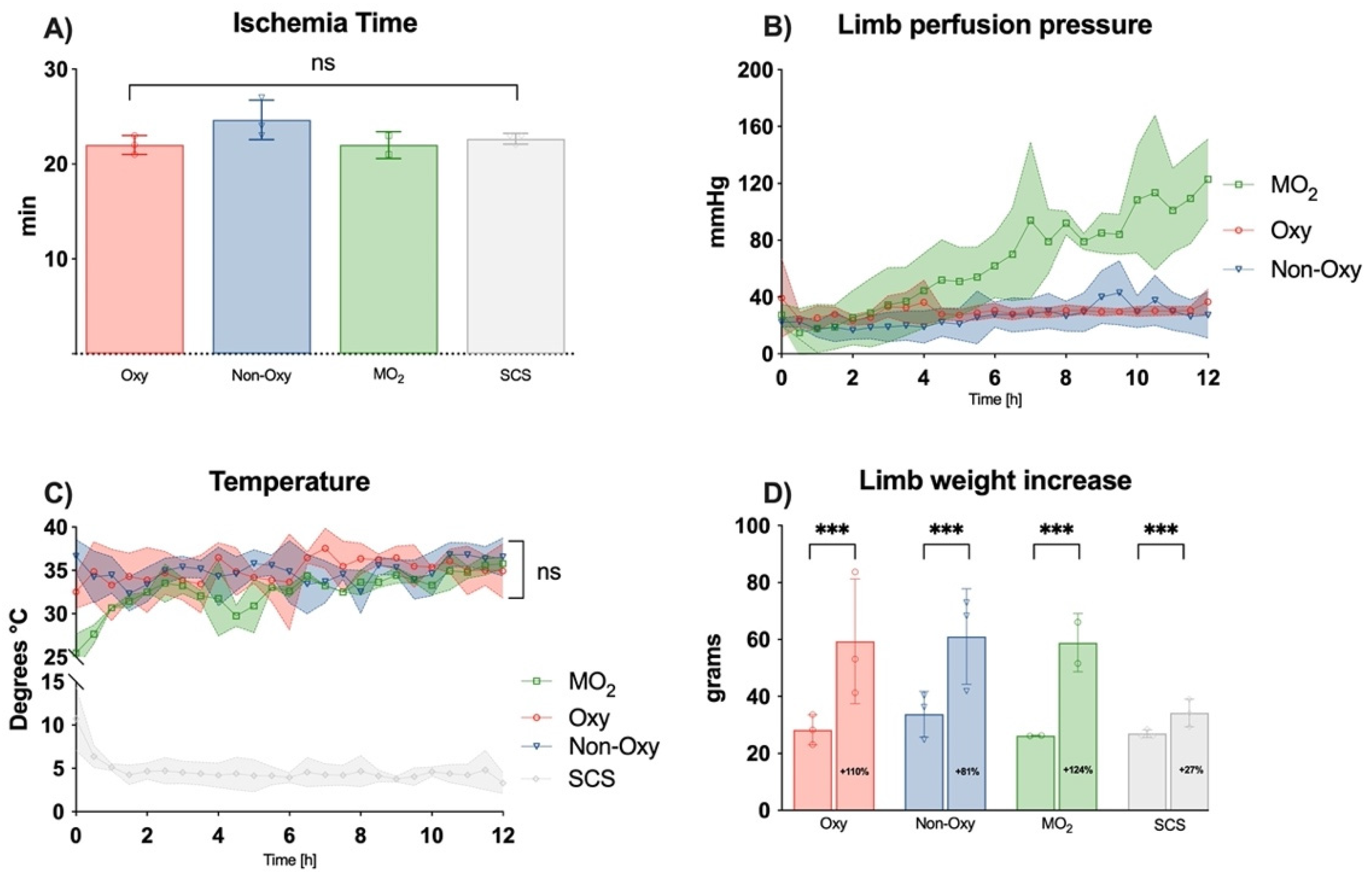
Over the course of the experiment, a substantial weight gain was observed in all perfusion groups, which is in accordance with previous animal experiments by our group in a large animal model [
5]. Weight increase of the perfused limbs ranged from 84–121% and was similar to the results of other working groups with similar perfusion settings (110–130%) [
12]. As shown in previous experiments, weight gain was linked to interstitial edema and was higher in groups with acellular solutions enriched with dextrane as compared to solutions with different colloids [
5]. In a study with human allografts and a pressure-controlled perfusion model, only minimal weight gain was observed after 24 h with a colloidal perfusate (+4.3%) [
2]. In the SCS group, the repeated irrigation of the extremity to prevent tissue from drying out might have led to an untypical weight gain.
Flow rates of perfusion were comparable to physiologic conditions in resting muscle, as previously published, and in accordance with our preliminary measurements with microvascular flow probes [
13,
14,
15]. Different research groups performed ex situ perfusion experiments with higher flow rates, such as 2.5 mL/min, and reported higher perfusion pressures of MO
2 100 mmHg in the femoral artery. Those values were not reached earlier than 6 h after perfusion in the MO
2 group in our experimental setting, which might play a role in the formation of edema.
