Efficacy and Risks of Different Treatments for Oral Hyperpigmentation: A Systematic Review and Network Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Study Selection
2.5. Data Items and Collection Process
2.6. Risk of Bias
2.7. Data Analysis
3. Results
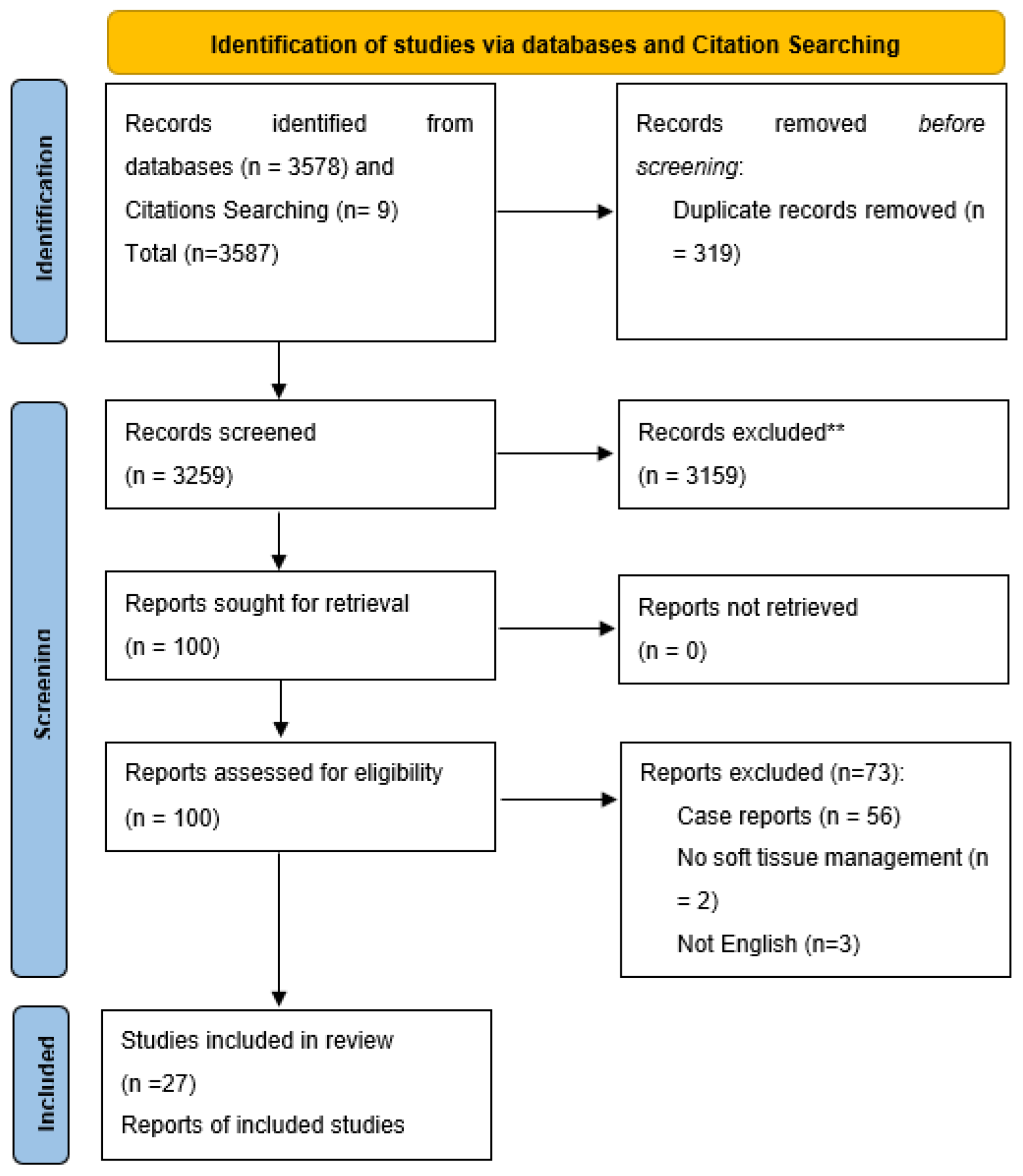
3.1. Study Selection
3.2. Characteristics of Included Studies
3.3. Risk of Bias
3.4. Network Meta-Analysis
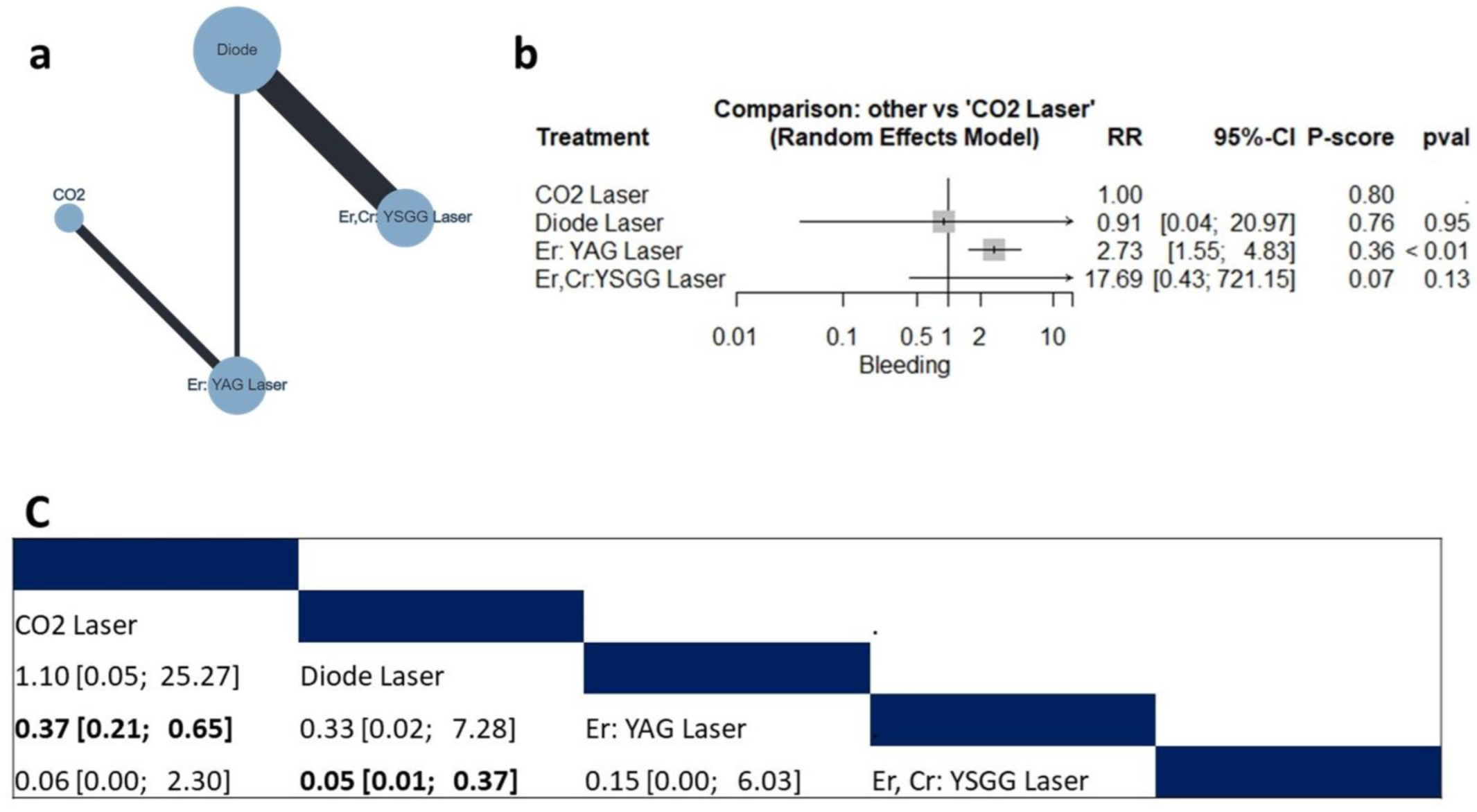
3.4.1. Bleeding Risk
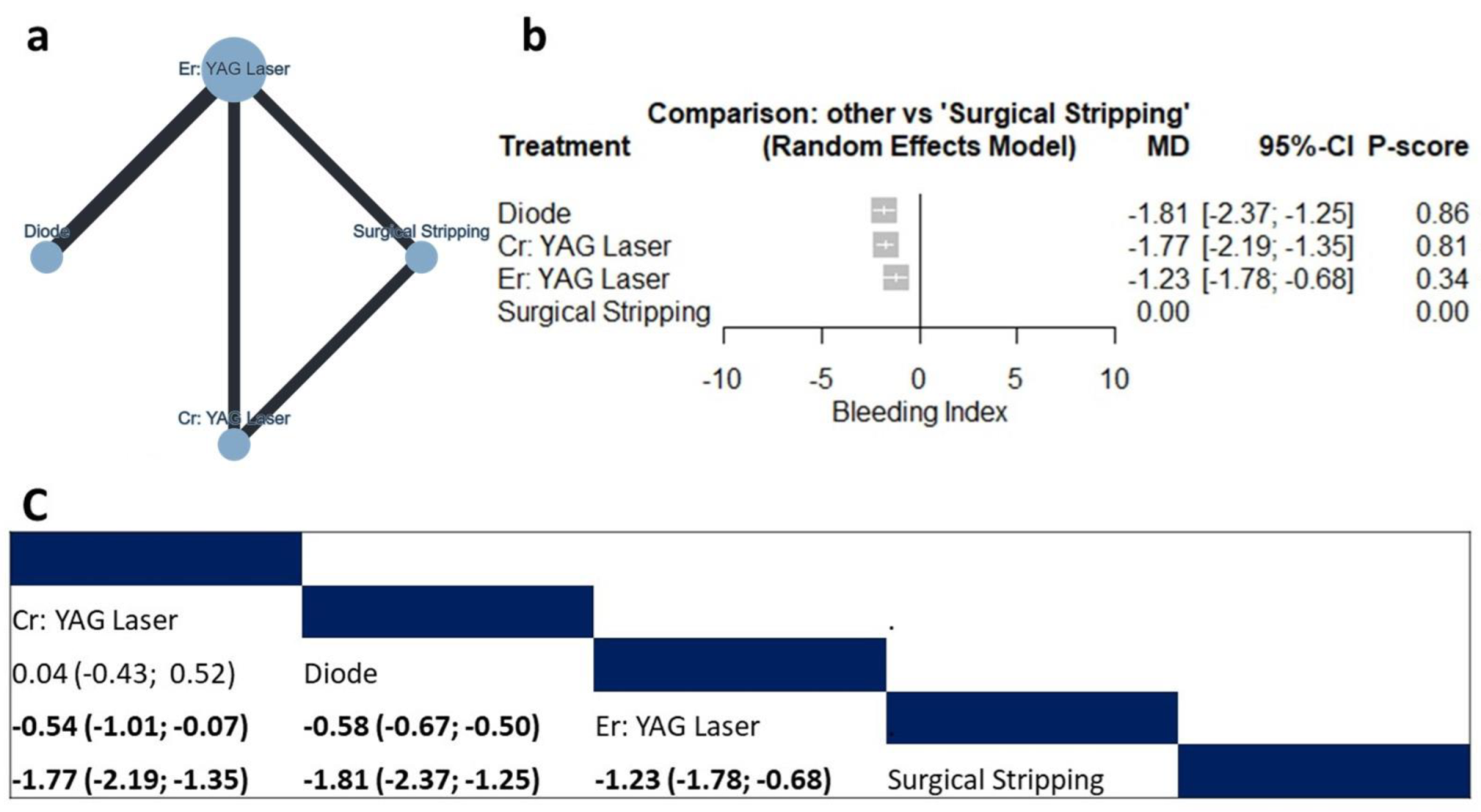
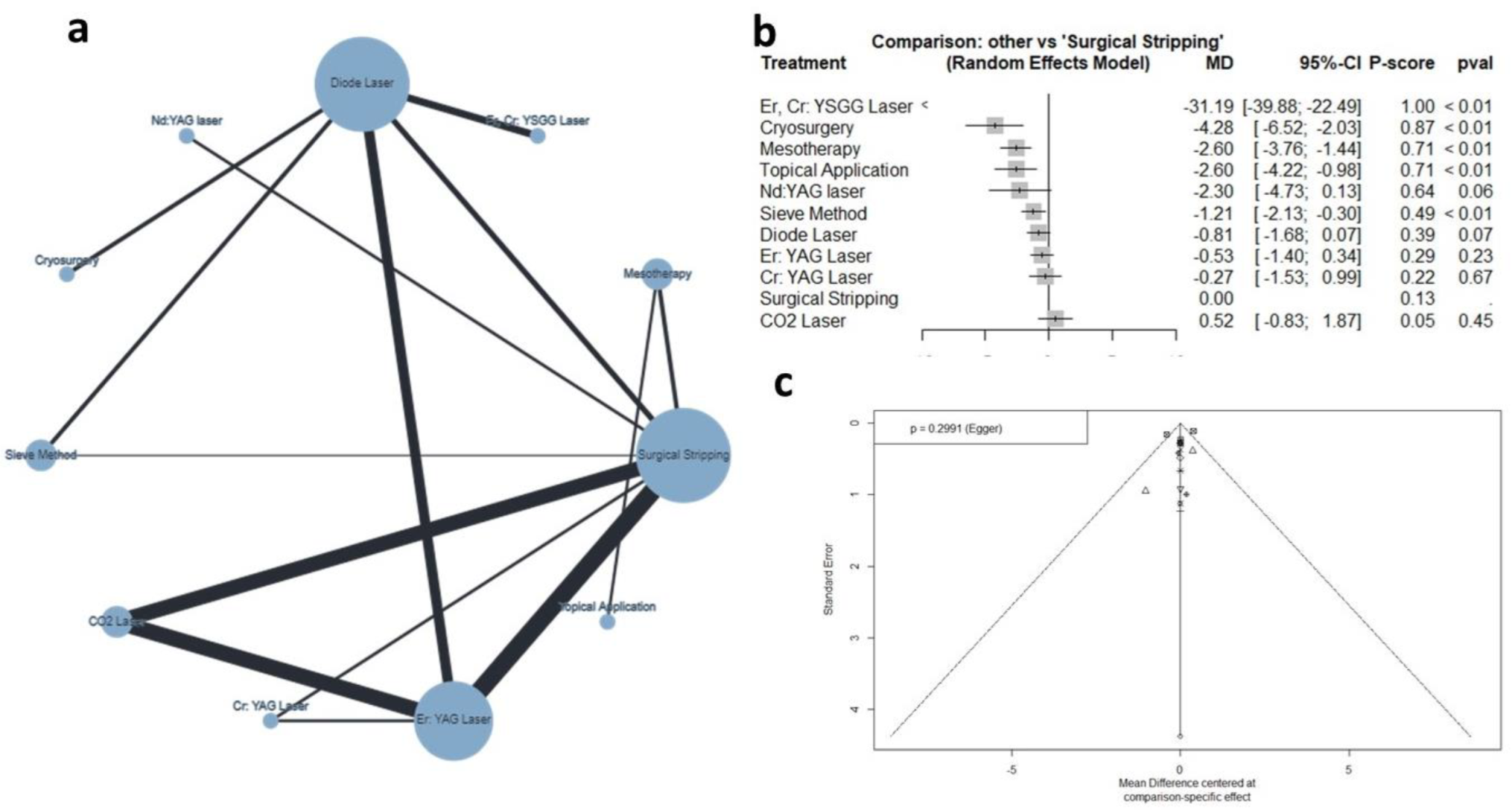
3.4.2. Bleeding Index
3.4.3. Postoperative Pain Score
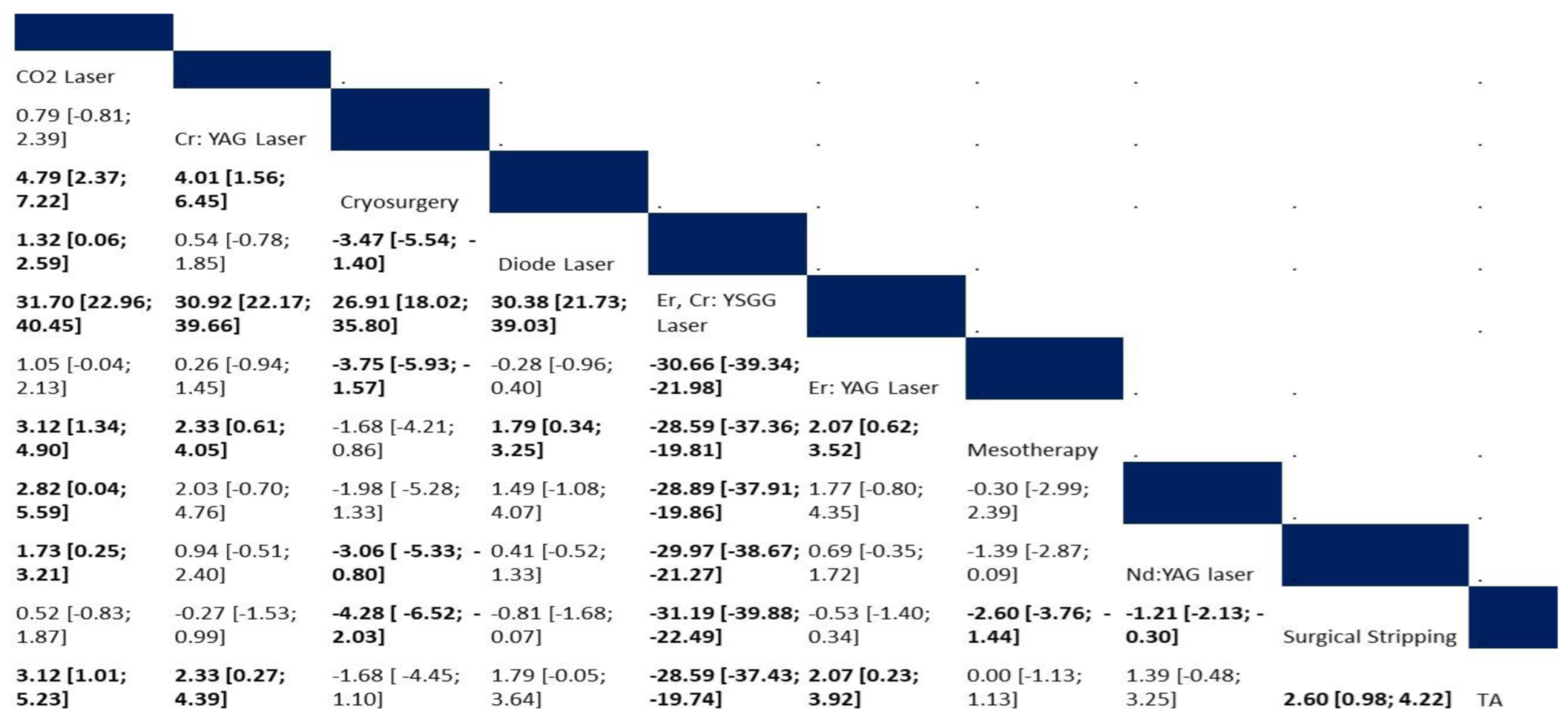
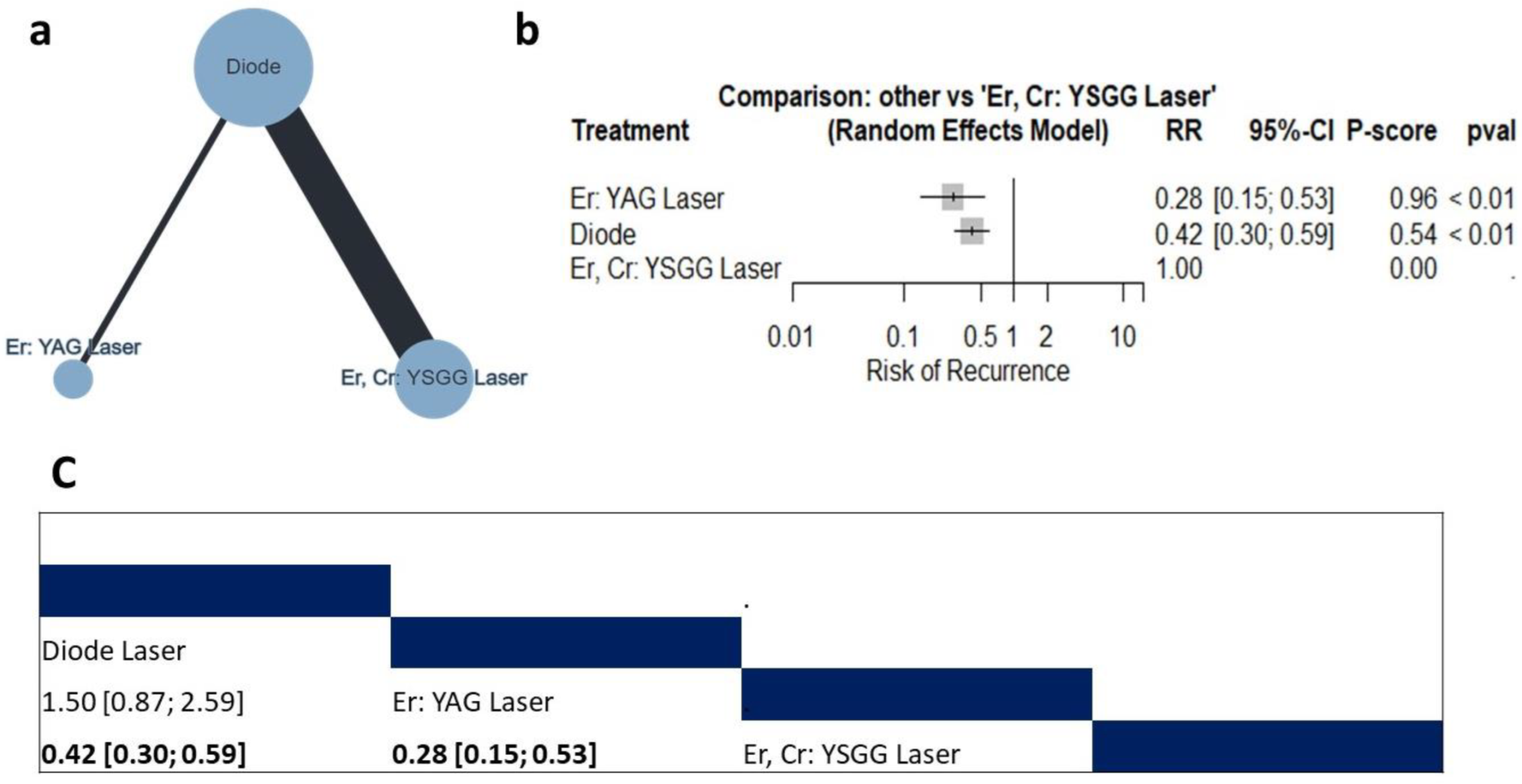
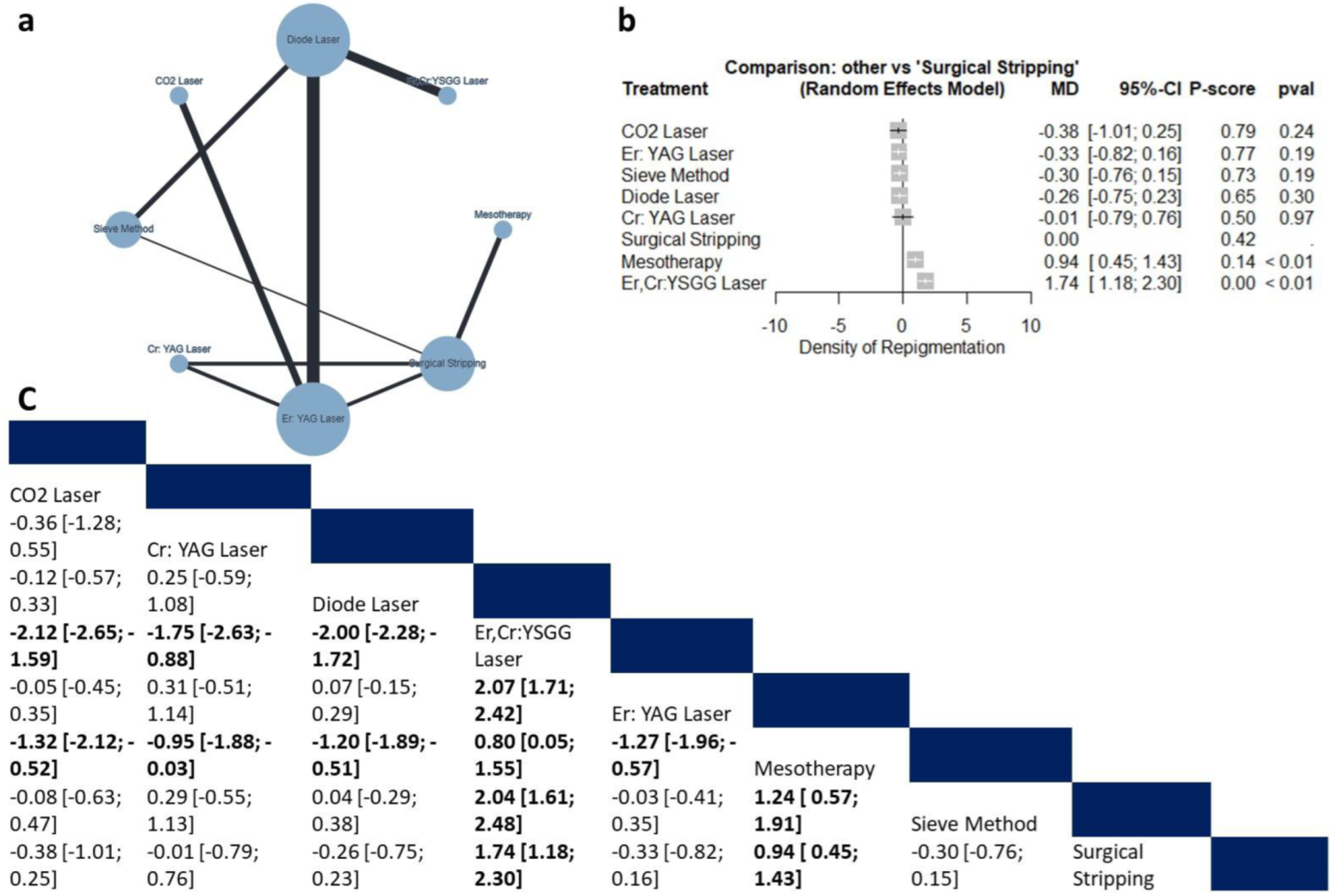
3.4.4. Recurrence
Risk of Recurrence
Density of Repigmentation
3.4.5. Summary of the Study Findings
4. Discussion
4.1. Bleeding
4.2. Postoperative Pain
4.3. Recurrence
4.4. Clinical Implications
4.5. Future Perspectives
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albuquerque, D.M.; Cunha, J.L.; Roza, A.L.; Arboleda, L.P.; Santos-Silva, A.R.; Lopes, M.A.; Vargas, P.A.; Jorge, J.; de Almeida, O.P.; Abrahão, A.C.; et al. Oral pigmented lesions: A retrospective analysis from Brazil. Med. Oral. Patol. Oral. Cir. Bucal 2021, 26, e284–e291. [Google Scholar] [CrossRef]
- Feller, L.; Masilana, A.; Khammissa, R.A.; Altini, M.; Jadwat, Y.; Lemmer, J. Melanin: The biophysiology of oral melanocytes and physiological oral pigmentation. Head. Face Med. 2014, 10, 8. [Google Scholar] [CrossRef]
- Sreeja, C.; Ramakrishnan, K.; Vijayalakshmi, D.; Devi, M.; Aesha, I.; Vijayabanu, B. Oral pigmentation: A review. J. Pharm. Bioallied Sci. 2015, 7, S403–S408. [Google Scholar] [CrossRef]
- Tarakji, B.; Umair, A.; Prasad, D.; Alsakran Altamimi, M. Diagnosis of oral pigmentations and malignant transformations. Singapore Dent. J. 2014, 35c, 39–46. [Google Scholar] [CrossRef]
- Rosebush, M.S.; Briody, A.N.; Cordell, K.G. Black and Brown: Non-neoplastic Pigmentation of the Oral Mucosa. Head. Neck Pathol. 2019, 13, 47–55. [Google Scholar] [CrossRef]
- Mallagray-Montero, M.C.; Moreno-López, L.A.; Cerero-Lapiedra, R.; Castro-Janeiro, M.; Madrigal-Martínez-Pereda, C. Medication related to pigmentation of oral mucosa. Med. Oral. Patol. Oral. Cir. Bucal 2022, 27, e230–e237. [Google Scholar] [CrossRef]
- Houlihan, O.A.; Rangaswamy, G.; McArdle, O. A rare case of melanotic hyperpigmentation of the tongue secondary to radiotherapy. Rep. Pract. Oncol. Radiother. 2021, 26, 320–323. [Google Scholar] [CrossRef]
- Farid, H.; Shinwari, M.S.; Khan, F.R.; Tanwir, F. Journey From Black To Pink Gums: Management Of Melanin Induced Physiological Gingival Hyper Pigmentation. J. Ayub Med. Coll. Abbottabad 2017, 29, 132–138. [Google Scholar]
- Abduljabbar, T.; Vohra, F.; Akram, Z.; Ghani, S.M.A.; Al-Hamoudi, N.; Javed, F. Efficacy of surgical laser therapy in the management of oral pigmented lesions: A systematic review. J. Photochem. Photobiol. B 2017, 173, 353–359. [Google Scholar] [CrossRef]
- Altayeb, W.; Hamadah, O.; Alhaffar, B.A.; Abdullah, A.; Romanos, G. Gingival depigmentation with diode and Er,Cr:YSGG laser: Evaluating re-pigmentation rate and patient perceptions. Clin. Oral. Investig. 2021, 25, 5351–5361. [Google Scholar] [CrossRef]
- Suryavanshi, P.; Dhadse, P.; Bhongade, M. Comparative evaluation of effectiveness of surgical blade, electrosurgery, free gingival graft, and diode laser for the management of gingival hyperpigmentation. J. Datta Meghe Inst. Med. Sci. Univ. 2017, 12, 133–137. [Google Scholar] [CrossRef]
- Jokar, L.; Bayani, M.; Hamidi, H.; Keivan, M.; Azari-Marhabi, S. A Comparison of 940 nm Diode Laser and Cryosurgery With Liquid Nitrogen in the Treatment of Gingival Physiologic Hyperpigmentation Using Split Mouth Technique: 12 Months Follow Up. J. Lasers Med. Sci. 2019, 10, 131–138. [Google Scholar] [CrossRef]
- Negi, R.; Gupta, R.; Dahiya, P.; Kumar, M.; Bansal, V.; Kaur Samlok, J. Ceramic soft tissue trimming bur: A new tool for gingival depigmentation. J. Oral. Biol. Craniofac Res. 2019, 9, 14–18. [Google Scholar] [CrossRef]
- Muruppel, A.M.; Pai, B.S.J.; Bhat, S.; Parker, S.; Lynch, E. Laser-Assisted Depigmentation-An Introspection of the Science, Techniques, and Perceptions. Dent. J. 2020, 8, 88. [Google Scholar] [CrossRef]
- Passeron, T.; Genedy, R.; Salah, L.; Fusade, T.; Kositratna, G.; Laubach, H.J.; Marini, L.; Badawi, A. Laser treatment of hyperpigmented lesions: Position statement of the European Society of Laser in Dermatology. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 987–1005. [Google Scholar] [CrossRef]
- Mosaddad, S.A.; Mahootchi, P.; Rastegar, Z.; Abbasi, B.; Alam, M.; Abbasi, K.; Fani-Hanifeh, S.; Amookhteh, S.; Sadeghi, S.; Soufdoost, R.S.; et al. Photodynamic Therapy in Oral Cancer: A Narrative Review. Photobiomodul Photomed. Laser Surg. 2023, 41, 248–264. [Google Scholar] [CrossRef]
- Nammour, S.; El Mobadder, M.; Namour, M.; Namour, A.; Rompen, E.; Maalouf, E.; Brugnera Junior, A.; Brugnera, A.P.; Vescovi, P.; Zeinoun, T. A Randomized Comparative Clinical Study to Evaluate the Longevity of Esthetic Results of Gingival Melanin Depigmentation Treatment Using Different Laser Wavelengths (Diode, CO(2), and Er:YAG). Photobiomodul Photomed. Laser Surg. 2020, 38, 167–173. [Google Scholar] [CrossRef]