In contrast to other publications reporting a subsequent increase in lactate levels within the perfusion solution in a hypothermic setting, our results demonstrate a decrease in lactate levels after a peak between hours 2–4 of perfusion in the MO
2 group to physiological levels [
16]. These findings are consistent with the trend shown by Gok et al. under near-normothermic conditions after perfusion for 6 h [
15]. Araki et al. found lactate levels decreased after one hour of perfusion until hour 6 in rat hindlimbs under normothermic conditions with extracellular-trehalose-Kyoto (ETK) solution with and without hemoglobin vesicles [
12]. Perfusate flow in the femoral artery was comparable at <1mL/min.
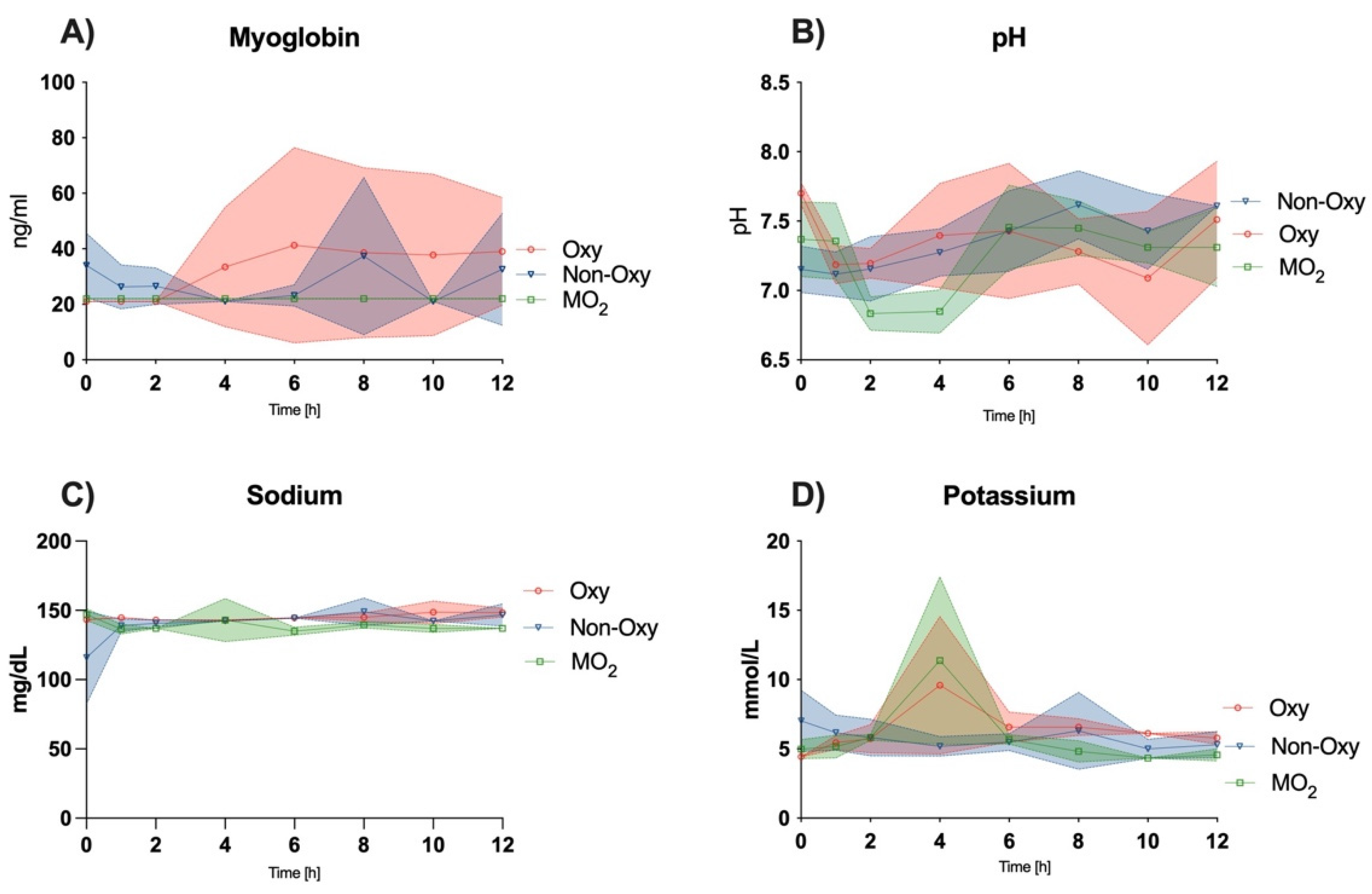
Potassium levels peaked after two hours of perfusion in the Oxy and MO2 groups and equilibrated at a level of mild hyperkalemia. Gok et al. reported similar values in a 6-h perfusion setting with hemofilter and an increase without hemofilter.
Other markers of skeletal muscle cell injury, e.g., CK, LDH, and myoglobin, increased to a greater extent in the Oxy and Non-Oxy groups when compared to the MO
2 group. Similar trends could be observed in a study by Herold et al., where LDH levels were found to rise and plateau after several hours of subnormothermic perfusion of adipofascial flaps in a rat model [
17]. These trends are analogous to previous large animal and human studies under hypothermic conditions and near-normothermic conditions, respectively [
2,
5,
18].
CO
2 and O
2 levels in the efflux appeared to be comparable to trends in the study by Araki et al. [
12].