- El-Mofty, M.; Elkot, S.; Ghoneim, A.; Yossri, D.; Ezzatt, O.M. Vitamin C mesotherapy versus topical application for gingival hyperpigmentation: A clinical and histopathological study. Clin. Oral. Investig. 2021, 25, 6881–6889. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Gholami, L.; Moghaddam, S.A.; Rigi Ladiz, M.A.; Molai Manesh, Z.; Hashemzehi, H.; Fallah, A.; Gutknecht, N. Comparison of gingival depigmentation with Er,Cr:YSGG laser and surgical stripping, a 12-month follow-up. Lasers Med. Sci. 2018, 33, 1647–1656. [Google Scholar] [CrossRef]
- Hegde, R.; Padhye, A.; Sumanth, S.; Jain, A.S.; Thukral, N. Comparison of surgical stripping; erbium-doped:yttrium, aluminum, and garnet laser; and carbon dioxide laser techniques for gingival depigmentation: A clinical and histologic study. J. Periodontol. 2013, 84, 738–748. [Google Scholar] [CrossRef]
- Ribeiro, F.V.; Cavaller, C.P.; Casarin, R.C.; Casati, M.Z.; Cirano, F.R.; Dutra-Corrêa, M.; Pimentel, S.P. Esthetic treatment of gingival hyperpigmentation with Nd:YAG laser or scalpel technique: A 6-month RCT of patient and professional assessment. Lasers Med. Sci. 2014, 29, 537–544. [Google Scholar] [CrossRef]
- Limpjaroenviriyakul, N.; Jurairattanaporn, N.; Kamanamool, N.; Rojhirunsakool, S.; Kanokrungsee, S.; Udompataikul, M. Low-fluence Q-switched Nd:YAG 1064-nm laser versus Q-switched Nd:YAG 532-nm laser in the treatment of hyperpigmented lips: A prospective, randomized, controlled, evaluator-blinded trial. Lasers Med. Sci. 2020, 35, 165–171. [Google Scholar] [CrossRef]
- Giannelli, M.; Formigli, L.; Bani, D. Comparative evaluation of photoablative efficacy of erbium: Yttrium-aluminium-garnet and diode laser for the treatment of gingival hyperpigmentation. A randomized split-mouth clinical trial. J. Periodontol. 2014, 85, 554–561. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, Q.L.D.; Do, T.T.; Truong, K.N.; Dang, Q.V.; Bui, M.T.N. Evaluation of Carbon Dioxide Laser-Assisted Treatment for Gingival Melanin Hyperpigmentation. Dent. J. 2022, 10, 238. [Google Scholar] [CrossRef]
- Arif, R.H.; Kareem, F.A.; Zardawi, F.M.; Al-Karadaghi, T.S. Efficacy of 980 nm diode laser and 2940 nm Er: YAG laser in gingival depigmentation: A comparative study. J. Cosmet. Dermatol. 2021, 20, 1684–1691. [Google Scholar] [CrossRef]
- Stas, Y.T.; Hassan, A.A.; Noor, N.F.; Samsudin, A.R.; Shaari, R. Surgical laser oral depigmentation procedure: An investigation on the optimum power settings using Er:YAG laser. Lasers Dent. Sci. 2018, 2, 181–192. [Google Scholar] [CrossRef]
- Houshmand, B.; Janbakhsh, N.; Khalilian, F.; Talebi Ardakani, M.R. Efficacy of Conventional Laser Irradiation Versus a New Method for Gingival Depigmentation (Sieve Method): A Clinical Trial. J. Lasers Med. Sci. 2017, 8, 88–94. [Google Scholar] [CrossRef][Green Version]
- Roshannia, B.; Nourellahi, M.; Ahmadpanahi, T.; Norouzifard, A.; Kojoori, S.S. Comparison of Bur Abrasion and CO2 Laser in Treatment of Gingival Pigmentation: 6 Months Follow-Up. Oral. Health Prev. Dent. 2021, 19, 321–326. [Google Scholar] [CrossRef]
- Surve, P.; Mudda, J.A.; Patil, V.A.; Desai, S.R.; Agarwal, P.; Mustafa, M. Gingival depigmentation using surgical scalpel and sieve method of diode laser techniques-a comparative clinical intervention study. J. Evol. Med. Dent. Sci. 2020, 9, 2063–2067. [Google Scholar] [CrossRef]
- Hawwam, S.; El-Makaky, Y. Dermoscopic findings in benign racial gingival melanin hyperpigmentation and evaluation of its surgical management. Egypt. J. Dermatol. Venerol. 2020, 40, 23–28. [Google Scholar] [CrossRef]
- AlShoubaki, R.E.; AlZahrani, A.S. Outcomes of gingival depigmentation among smokers and non-smokers: A comparative study. Int. J. Pharm. Res. Allied Sci. Internet 2018, 7, 148–155. [Google Scholar]
- Juliana, H.; Tarek, S. Comparative study of the effect of BlueM active oxygen gel and coe-pack dressing on postoperative surgical depigmentation healing. Saudi Dent. J. 2022, 34, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Koca-Ünsal, R.B.; Kasnak, G.; Fıratlı, E. Comparison of Diode Laser and Conventional Method in Treatment of Gingival Melanin Hyperpigmentation. Eur. Ann. Dent. Sci. 2021, 48, 95–100. [Google Scholar] [CrossRef]
- Bakhshi, M.; Mojahedi, S.M.; Asnaashari, M.; Rahmani, S.; Namdari, M. Gingival depigmentation by Er,Cr:YSGG laser and diode laser: A split mouth, clinical trial study. Laser Ther. 2018, 27, 203–213. [Google Scholar] [CrossRef]
- El Shenawy, H.M.; Nasry, S.A.; Zaky, A.A.; Quriba, M.A. Treatment of Gingival Hyperpigmentation by Diode Laser for Esthetical Purposes. Open Access Maced. J. Med. Sci. 2015, 3, 447–454. [Google Scholar] [CrossRef]
- Kishore, A.; Kathariya, R.; Deshmukh, V.; Vaze, S.; Khalia, N.; Dandgaval, R. Effectiveness of Er:YAG and CO2 lasers in the management of gingival melanin hyperpigmentation. Oral. Health Dent. Manag. 2014, 13, 486–491. [Google Scholar]
- Jnaid Harb, Z.K.; El-Sayed, W.; Alkhabuli, J. Gingival Depigmentation Using Diode 980 nm and Erbium-YAG 2940 nm Lasers: A Split-Mouth Clinical Comparative Study. Int. J. Dent. 2021, 2021, 9424793. [Google Scholar] [CrossRef]
- Chaudhary, D.S.; Parwani, S.R.; Barkhade, S.; Gajbhiye, M.; Parwani, R.; Sikka, G.; Kawadkar, K.; Soni, N.J.; Armogida, N.G.; Dadlani, H.; et al. Physiological Gingival Melanin Hyperpigmentation Treatment with Injectable Vitamin C and Scalpel Technique: A Randomised Controlled Clinical Trial. Int. J. Dent. 2023, 2023, 4586923. [Google Scholar] [CrossRef]
- Yussif, N.M.; Abdel Rahman, A.R.; Elbarbary, A.M. Minimally invasive non-surgical locally injected vitamin C versus the conventional surgical depigmentation in treatment of gingival hyperpigmentation of the anterior esthetic zone: A prospective comparative study. Clin. Nutr. Exp. 2019, 24, 54–65. [Google Scholar] [CrossRef]
- Grover, H.S.; Dadlani, H.; Bhardwaj, A.; Yadav, A.; Lal, S. Evaluation of patient response and recurrence of pigmentation following gingival depigmentation using laser and scalpel technique: A clinical study. J. Indian. Soc. Periodontol. 2014, 18, 586–592. [Google Scholar] [CrossRef]
- Kathariya, R.; Pradeep, A.R. Split mouth de-epithelization techniques for gingival depigmentation: A case series and review of literature. J. Indian. Soc. Periodontol. 2011, 15, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, M.R.; Ahrari, F.; Dokouhaki, S.; Fallahrastegar, A.; Ghasemzadeh, A. Effectiveness of an 810-nm Diode Laser in Addition to Non-surgical Periodontal Therapy in Patients With Chronic Periodontitis: A Randomized Single-Blind Clinical Trial. J. Lasers Med. Sci. 2021, 12, e37. [Google Scholar] [CrossRef] [PubMed]
- Kamar Affendi, N.H.; Ahmad, R.; Vahidi, F.; Hassan, M.Z.; Rahimi, S.N. The Integration of a Dual-Wavelength Super Pulsed Diode Laser for Consistent Tissue Ablation in the Esthetic Zone: A Case Series. Case Rep. Dent. 2020, 2020, 8883156. [Google Scholar] [CrossRef]
- Gutiérrez-Corrales, A.; Rizcala-Orlando, Y.; Montero-Miralles, P.; Volland, G.; Gutiérrez-Pérez, J.L.; Torres-Lagares, D.; Serrera-Figallo, M.A. Comparison of diode laser—Oral tissue interaction to different wavelengths. In vitro study of porcine periodontal pockets and oral mucosa. Med. Oral. Patol. Oral. Cir. Bucal 2020, 25, e224–e232. [Google Scholar] [CrossRef]
- Michalik, M.; Szymańczyk, J.; Stajnke, M.; Ochrymiuk, T.; Cenian, A. Medical Applications of Diode Lasers: Pulsed versus Continuous Wave (cw) Regime. Micromachines 2021, 12, 710. [Google Scholar] [CrossRef]
- Moeintaghavi, A.; Ahrari, F.; Fallahrastegar, A.; Salehnia, A. Comparison of the Effectiveness of CO2 and Diode Lasers for Gingival Melanin Depigmentation: A Randomized Clinical Trial. J. Lasers Med. Sci. 2022, 13, e8. [Google Scholar] [CrossRef]
- Azzeh, M.M. Treatment of gingival hyperpigmentation by erbium-doped:yttrium, aluminum, and garnet laser for esthetic purposes. J. Periodontol. 2007, 78, 177–184. [Google Scholar] [CrossRef]
- Murthy, M.B.; Kaur, J.; Das, R. Treatment of gingival hyperpigmentation with rotary abrasive, scalpel, and laser techniques: A case series. J. Indian. Soc. Periodontol. 2012, 16, 614–619. [Google Scholar] [CrossRef]
- Alhabashneh, R.; Darawi, O.; Khader, Y.S.; Ashour, L. Gingival depigmentation using Er:YAG laser and scalpel technique: A six-month prospective clinical study. Quintessence Int. 2018, 49, 113–122. [Google Scholar] [CrossRef]
- Beşiroğlu-Turgut, E.; Kayaaltı-Yüksek, S. Comparison of Er,Cr:YSGG laser and diode laser in the treatment of gingival melanin pigmentation: A randomized clinical trial. Lasers Med. Sci. 2023, 38, 79. [Google Scholar] [CrossRef] [PubMed]
- Suthprasertporn, S. Treatment of gingival melanin hyperpigmentation by Er, Cr: YSGG laser: Report of 2 cases. Thai J. Periodontol. 2007, 1, 46–55. [Google Scholar]
- Moneim, R.A.A.; El Deeb, M.; Rabea, A.A. Gingival pigmentation (cause, treatment and histological preview). Future Dent. J. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Parvez, M. Comparative Evaluation of Split Thickness Epithelial Excision and Cryosurgery for the Treatment of Gingival Pigmentation; Rajiv Gandhi University of Health Sciences: Karnataka, India, 2006. [Google Scholar]
- Narayankar, S.D.; Deshpande, N.C.; Dave, D.H.; Thakkar, D.J. Comparative evaluation of gingival depigmentation by tetrafluroethane cryosurgery and surgical scalpel technique. A randomized clinical study. Contemp. Clin. Dent. 2017, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Agrawal, N.; Reddy, N. Gingival depigmentation: A case report. People’s J. Sci. Res. 2010, 3, 27–29. [Google Scholar]
- Gul, M.; Hameed, M.H.; Nazeer, M.R.; Ghafoor, R.; Khan, F.R. Most effective method for the management of physiologic gingival hyperpigmentation: A systematic review and meta-analysis. J. Indian Soc. Periodontol. 2019, 23, 203. [Google Scholar] [CrossRef]
- Suragimath, G.; Lohana, M.H.; Varma, S. A split mouth randomized clinical comparative study to evaluate the efficacy of gingival depigmentation procedure using conventional scalpel technique or diode laser. J. Lasers Med. Sci. 2016, 7, 227. [Google Scholar] [CrossRef]
- Raghavendra, R.N.; Ragul, M.; Nabeeh, A.-Q.; Ravi, K.; Tikare, S.; Pasupuleti, M.K. Clinical effectiveness of gingival depigmentation using conventional surgical scrapping and diode laser technique: A quasi experimental study. Glob. J. Health Sci. 2016, 9, 296. [Google Scholar] [CrossRef]
- Raaman, A.R.; Pratebha, B.; Jananni, M.; Saravanakumar, R. Comparison of efficacy of depigmentation of gingiva in terms of ImageJ intensity values and surface area of repigmentation using scalpel and diode laser. Int. J. Oral. Health Sci. 2016, 6, 59–64. [Google Scholar]
- Becker, D.E. Pain management: Part 1: Managing acute and postoperative dental pain. Anesth. Prog. 2010, 57, 67–79. [Google Scholar] [CrossRef]
| Study ID | Study Design | Interventions | Sample Size | Age | Males | Pigmented Lesions | Location | Follow-Up |
|---|---|---|---|---|---|---|---|---|
| Altayeb et al., 2021 [10] | RCT | Diode 940 nm and Er,Cr: YSGG 2780 nm | 60 | 21–43 | 22 | Physiological pigmentation | Upper gingiva | 2 years |
| Gholami et al., 2018 [20] | RCT | Er,Cr 2 settings/surgical stripping | 36 arches | 18+ | 12 | Physiological pigmentation | Upper and lower gingiva | 12 months |
| Hegde et al., 2013 [21] | CCT | Surgical stripping/carbon dioxide laser/Er YAG | 35 | 18–50 | 15 | Physiological pigmentation | Upper and lower gingiva | 6 months |
| Ribeiro et al., 2014 [22] | RCT | Nd YAG/scalpel stripping | 11 | NA | NA | Physiological pigmentation | Upper anterior gingiva | 6 months |
| Limpjaroenviriyak ul et al., 2020 [23] | RCT | QS 532 nm/LFQS 1064 nm both Nd YAG | 30 | 20+ | 8 | Multifactorial | Lip | 4 weeks |
| Giannelli et al., 2014 [24] | RCT | Er YAG/diode lasers | 21 | 18–40 | 10 | Physiological pigmentation | Upper and lower gingiva | 6 months |
| Tran et al., 2022 [25] | Cross-sectional | CO2 laser | 38 | 20–24 | 19 | Physiological pigmentation | Upper anterior gingiva | 6 months |
| Ranj Arif et al., 2020 [26] | RCT | Er YAG 2940/diode lasers 940 | 20 | 18–35 | 16 | Physiological pigmentation | Upper and lower anterior gingiva | 6 months |
| Rohini Negi et al., 2018 [13] | RCT | Diode laser/ceramic soft tissue trimming bur | 20 | 20–40 | NA | Physiological pigmentation | Upper anterior gingiva | 6 months |
| Stas et al., 2018 [27] | RCT | Er YAG (4 different settings) | 40 | 18–50 | NA | Physiological pigmentation | Upper and lower gingiva | 6 months |
| Suryavanshi et al., 2017 [11] | Non-RCT | Surgical blade/ electrosurgery/ FGG/diode laser | 40 | NA | NA | Physiological pigmentation | Upper anterior gingiva | 3 months |
| Houshmand et al., 2017 [28] | Non-RCT | Diode laser 940 (2 methods, sieve or conventional) | 15 | Mean 33 | 5 | Physiological pigmentation | Upper and lower anterior gingiva | 3 months |
| Roshannia et al., 2021 [29] | Non-RCT | Diamond bur abrasion/CO2 laser | 12 | 18–40 | NA | Physiological pigmentation | Upper and lower anterior gingiva | 6 months |
| Surve et al., 2020 [30] | RCT | Surgical blade/sieve method diode laser | 5 | 18–40 | NA | Physiological pigmentation | Anterior gingiva | 1 year |
| Hawwam et al., 2020 [31] | Case series | Surgical blade | 20 | 23–30 | 11 | Physiological pigmentation | Upper and lower gingiva | 1 year |
| AlShoubaki et al., 2018 [32] | Cohort | Rotary diamond bur abrasion | 34 | 19–45 | 10 | Smokers’ melanosis | Upper and lower gingiva | 6 months |
| Jokar et al., 2019 [12] | RCT | Cryosurgery/Diode laser 940 | 15 | 17–35 | 4 | Physiological pigmentation | Upper anterior gingiva | 1 year |
| Juliana et al., 2022 [33] | RCT | Blue M gel/Coe pack, both after the surgical blade | 20 | 20–38 | NA | Physiological pigmentation | Upper anterior gingiva | 4 weeks |
| Koca-Ünsal et al., 2021 [34] | RCT | Surgical blade/Diode laser 810 | 16 | 23–41 | 8 | Physiological pigmentation | Anterior gingiva | 1 week |
| Bakhshi et al., 2018 [35] | Non-RCT | Er CR YSGG/diode | 14 | 15–39 | 5 | Physiological pigmentation | Upper and lower gingiva | 6 months |
| El Shenawy et al., 2015 [36] | Case series | Diode 980 | 15 | 15–40 | 7 | Physiological pigmentation | Upper and lower anterior gingiva | 3 months |
| Kishore et al., 2014 [37] | RCT | Er YAG/CO2 laser | 20 | 18–30 | 10 | Physiological pigmentation | Upper anterior gingiva | 6 months |
| Harb et al., 2021 [38] | RCT | Diode 980 nm/Er YAG | 12 | 28.6 ± 7.8 | 7 | Physiological pigmentation | Upper and lower gingiva | 6 month |
| El-Mofty et al., 2021 [18] | RCT | Injection and topical vitamin C | 20 | 18–40 | 4 | Physiological pigmentation | Upper and lower anterior gingiva | 6 months |
| Chaudhary et al., 2023 [39] | RCT | Surgical blade/intraepidermal injection of vitamin C | 30 | 18–40 | 18 | Physiological pigmentation | Upper and lower anterior gingiva | 3 months |
| Yussif et al., 2019 [40] | Non-RCT | Vitamin C injection intraepidermal/surgical blade | 30 | 18+ | NA | Physiological pigmentation | Upper and lower anterior gingiva | 9 months |
| Grover et al., 2014 [41] | Non-RCT | Surgical blade/diode laser 800–980 nm | 20 | 15–35 | 11 | Physiological pigmentation | Upper and lower anterior gingiva | 3 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jazzar, A.; AlDehlawi, H. Efficacy and Risks of Different Treatments for Oral Hyperpigmentation: A Systematic Review and Network Meta-Analysis. J. Clin. Med. 2023, 12, 6567. https://doi.org/10.3390/jcm12206567
Jazzar A, AlDehlawi H. Efficacy and Risks of Different Treatments for Oral Hyperpigmentation: A Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine. 2023; 12(20):6567. https://doi.org/10.3390/jcm12206567
Chicago/Turabian StyleJazzar, Ahoud, and Hebah AlDehlawi. 2023. "Efficacy and Risks of Different Treatments for Oral Hyperpigmentation: A Systematic Review and Network Meta-Analysis" Journal of Clinical Medicine 12, no. 20: 6567. https://doi.org/10.3390/jcm12206567
APA StyleJazzar, A., & AlDehlawi, H. (2023). Efficacy and Risks of Different Treatments for Oral Hyperpigmentation: A Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine, 12(20), 6567. https://doi.org/10.3390/jcm12206567







